Double Trouble on the Lower Leg—Unique Human Coinfection with Echinococcus granulosus and Echinococcus multilocularis Without Liver Involvement
Abstract
1. Introduction
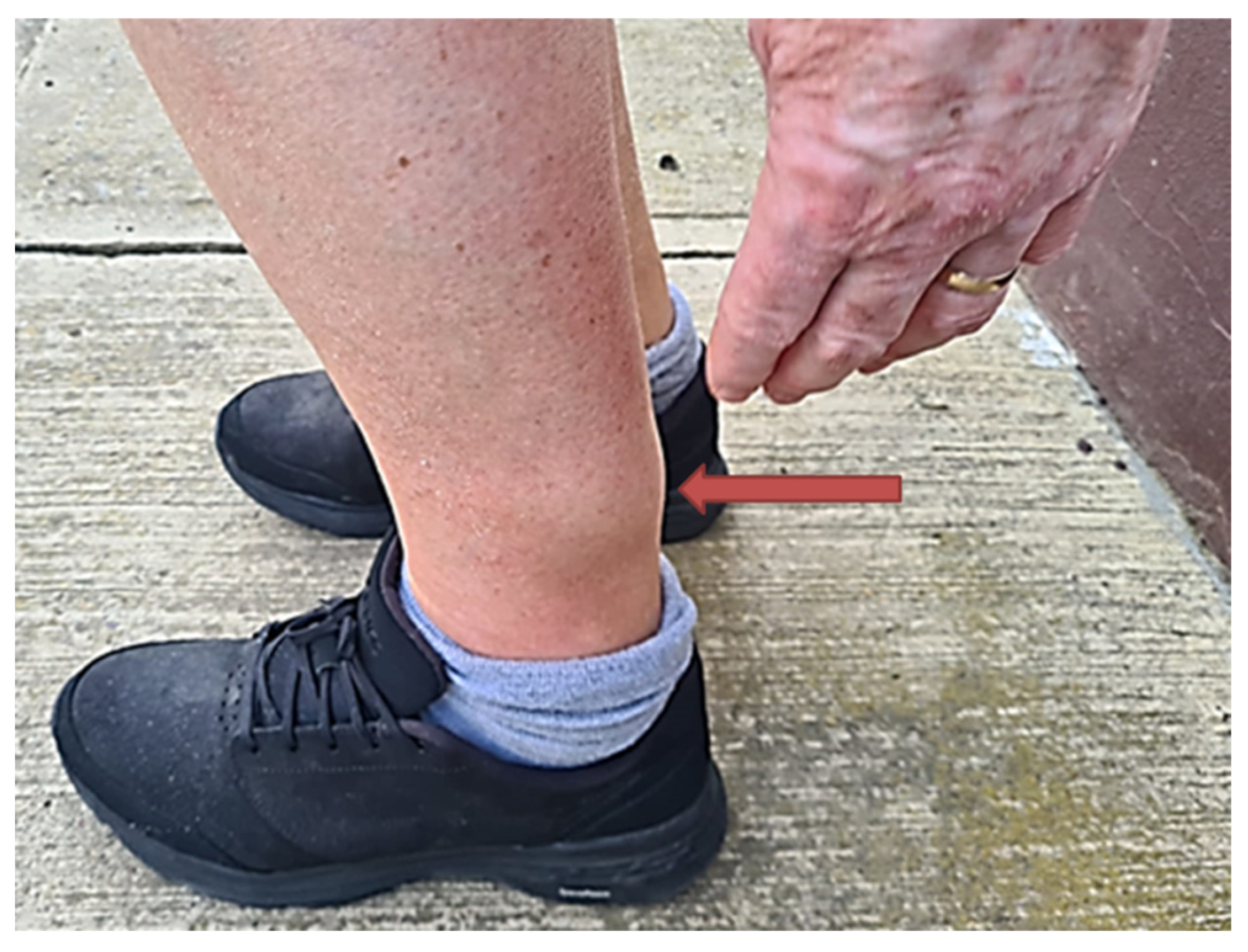
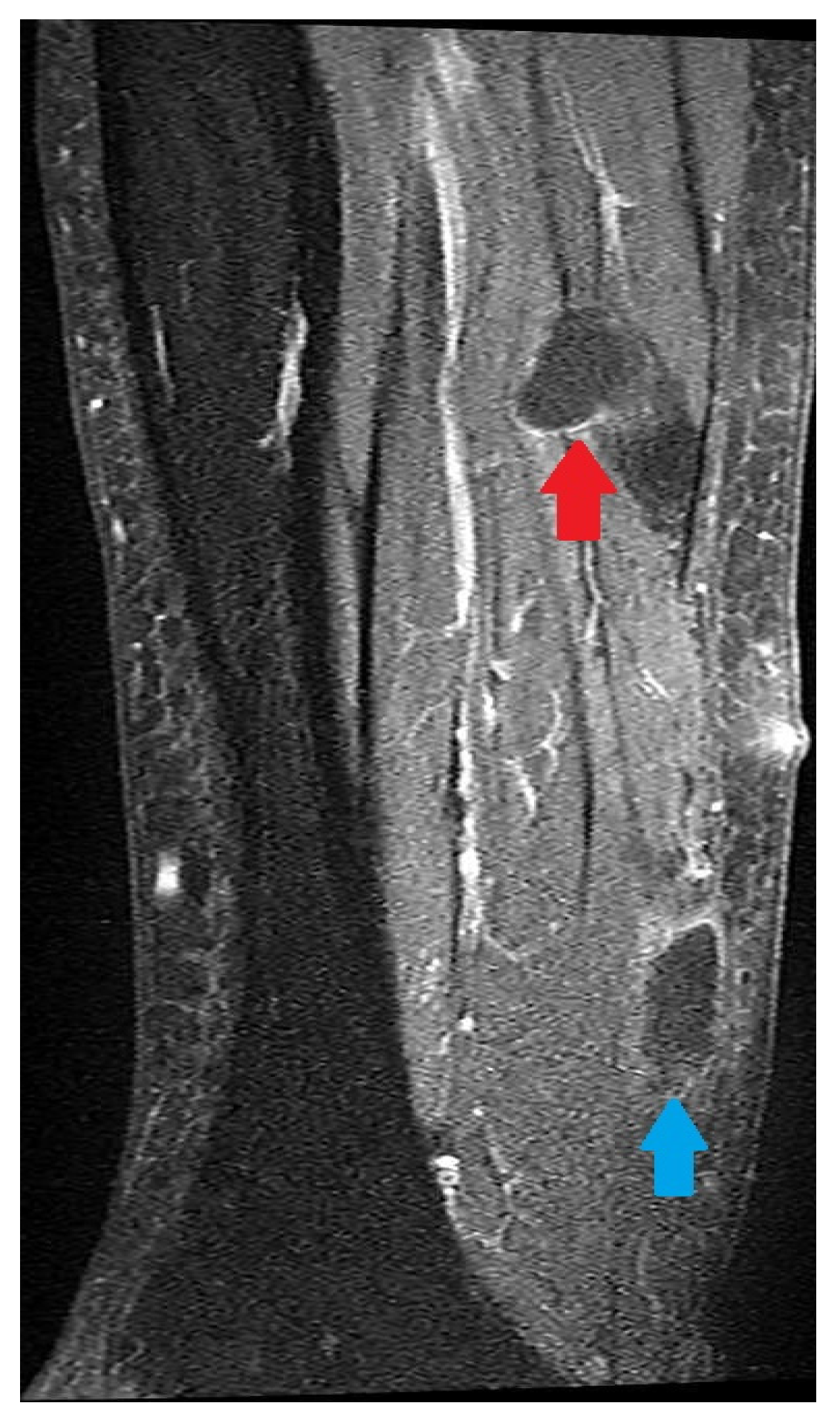
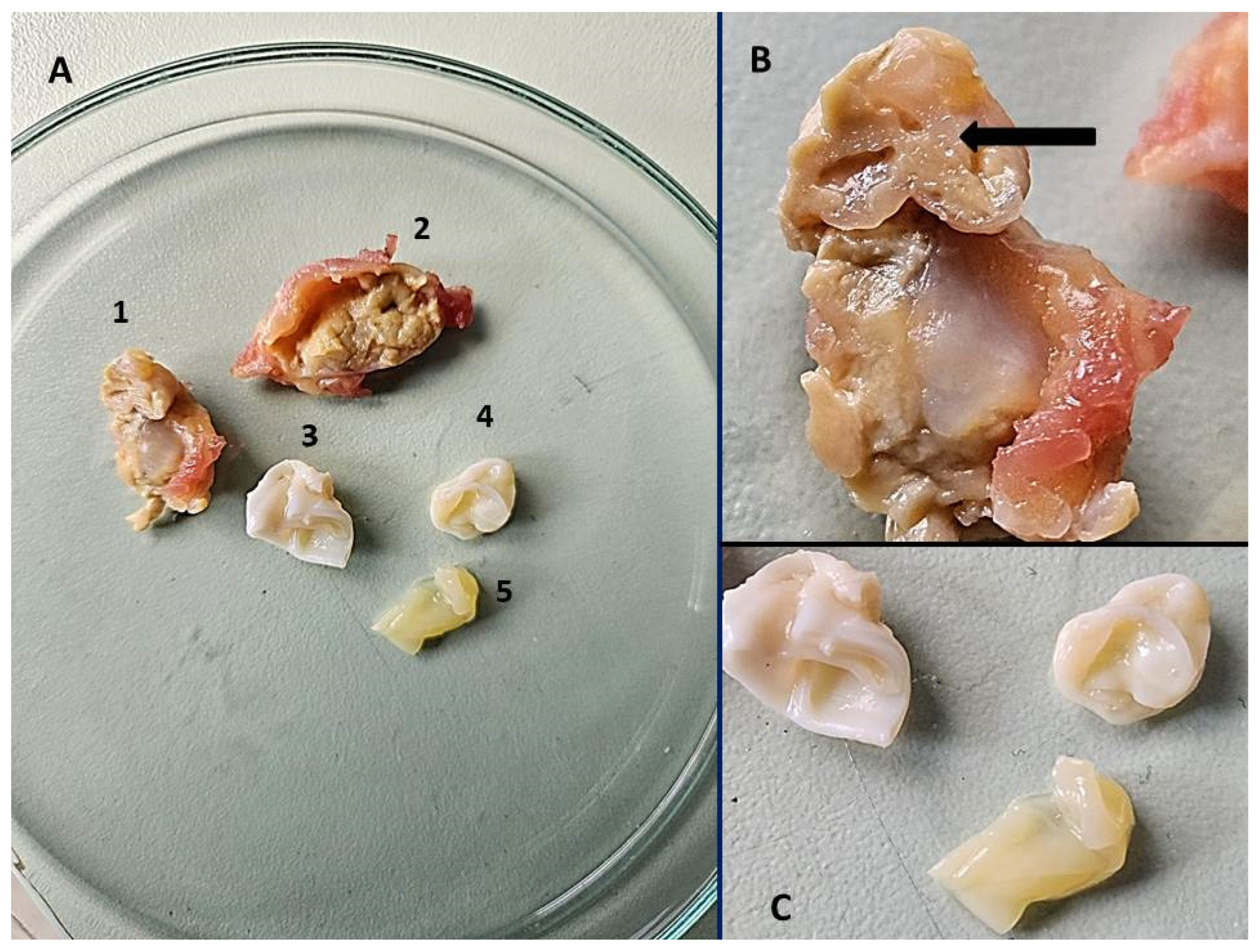
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conraths, F.J.; Probst, C.; Possenti, A.; Boufana, B.; Saulle, R.; La Torre, G.; Busani, L.; Casulli, A. Potential risk factors associated with human alveolar echinococcosis: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005801. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Rinaldi, L.; Rojas, C.A.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar] [CrossRef] [PubMed]
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; De La Rue, M. Ecology and life cycle patterns of Echinococcus species. Adv. Parasitol. 2017, 95, 213–314. [Google Scholar] [CrossRef]
- Oksanen, A.; Siles-Lucas, M.; Karamon, J.; Possenti, A.; Conraths, F.J.; Romig, T.; Wysocki, P.; Mannocci, A.; Mipatrini, D.; La Torre, G.; et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: A systematic review and meta-analysis. Parasit. Vectors 2016, 9, 519. [Google Scholar] [CrossRef]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef]
- Kern, P.; Da Silva, A.M.; Akhan, O.; Müllhaupt, B.; Vizcaychipi, K.A.; Budke, C.; Vuitton, D.A. The echinococcoses. Adv. Parasitol. 2017, 96, 259–369. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Qi, X.; Yang, L.; Duan, X.; Fang, B.; Gongsang, Q.; Bartholomot, B.; Vuitton, D.A.; Wen, H.; Craig, P.S. Human cystic and alveolar echinococcosis in the Tibet Autonomous Region (TAR), China. J. Helminthol. 2015, 89, 671–679. [Google Scholar] [CrossRef]
- Wen, H.; Tian, W.L.; Zou, P.F.; Xiang, M.X. A rare case of mixed cystic and alveolar hydatidosis. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 290–291. [Google Scholar] [CrossRef]
- Morović, M. Human hydatidosis in Dalmatia, Croatia. Epidemiol. Infect. 1997, 119, 271–276. [Google Scholar] [CrossRef]
