The Frontier of Entomo-Virology: Applications and Tools for Virus and Vector Surveillance
Abstract
1. Introduction
2. What Is Entomo-Virological Surveillance?
3. How Do Mosquitoes Participate in the Maintenance and Transmission of Arboviruses in the Environment?
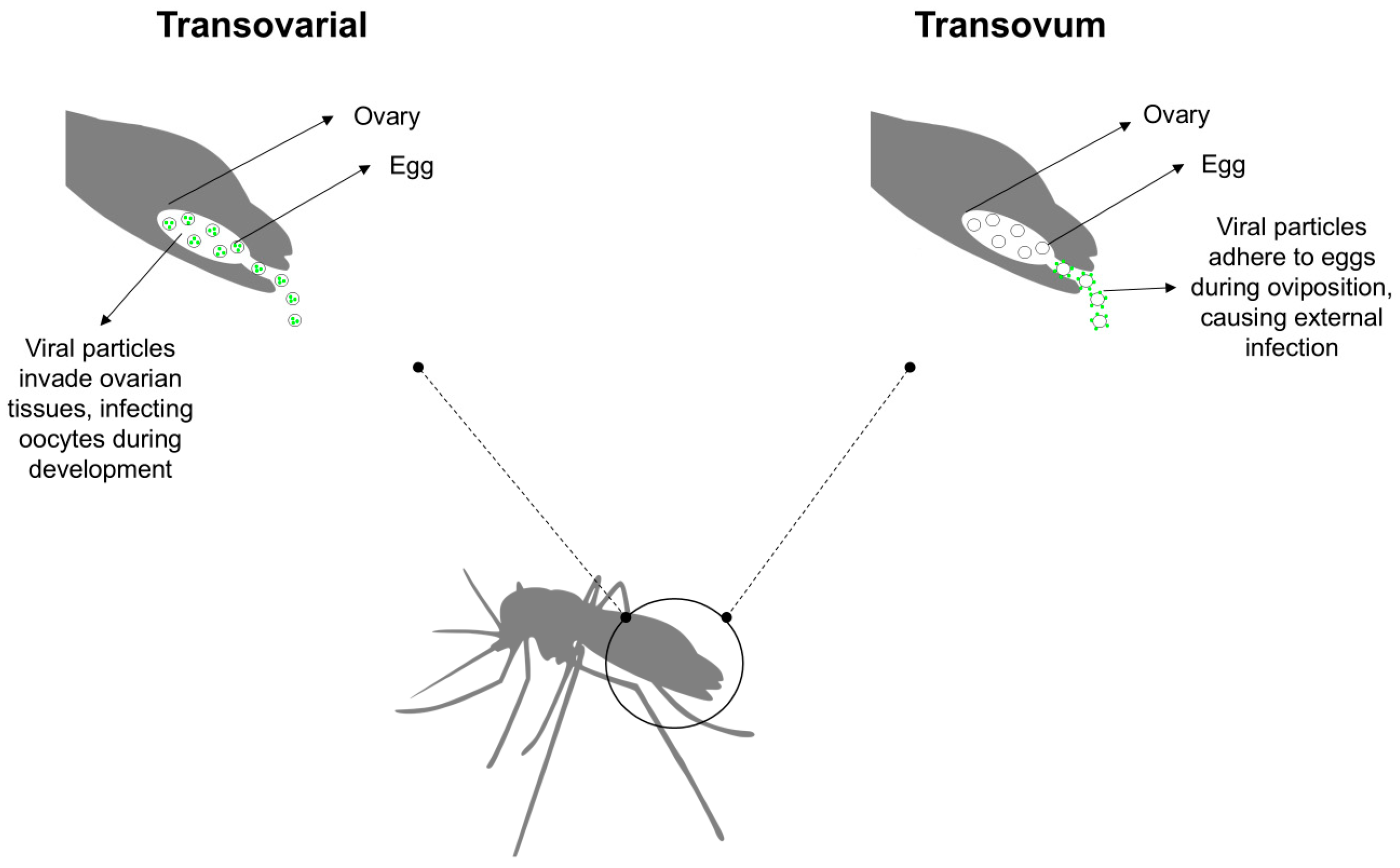
4. How Does the Prolonged Viability of Mosquito Eggs Influence Viral Transmission Dynamics?
5. Methodologies Applied to Entomo-Virological Surveillance
- n = total number of pools tested;
- x = number of positive pools;
- m = pool size.
