Exophiala Bloodstream Infections in Humans—A Narrative Review
Abstract
1. Introduction
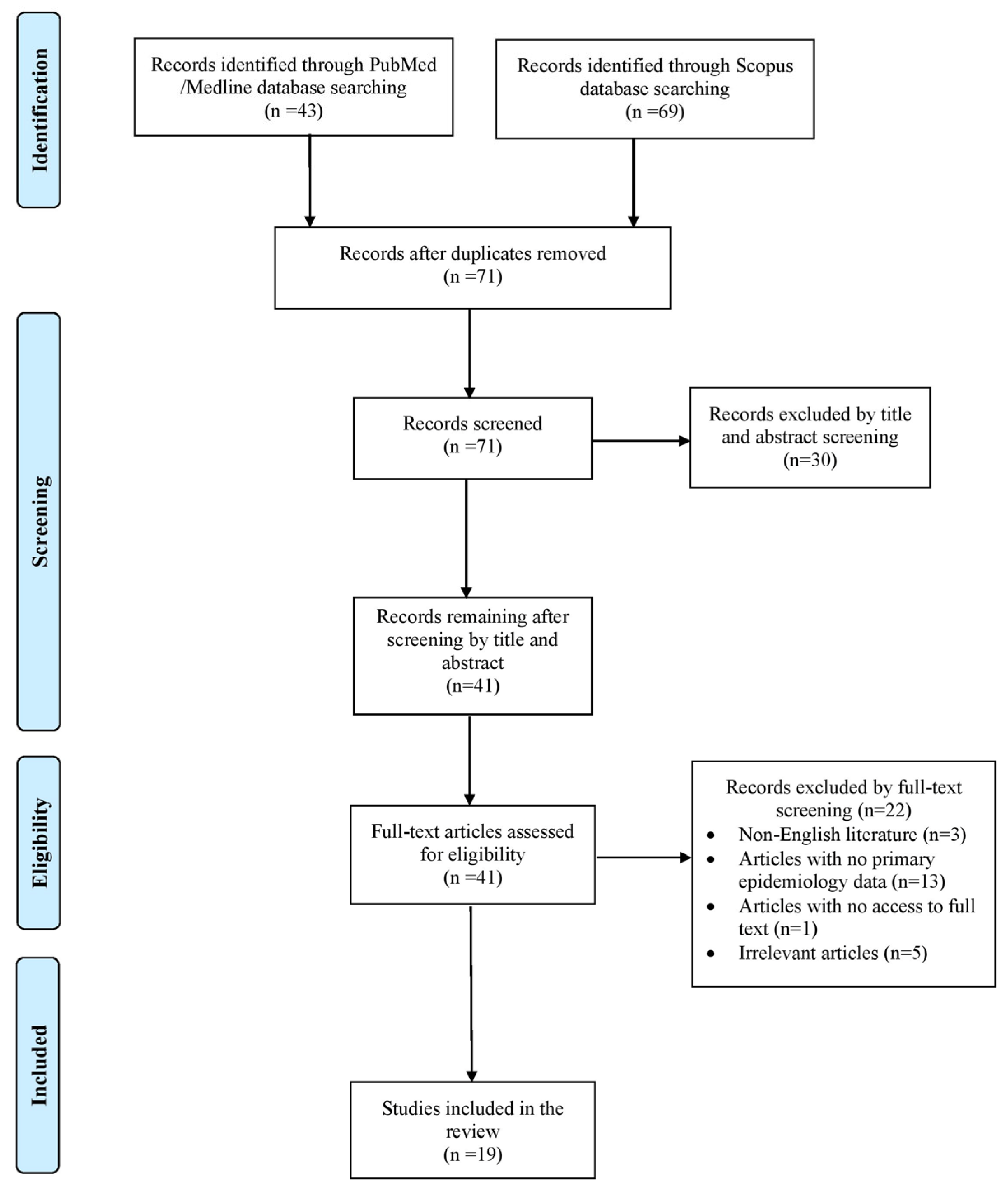
2. Materials and Methods
2.1. Search Strategy and Inclusion and Exclusion Criteria
2.2. Data Extraction and Definition
3. Results
3.1. Included Studies’ Characteristics
3.2. Epidemiology of Exophiala spp. Fungemia
3.3. Antifungal Resistance and Microbiology of Exophiala spp. Fungemia
3.4. Clinical Presentation of Exophiala spp. Fungemia
3.5. Treatment and Outcome of Exophiala spp. Fungemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyedmousavi, S.; Netea, M.G.; Mouton, J.W.; Melchers, W.J.G.; Verweij, P.E.; de Hoog, G.S. Black yeasts and their filamentous relatives: Principles of pathogenesis and host defense. Clin. Microbiol. Rev. 2014, 27, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.; de Hoog, G.S.; de Boer, A.G.; de Crom, I.; Haase, G. High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 2002, 45, 373–377. [Google Scholar] [CrossRef]
- Hohl, P.E.; Holley, H.P.; Prevost, E.; Ajello, L.; Padhye, A.A. Infections due to Wangiella dermatitidis in humans: Report of the first documented case from the United States and a review of the literature. Rev. Infect. Dis. 1983, 5, 854–864. [Google Scholar] [CrossRef]
- Mpakosi, A.; Siopi, M.; Demetriou, M.; Falaina, V.; Theodoraki, M.; Meletiadis, J. A fatal neonatal case of fungemia due to Exophiala dermatitidis-case report and literature review. BMC Pediatr. 2022, 22, 482. [Google Scholar] [CrossRef] [PubMed]
- de León, L.R.; Moreno-Perlín, T.; Castillo-Marenco, T.; Sánchez-Carbente, M.d.R.; Gostinčar, C.; Ramírez-Durán, N.; Ocaña, A.M.F.; Sánchez, N.C. Polyextremotolerant, opportunistic, and melanin-driven resilient black yeast Exophiala dermatitidis in environmental and clinical contexts. Sci. Rep. 2025, 15, 6472. [Google Scholar] [CrossRef]
- Chalkias, S.; Alonso, C.D.; Levine, J.D.; Wong, M.T. Emerging pathogen in immunocompromised hosts: Exophiala dermatitidis mycosis in graft-versus-host disease. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2014, 16, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Gotoh, A.; Shirane, S.; Hamano, Y.; Hirai, Y.; Shimizu, M.; Nakamura, A.; Matsumoto, K.; Morita, K.; Mori, T.; et al. Breakthrough Exophiala dermatitidis infection during prophylactic administration of micafungin during second umbilical cord blood transplantation after graft failure. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2018, 20, e12833. [Google Scholar] [CrossRef]
- Simpson, A.J.; Nightingale, J.M. Intravascular line infection with Exophiala dermatitidis. Lancet Lond. Engl. 1995, 345, 67. [Google Scholar] [CrossRef]
- LaRocco, A.; Netzer, R.C. Central venous catheters as a risk factor for disseminated phaeohyphomycosis? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 35, 1022–1023, author reply 1023–1024. [Google Scholar] [CrossRef]
- Ahamad, A.; Tehreem, B.; Farooqi, M.; Maramara, B. Case report and literature review: Double jeopardy-Exophiala dermatitidis and Mycobacterium canariasense central line-associated bloodstream infection in a patient. Access Microbiol. 2022, 4, 000347. [Google Scholar] [CrossRef]