- Beck, R.; Mihaljević, Ž.; Brezak, R.; Bosnić, S.; Janković, I.L.; Deplazes, P. First detection of Echinococcus multilocularis in Croatia. Parasitol. Res. 2017, 117, 617–621. [Google Scholar] [CrossRef]
- Dušek, D.; Vince, A.; Kurelac, I.; Papić, N.; Višković, K.; Deplazes, P.; Beck, R. Human alveolar echinococcosis, Croatia. Emerg. Infect. Dis. 2019, 26, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Topić, M.B.; Papić, N.; Višković, K.; Sviben, M.; Kanižaj, T.F.; Jadrijević, S.; Jurković, D.; Beck, R. Emergence of Echinococcus multilocularis in Central Continental Croatia: A Human Case Series and Update on Prevalence in Foxes. Life 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.; Blair, D.; Mcmanus, D. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992, 54, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, D.; Deplazes, P.; Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007, 134, 911–920. [Google Scholar] [CrossRef]
- Stieger, C.; Hegglin, D.; Schwarzenbach, G.; Mathis, A.; Deplazes, P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology 2002, 124, 631–640. [Google Scholar] [CrossRef]
- Le, T.H.; Pearson, M.S.; Blair, D.; Dai, N.; Zhang, L.H.; Mcmanus, D.P. Complete mitochondrial genomes confirm the distinctiveness of the horse-dog and sheep-dog strains of Echinococcus granulosus. Parasitology 2002, 124, 97–112. [Google Scholar] [CrossRef]
- Xiao, N.; Qiu, J.; Nakao, M.; Li, T.; Yang, W.; Chen, X.; Schantz, P.M.; Craig, P.S.; Ito, A. Echinococcus shiquicus n. sp., a taeniid cestode from Tibetan fox and plateau pika in China. Int. J. Parasitol. 2005, 35, 693–701. [Google Scholar] [CrossRef]
- Reuter, S.; Seitz, H.M.; Kern, P.; Junghanss, T. Extrahepatic Alveolar Echinococcosis without Liver Involvement: A Rare Manifestation. Infection 2000, 28, 187–192. [Google Scholar] [CrossRef]
- Ran, B.; Wang, M.; Jian, W.; Jiang, T.; Zhang, R.; Guo, Q.; Zhang, W.; Wen, H.; Shao, Y.; Aji, T. Simultaneous occurrence of hepatic alveolar and cystic echinococcosis. J. Helminthol. 2019, 94, e80. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Arif, S.H. Hydatid cyst of the calf presenting as painless mass: A case report. Int. J. Surg. Case Rep. 2019, 60, 273–275. [Google Scholar] [CrossRef]