6. The Role of Entomo-Virological Surveillance in Understanding the Multi-Vector Transmission of Arboviruses
7. Entomology–Virology for Strengthening Border and Port Surveillance
8. Challenges in Implementing Entomo-Virological Surveillance for Public Health
9. The Arbovirus Diagnostic Laboratory Network of the Americas (RELDA)
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CDC LT | CDC light traps |
| CHIKV | Alphavirus chikungunya |
| COI | Cytochrome oxidase 1 |
| Ct | Cycle threshold |
| CytB | Cytochrome oxidase B |
| DENV | Orthoflavivirus denguei |
| DNA | Deoxyribonucleic acid |
| EIP | Extrinsic incubation period |
| IVM | Integrated vector management |
| MEB | Midgut escape barrier |
| MIB | Midgut infection barrier |
| MIR | Minimum infection rate |
| MLE | Maximum likelihood estimate |
| NGS | Next-generation sequencing |
| OROV | Orthobunyavirus oropoucheense |
| PAHO | Pan American Health Organization |
| RELDA | Americas Arbovirus Diagnostic Network |
| RELEVA | Entomo-Virological Laboratory Network |
| RNA | Ribonucleic acid |
| rRNA | Ribosomal RNA |
| RT-qPCR | Reverse transcription–quantitative polymerase chain reaction |
| SGEB | Salivary gland escape barrier |
| SGIB | Salivary gland infection barrier |
| USUV | Orthoflavivirus usutuense |
| ViGenDa | Arbovirus Genomic Surveillance Platform |
| WHO | World Health Organization |
| WNV | Orthoflavivirus nilense |
| YFV | Orthoflavivirus flavi |
| ZIKV | Orthoflavivirus zikaense |
References
- World Health Organization. UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. In Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151297-8. [Google Scholar]
- Donalisio, M.R.; Freitas, A.R.R.; Zuben, A.P.B.V. Arboviruses Emerging in Brazil: Challenges for Clinic and Implications for Public Health. Rev. Saúde Pública 2017, 51, 30. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, L.; Matthews, E.; Piquet, A.L.; Henao-Martinez, A.; Franco-Paredes, C.; Tyler, K.L.; Beckham, D.; Pastula, D.M. Nervous System Manifestations of Arboviral Infections. Curr. Trop. Med. Rep. 2022, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The Global Economic Burden of Dengue: A Systematic Analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Lequime, S.; Lambrechts, L. Vertical Transmission of Arboviruses in Mosquitoes: A Historical Perspective. Infect. Genet. Evol. 2014, 28, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.; Shearer, F.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef]
- Tsioka, K.; Gewehr, S.; Pappa, S.; Kalaitzopoulou, S.; Stoikou, K.; Mourelatos, S.; Papa, A. West Nile Virus in Culex Mosquitoes in Central Macedonia, Greece, 2022. Viruses 2023, 15, 224. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, S.K.; Singh, H. Surveillance of Zika and Dengue Viruses in Field-Collected Aedes aegypti Mosquitoes from Different States of India. Virology 2022, 574, 96–101. [Google Scholar] [CrossRef]
- Ferreira-de-Brito, A.; Ribeiro, I.P.; Miranda, R.M.D.; Fernandes, R.S.; Campos, S.S.; Silva, K.A.B.D.; Castro, M.G.D.; Bonaldo, M.C.; Brasil, P.; Lourenço-de-Oliveira, R. First Detection of Natural Infection of Aedes aegypti with Zika Virus in Brazil and throughout South America. Mem. Inst. Oswaldo Cruz 2016, 111, 655–658. [Google Scholar] [CrossRef]
- Aragão, C.F.; Pinheiro, V.C.S.; Nunes Neto, J.P.; Silva, E.V.P.D.; Pereira, G.J.G.; Nascimento, B.L.S.D.; Castro, K.D.S.; Maia, A.M.; Catete, C.P.; Martins, L.C.; et al. Natural Infection of Aedes aegypti by Chikungunya and Dengue Type 2 Virus in a Transition Area of North-Northeast Brazil. Viruses 2019, 11, 1126. [Google Scholar] [CrossRef]