- Maraki, S.; Katzilakis, N.; Neonakis, I.; Stafylaki, D.; Meletiadis, J.; Hamilos, G.; Stiakaki, E. Exophiala dermatitidis Central Line-Associated Bloodstream Infection in a Child with Ewing’s Sarcoma: Case Report and Literature Review on Paediatric Infections. Mycopathologia 2022, 187, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Tzar, M.N.; Meor Jamaludin, W.H.B.; Abdul Wahab, A.; Ding, C.H. Exophiala dermatitidis, ‘the real black fungus’ fungemia in a patient with COVID-19. IDCases 2022, 27, e01428. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Murakami, H.; Ishibana, Y.; Matsubara, Y.; Yaguchi, T.; Kamei, K. Challenges in the diagnosis and management of central line-associated blood stream infection due to Exophiala dermatitidis in an adult cancer patient. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2021, 27, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nandakumar, A.; Nair, S.; Singh, A.; Shashindran, N.; Thulasidharan, S.; Subhash, K.; Ramachandran, A.; Chowdhary, A. Exophiala dermatitidis as a cause of central line associated bloodstream infection in an infant: Case report and literature review. Rev. Iberoam. Micol. 2021, 38, 12–15. [Google Scholar] [CrossRef]
- Yoshida, T.; Tachita, T.; Fujinami, H.; Oshima, Y.; Sasaki, H.; Marumo, Y.; Narita, T.; Ito, A.; Ri, M.; Kusumoto, S.; et al. Exophiala dermatitidis Fungemia Diagnosed Using Time-of-flight Mass Spectrometry during Chemotherapy for Malignant Lymphoma and Successful Treatment with Voriconazole. Intern. Med. Tokyo Jpn. 2019, 58, 2219–2224. [Google Scholar] [CrossRef]
- Vila, A.; Jahan, C.; Rivero, C.; Amadio, C.; Ampuero, A.; Pagella, H. Central line associated blood stream infection (CLABSI) due to Exophiala dermatitidis in an adult patient: Case report and review. Med. Mycol. Case Rep. 2019, 24, 33–36. [Google Scholar] [CrossRef]
- Hagiya, H.; Maeda, T.; Kusakabe, S.; Kawasaki, K.; Hori, Y.; Kimura, K.; Ueda, A.; Yoshioka, N.; Sunada, A.; Nishi, I.; et al. A fatal case of Exophiala dermatitidis disseminated infection in an allogenic hematopoietic stem cell transplant recipient during micafungin therapy. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2019, 25, 463–466. [Google Scholar] [CrossRef]
- Al-Obaid, I.; Ahmad, S.; Khan, Z.U.; Dinesh, B.; Hejab, H.M. Catheter-associated fungemia due to Exophiala oligosperma in a leukemic child and review of fungemia cases caused by Exophiala species. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2006, 25, 729–732. [Google Scholar] [CrossRef]
- Guarro, J.; Nucci, M.; Akiti, T.; Gené, J.; Cano, J.; Barreiro, M.D.G.C.; Aguilar, C. Phialemonium fungemia: Two documented nosocomial cases. J. Clin. Microbiol. 1999, 37, 2493–2497. [Google Scholar] [CrossRef]
- Nachman, S.; Alpan, O.; Malowitz, R.; Spitzer, E.D. Catheter-associated fungemia due to Wangiella (Exophiala) dermatitidis. J. Clin. Microbiol. 1996, 34, 1011–1013. [Google Scholar] [CrossRef]