- Notarnicola, A.; Moretti, L.; Panella, A.; Margari, A.G.G.; Cimino, A.; Pesce, V.; Moretti, B. Case report of a primary multiloculate muscular cystic hydatidosis. Musculoskelet. Surg. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- Samsami, M.; Qaderi, S.; Bagherpour, J.Z.; Lucero-Prisno, D.E. A case report of primary isolated extrahepatic hydatid cyst of the soft tissues of the breast and thigh. Int. J. Surg. Case Rep. 2021, 79, 475–478. [Google Scholar] [CrossRef]
- Arian, M.; Kazerani, M. Primary hydatid cyst in the adductor muscles of thigh: A case report. Clin. Case Rep. 2022, 10, e6664. [Google Scholar] [CrossRef]
- Shetty, V.; Shetty, K.S.; Ali, I.M. Hydatid cyst in the thigh: An unusual extra-hepatic site. Cureus 2024, 16, e67929. [Google Scholar] [CrossRef] [PubMed]
- Kaya, O.; Gönder, N. An unusual cause of insidious back and shoulder pain in a man: A case report. Iran. J. Parasitol. 2024, 19, 123–127. [Google Scholar] [CrossRef]
- Gurcan, M.; Ergul, R.B.; Degirmenci, E.; Dursun, M.; Kadıoğlu, A. A rare presentation of hydatid cyst: A case report of uncommon localization in the pelvic region and a review of current literature. Cureus 2024, 16, e60312. [Google Scholar] [CrossRef] [PubMed]
- Seyedsadeghi, M.; Ghobadi, J.; Haghshenas, N.; Habibzadeh, A. Gluteal hydatid cyst: A case report. Iran. J. Parasitol. 2019, 14, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Ciobotaru, O.C.; Duca, O.-M.; Ciobotaru, O.R.; Stamate, E.; Piraianu, A.I.; Dumitrascu, A.G.; Constantin, G.B.; Matei, M.N.; Voinescu, D.C.; Luchian, S.-A. Hydatid Cysts of the Psoas Muscle: Insights from the Past Five Years. Life 2024, 14, 1331. [Google Scholar] [CrossRef]
- Nell, M.; Burgkart, R.H.; Gradl, G.; Von Eisenhart-Rothe, R.; Schaeffeler, C.; Trappe, D.; Da Costa, C.P.; Gradinger, R.; Kirchhoff, C. Primary extrahepatic alveolar echinococcosis of the lumbar spine and the psoas muscle. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 13. [Google Scholar] [CrossRef]
- Merkle, E.M.; Kramme, E.; Vogel, J.; Krämer, S.; Schulte, M.; Usadel, S.; Kern, P.; Brambs, H.J. Bone and soft tissue manifestations of alveolar echinococcosis. Skelet. Radiol. 1997, 26, 289–292. [Google Scholar] [CrossRef]
- Song, T.; Peng, S.; Zhou, X.; Jiang, L.; Zhang, J. Case Report: Diagnosis of vertebral alveolar echinococcosis upon next-generation sequencing in a suspected tuberculosis. Front. Surg. 2022, 9, 984640. [Google Scholar] [CrossRef]
- Koppen, T.; Barth, T.F.E.; Eichhorn, K.W.; Gabrielpillai, J.; Kader, R.; Bootz, F.; Send, T. Alveolar Echinococcosis of the Parotid Gland—An Ultra Rare Location Reported from Western Europe. Pathogens 2021, 10, 426. [Google Scholar] [CrossRef]
- Keutgens, A.; Simoni, P.; Detrembleur, N.; Frippiat, F.; Giot, J.-B.; Spirlet, F.; Aghazarian, S.; Descy, J.; Meex, C.; Huynen, P.; et al. Fatal alveolar echinococcosis of the lumbar spine. J. Clin. Microbiol. 2012, 51, 688–691. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; A, J.; Guo, Y.; Yang, J.; Naveed, A.; Gao, W. Co-Occurrence of cystic and alveolar echinococcosis in the liver: A case report. Iran. J. Parasitolog. 2021, 16, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; An, X.Q.; Chai, J.P.; Yang, J.Y.; A, J.D.; A, X.R. Coinfection with hepatic cystic and alveolar echinococcosis with abdominal wall abscess and sinus tract formation: A case report. World J. Hepatol. 2024, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, C.; Ye, H.; Wang, Z.; Wang, Z.; Zhou, Y.; Wang, H.; Zhang, B.; Pang, M.; Zhou, H.; et al. Diagnosis and treatment of a case of hepatic mixed echinococcosis infection combined with distant organ metastasis. J. Int. Med. Res. 2019, 48, 300060519851651. [Google Scholar] [CrossRef] [PubMed]
- A, J.D.; Chai, J.P.; Wang, H.; Gao, W.; Peng, Z.; Zhao, S.Y.; A, X.R. Diagnosis and treatment of mixed infection of hepatic cystic and alveolar echinococcosis: Four case reports. World J. Clin. Cases 2020, 8, 3911–3919. [Google Scholar] [CrossRef]
- Meštrović, T.; Sviben, M.; Jurišić, A.; Stevanovski, F.; Beck, R.; Balen Topić, M. A ticking time bomb? A position paper on the rising and neglected threat of alveolar echinococcosis in the Republic of Croatia. Clin. Microbiol. Infect. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Kapel, C.M.O.; Torgerson, P.R.; Thompson, R.C.A.; Deplazes, P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006, 36, 79–86. [Google Scholar] [CrossRef]