- Nakatani, R.; Ashiarai, M.; Yoshihara, H.; Yada, K.; Nozaki, T.; Ushigusa, T.; Mori, N.; Hasegawa, D. Multidisciplinary management of disseminated Exophiala dermatitidis mycosis in an infant with mixed phenotype acute leukemia: A case report. BMC Infect. Dis. 2022, 22, 797. [Google Scholar] [CrossRef]
- Kabel, P.J.; Illy, K.E.; Holl, R.A.; Buiting, A.G.; Wintermans, R.G. Nosocomial intravascular infection with Exophiala dermatitidis. Lancet Lond. Engl. 1994, 344, 1167–1168. [Google Scholar] [CrossRef]
- Vasquez, A.M.; Zavasky, D.; Chow, N.A.; Gade, L.; Zlatanic, E.; Elkind, S.; Litvintseva, A.P.; Pappas, P.G.; Perfect, J.R.; Revankar, S.G.; et al. Management of an Outbreak of Exophiala dermatitidis Bloodstream Infections at an Outpatient Oncology Clinic. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 959–962. [Google Scholar] [CrossRef]
- Horré, R.; Schaal, K.P.; Siekmeier, R.; Sterzik, B.; de Hoog, G.S.; Schnitzler, N. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respir. Int. Rev. Thorac. Dis. 2004, 71, 360–366. [Google Scholar]
- Borman, A.M.; Fraser, M.; Szekely, A.; Larcombe, D.E.; Johnson, E.M. Rapid Identification of Clinically Relevant Members of the Genus Exophiala by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and Description of Two Novel Species, Exophiala campbellii and Exophiala lavatrina. J. Clin. Microbiol. 2017, 55, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Queiroz-Telles, F.; Haase, G.; Fernandez-Zeppenfeldt, G.; Angelis, D.A.; Ende, A.H.G.V.D.; Matos, T.; Peltroche-Llacsahuanga, H.; A Pizzirani-Kleiner, A.; Rainer, J.; et al. Black fungi: Clinical and pathogenic approaches. Med. Mycol. 2000, 38 (Suppl. S1), 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, J.; Novak Babič, M.; Zalar, P.; Gunde-Cimerman, N. The Black Yeast Exophiala dermatitidis and Other Selected Opportunistic Human Fungal Pathogens Spread from Dishwashers to Kitchens. PLoS ONE 2016, 11, e0148166. [Google Scholar] [CrossRef]
- Tesei, D.; Marzban, G.; Marchetti-Deschmann, M.; Tafer, H.; Arcalis, E.; Sterflinger, K. Proteome of tolerance fine-tuning in the human pathogen black yeast Exophiala dermatitidis. J. Proteom. 2015, 128, 39–57. [Google Scholar] [CrossRef]
- Chowdhary, A.; Perfect, J.; de Hoog, G.S. Black Molds and Melanized Yeasts Pathogenic to Humans. Cold Spring Harb. Perspect. Med. 2014, 5, a019570. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy—United States, July–November 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1109–1112. [Google Scholar]
- Kirchhoff, L.; Olsowski, M.; Zilmans, K.; Dittmer, S.; Haase, G.; Sedlacek, L.; Steinmann, E.; Buer, J.; Rath, P.-M.; Steinmann, J. Biofilm formation of the black yeast-like fungus Exophiala dermatitidis and its susceptibility to antiinfective agents. Sci. Rep. 2017, 7, 42886. [Google Scholar] [CrossRef]