- Schmidberger, J.; Uhlenbruck, J.; Schlingeloff, P.; Maksimov, P.; Conraths, F.J.; Mayer, B.; Kratzer, W. Dog ownership and risk for alveolar echinococcosis, Germany. Emerg. Infect. Dis. 2022, 28, 1597–1605. [Google Scholar] [CrossRef]
- Balog, T.; Nagy, G.; Halász, T.; Csányi, E.; Zomborszky, Z.; Csivincsik, Á. The occurrence of Echinococcus spp. in golden jackal (Canis aureus) in southwestern Hungary: Should we need to rethink its expansion? Parasitol. Int. 2020, 80, 102214. [Google Scholar] [CrossRef] [PubMed]
- Stronen, A.V.; Konec, M.; Boljte, B.; Bošković, I.; Gačić, D.; Galov, A.; Heltai, M.; Jelenčič, M.; Kljun, F.; Kos, I.; et al. Population genetic structure in a rapidly expanding mesocarnivore: Golden jackals in the Dinaric-Pannonian region. Glob. Ecol. Conserv. 2021, 28, e01707. [Google Scholar] [CrossRef]
- Sindičić, M.; Bujanić, M.; Štimac, I.; Martinković, F.; Tuškan, N.; Špehar, M.; Konjević, D. First identification of Echinococcus multilocularis in golden jackals in Croatia. Acta Parasitol. 2018, 63, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Soboslay, P.; Ortona, E.; Wang, J.; Siracusano, A.; Vuitton, D.A. Immunology of Alveolar and Cystic echinococcosis (AE and CE). Adv. Parasitol. 2016, 96, 1–54. [Google Scholar] [CrossRef]
- Siles-Lucas, M.; Casulli, A.; Conraths, F.J.; Müller, N. Laboratory Diagnosis of Echinococcus spp. in Human Patients and Infected Animals. Adv. Parasitol. 2017, 96, 159–257. [Google Scholar] [CrossRef]
- Guo, H.; Liu, W.; Wang, J.; Xing, Y. Extrahepatic alveolar echinococcus on multi-slice computed tomography and magnetic resonance imaging. Sci. Rep. 2021, 11, 9409. [Google Scholar] [CrossRef]
- Şimşek, S.; Hattapoğlu, S. Intramuscular hydatid cyst in the lower extremity: Report of three cases. Rev. Soc. Bras. Med. Trop. 2021, 54, e02552021. [Google Scholar] [CrossRef]
| August 2023 | February 2024 | March 2024 | May 2024 | June 2024 | September 2024 | |
|---|---|---|---|---|---|---|