- Nucci, M.; Akiti, T.; Barreiros, G.; Silveira, F.; Revankar, S.G.; Wickes, B.L.; Sutton, D.A.; Patterson, T.F. Nosocomial outbreak of Exophiala jeanselmei fungemia associated with contamination of hospital water. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2002, 34, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Gamaletsou, M.N. Treatment of fungal disease in the setting of neutropenia. Hematology 2013, 2013, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.S.; Almaghrabi, R.S. Complications of hematopoietic stem transplantation: Fungal infections. Hematol. Oncol. Stem Cell Ther. 2017, 10, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Desai, J.V.; Ricotta, E.E.; Swamydas, M.; Deming, C.; Conlan, S.; Quinones, M.; Matei-Rascu, V.; Sheriff, L.; Lecky, D.; et al. Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria. Cell Host Microbe 2022, 30, 1020–1033.e6. [Google Scholar] [CrossRef]
- Guenezan, J.; Drugeon, B.; Marjanovic, N.; Mimoz, O. Treatment of central line-associated bloodstream infections. Crit. Care Lond. Engl. 2018, 22, 303. [Google Scholar] [CrossRef]
- Opilla, M. Epidemiology of bloodstream infection associated with parenteral nutrition. Am. J. Infect. Control 2008, 36, e5–e8. [Google Scholar] [CrossRef]
- Blumberg, H.M.; Jarvis, W.R.; Soucie, J.M.; Edwards, J.E.; Patterson, J.E.; Pfaller, M.A.; Rangel-Frausto, M.S.; Rinaldi, M.G.; Saiman, L.; Wiblin, R.T.; et al. Risk Factors for Candidal Bloodstream Infections in Surgical Intensive Care Unit Patients: The NEMIS Prospective Multicenter Study. Clin. Infect. Dis. 2001, 33, 177–186. [Google Scholar] [CrossRef]
- Percival, S.L.; Williams, D.W. Ventilator-Associated Pneumonia, Endotracheal Tubes and Biofilms. In Biofilms in Infection Prevention and Control [Internet]; Elsevier: Amsterdam, The Netherlands, 2014; pp. 199–208. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780123970435000128 (accessed on 26 June 2025).
- Rodrigues, M.E.; Lopes, S.P.; Pereira, C.R.; Azevedo, N.F.; Lourenço, A.; Henriques, M.; Pereira, M.O.; Sturtevant, J. Polymicrobial Ventilator-Associated Pneumonia: Fighting In Vitro Candida albicans-Pseudomonas aeruginosa Biofilms with Antifungal-Antibacterial Combination Therapy. PLoS ONE 2017, 12, e0170433. [Google Scholar] [CrossRef]
- Messing, B.; Peitra-Cohen, S.; Debure, A.; Beliah, M.; Bernier, J.J. Antibiotic-lock technique: A new approach to optimal therapy for catheter-related sepsis in home-parenteral nutrition patients. JPEN J. Parenter. Enteral. Nutr. 1988, 12, 185–189. [Google Scholar] [CrossRef]
- Wang, C.; Xing, H.; Jiang, X.; Zeng, J.; Liu, Z.; Chen, J.; Wu, Y. Cerebral Phaeohyphomycosis Caused by Exophiala dermatitidis in a Chinese CARD9-Deficient Patient: A Case Report and Literature Review. Front. Neurol. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, L.; Olsowski, M.; Rath, P.M.; Steinmann, J. Exophiala dermatitidis: Key issues of an opportunistic fungal pathogen. Virulence 2019, 10, 984–998. [Google Scholar] [CrossRef]
- Lang, R.; Minion, J.; Skinner, S.; Wong, A. Disseminated Exophiala dermatitidis causing septic arthritis and osteomyelitis. BMC Infect. Dis. 2018, 18, 255. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Hollis, R.J.; Jones, R.N.; SENTRY Participants Group. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: Report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 2002, 46, 1032–1037. [Google Scholar] [CrossRef]