| Surgery | Incision and biopsy on the lesion of the calf | Surgical excision of lessions of the calf | ||||
| Radiology | MRI of the head | MSCT of the calf Chest X-ray | MRI of the leg; MSCT of abdomen and pelvis | |||
| Histopathology | Histopathologically described as eosinophilic amorphous material | |||||
| Molecular analysis | PCR and sequencing from paraffin blocks collected in February 2024 confirmed E. granulosus | PCR and sequencing, confirmed E. granulosus and E. multilocularis | ||||
| Serology | EIA, WB positive for E. granulosus and E. multilocularis | |||||
| Treatment | Multiple antibiotic courses due to suspected abscess | Albendazol treatment 2 × 400 mg | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, D.; Balen Topić, M.; Višković, K.; Papić, N.; Žic, R.; Sviben, M.; Meštrović, T.; Baković Kovačević, A.; Beck, R. Double Trouble on the Lower Leg—Unique Human Coinfection with Echinococcus granulosus and Echinococcus multilocularis Without Liver Involvement. Pathogens 2025, 14, 343. https://doi.org/10.3390/pathogens14040343
Beck D, Balen Topić M, Višković K, Papić N, Žic R, Sviben M, Meštrović T, Baković Kovačević A, Beck R. Double Trouble on the Lower Leg—Unique Human Coinfection with Echinococcus granulosus and Echinococcus multilocularis Without Liver Involvement. Pathogens. 2025; 14(4):343. https://doi.org/10.3390/pathogens14040343
Chicago/Turabian StyleBeck, David, Mirjana Balen Topić, Klaudija Višković, Neven Papić, Rado Žic, Mario Sviben, Tomislav Meštrović, Adrijana Baković Kovačević, and Relja Beck. 2025. "Double Trouble on the Lower Leg—Unique Human Coinfection with Echinococcus granulosus and Echinococcus multilocularis Without Liver Involvement" Pathogens 14, no. 4: 343. https://doi.org/10.3390/pathogens14040343
APA StyleBeck, D., Balen Topić, M., Višković, K., Papić, N., Žic, R., Sviben, M., Meštrović, T., Baković Kovačević, A., & Beck, R. (2025). Double Trouble on the Lower Leg—Unique Human Coinfection with Echinococcus granulosus and Echinococcus multilocularis Without Liver Involvement. Pathogens, 14(4), 343. https://doi.org/10.3390/pathogens14040343