- Scott, L.J.; Simpson, D. Voriconazole: A review of its use in the management of invasive fungal infections. Drugs 2007, 67, 269–298. [Google Scholar] [CrossRef]
- Gülmez, D.; Doğan, Ö.; Boral, B.; Döğen, A.; İlKit, M.; de Hoog, G.S.; Arikan-Akdagli, S. In vitro activities of antifungal drugs against environmental Exophiala isolates and review of the literature. Mycoses 2018, 61, 561–569. [Google Scholar] [CrossRef]
| Characteristic | All Patients (n = 32) | Survived (n = 21) | Died (n =11) |
|---|---|---|---|
| Age, years, median | 58 | 59.5 | 57 |
| Male gender, n (%) | 21 (65.6) | 12 (57.1) | 9 (81.8) |
| Predisposing factors | |||
| Central venous catheter, n (%) | 31 (96.9) | 21 (100) | 10 (90.9) |
| Malignancy, n (%) | 25 (78.1) | 17 (81) | 8 (72.7) |
| Immunosuppression, n (%) | 10 (31.3) | 6 (28.6) | 4 (36.4) |
| Neutropenia, n (%) | 8 (25) | 5 (23.8) | 3 (27.3) |
| HCT, n (%) | 4 (12.5) | 1 (4.8) | 3 (27.3) |
| TPN, n (%) | 4 (12.5) | 2 (9.5) | 2 (18.2) |
| Polymicrobial infection, n (%) | 5 (15.6) | 4 (19) | 1 (9.1) |
| Clinical characteristics | |||
| Fever, n (%) | 16 (50) | 9 (42.9) | 7 (63.6) |
| Organ dysfunction, n (%) | 7 (21.9) | 1 (4.8) | 6 (54.5) |
| Treatment | |||
| Voriconazole, n (%) | 20 (62.5) | 15 (71.4) | 5 (45.5) |
| Amphotericin B, n (%) | 10 (31.3) | 4 (19) | 6 (54.5) |
| Micafungin, n (%) | 9 (28.1) | 3 (14.3) | 6 (54.5) |
| Itraconazole, n (%) | 6 (18.8) | 4 (19) | 2 (18.2) |
| Fluconazole, n (%) | 4 (12.5) | 3 (14.3) | 1 (9.1) |
| Outcomes | |||
| Deaths due to infection, n (%) | 8 (25) | NA | NA |
| Deaths overall, n (%) | 11 (34.4) | NA | NA |
| Antifungal Agent | Number of Patients | Resistance (%) |
|---|---|---|
| Micafungin | 9/9 | 100 |
| Anidulafungin | 4/5 | 80 |
| Caspofungin | 7/9 | 77.8 |
| Fluconazole | 5/10 | 50 |
| Flucytosine | 3/7 | 42.9 |
| Amphotericin B | 1/11 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziogou, A.; Giannakodimos, A.; Giannakodimos, I.; Baliou, S.; Tsantes, A.G.; Ioannou, P. Exophiala Bloodstream Infections in Humans—A Narrative Review. Pathogens 2025, 14, 706. https://doi.org/10.3390/pathogens14070706
Ziogou A, Giannakodimos A, Giannakodimos I, Baliou S, Tsantes AG, Ioannou P. Exophiala Bloodstream Infections in Humans—A Narrative Review. Pathogens. 2025; 14(7):706. https://doi.org/10.3390/pathogens14070706
Chicago/Turabian StyleZiogou, Afroditi, Alexios Giannakodimos, Ilias Giannakodimos, Stella Baliou, Andreas G. Tsantes, and Petros Ioannou. 2025. "Exophiala Bloodstream Infections in Humans—A Narrative Review" Pathogens 14, no. 7: 706. https://doi.org/10.3390/pathogens14070706
APA StyleZiogou, A., Giannakodimos, A., Giannakodimos, I., Baliou, S., Tsantes, A. G., & Ioannou, P. (2025). Exophiala Bloodstream Infections in Humans—A Narrative Review. Pathogens, 14(7), 706. https://doi.org/10.3390/pathogens14070706