- Teixeira, A.F.; De Brito, B.B.; Correia, T.M.L.; Viana, A.I.S.; Carvalho, J.C.; Da Silva, F.A.F.; Santos, M.L.C.; Da Silveira, E.A.; Neto, H.P.G.; Da Silva, N.M.P.; et al. Simultaneous Circulation of Zika, Dengue, and Chikungunya Viruses and Their Vertical Co-Transmission among Aedes aegypti. Acta Trop. 2021, 215, 105819. [Google Scholar] [CrossRef]
- Almeida-Souza, P.A.; Oliveira, C.H.D.; Brito, L.P.; Teixeira, T.D.J.; Celestino, I.A.; Penha, G.B.; Dos Santos, R.M.; Mendes, W.M.; Ribeiro, B.M.; Campos, F.S.; et al. High Frequencies of Kdr Mutation and Chikungunya Infection in Aedes aegypti Population from Minas Gerais, Brazil. Pathogens 2024, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, I.C.; Gibson, G.; Ayllón, T.; De Medeiros Tavares, A.; De Araújo, J.M.G.; Da Silva Monteiro, E.; Rodrigues Aguiar, A.; De Oliveira, J.V.; De Paiva, A.A.P.; Wana Bezerra Pereira, H.; et al. Entomo-Virological Surveillance Strategy for Dengue, Zika and Chikungunya Arboviruses in Field-Caught Aedes Mosquitoes in an Endemic Urban Area of the Northeast of Brazil. Acta Trop. 2019, 197, 105061. [Google Scholar] [CrossRef]
- Cruz, A.C.R.; Hernández, L.H.A.; Aragão, C.F.; Da Paz, T.Y.B.; Da Silva, S.P.; Da Silva, F.S.; De Aquino, A.A.; Cereja, G.J.G.P.; Nascimento, B.L.S.D.; Rosa Junior, J.W.; et al. The Importance of Entomo-Virological Investigation of Yellow Fever Virus to Strengthen Surveillance in Brazil. Trop. Med. Infect. Dis. 2023, 8, 329. [Google Scholar] [CrossRef]
- Pinheiro, G.G.; Rocha, M.N.; De Oliveira, M.A.; Moreira, L.A.; Andrade Filho, J.D. Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil. Insects 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.V.S.D.; Ribeiro, I.P.; Ferreira-de-Brito, A.; Santos, A.A.C.D.; Miranda, R.M.D.; Bonelly, I.D.S.; Neves, M.S.A.S.; Bersot, M.I.; Santos, T.P.D.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys Are the Primary Vectors in the Major Yellow Fever Outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Cunha, M.S.; Faria, N.R.; Caleiro, G.S.; Candido, D.S.; Hill, S.C.; Claro, I.M.; Da Costa, A.C.; Nogueira, J.S.; Maeda, A.Y.; Da Silva, F.G.; et al. Genomic Evidence of Yellow Fever Virus in Aedes scapularis, Southeastern Brazil, 2016. Acta Trop. 2020, 205, 105390. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, L.M.D.A.; Motta, M.D.A.; Erbisti, R.S.; Abreu, F.V.S.D.; Nascimento-Pereira, A.C.; Ferreira-de-Brito, A.; Neves, M.S.A.S.; Pereira, G.R.; Pereira, G.R.; Santos, C.B.D.; et al. Back to Where It Was First Described: Vectors of Sylvatic Yellow Fever Transmission in the 2017 Outbreak in Espírito Santo, Brazil. Viruses 2022, 14, 2805. [Google Scholar] [CrossRef]
- De Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; Da, C.; Cardoso, J.; Gonçalves-dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; Almeida, M.A.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar] [CrossRef]
- Cevallos, V.; Ponce, P.; Waggoner, J.J.; Pinsky, B.A.; Coloma, J.; Quiroga, C.; Morales, D.; Cárdenas, M.J. Zika and Chikungunya Virus Detection in Naturally Infected Aedes aegypti in Ecuador. Acta Trop. 2018, 177, 74–80. [Google Scholar] [CrossRef]
- Wang, T.; Fan, Z.-W.; Ji, Y.; Chen, J.-J.; Zhao, G.-P.; Zhang, W.-H.; Zhang, H.-Y.; Jiang, B.-G.; Xu, Q.; Lv, C.-L.; et al. Mapping the Distributions of Mosquitoes and Mosquito-Borne Arboviruses in China. Viruses 2022, 14, 691. [Google Scholar] [CrossRef]
- Fairbanks, E.L.; Daly, J.M.; Tildesley, M.J. Modelling the Influence of Climate and Vector Control Interventions on Arbovirus Transmission. Viruses 2024, 16, 1221. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Sousa, A.R.; Lange, M.; Mucci, L.F.; Marrelli, M.T.; Grimm, V. Modelling the Transmission and Spread of Yellow Fever in Forest Landscapes with Different Spatial Configurations. Ecol. Model. 2024, 489, 110628. [Google Scholar] [CrossRef]
- World Health Organization. Handbook for Integrated Vector Management; World Health Organization: Geneva, Switzerland, 2012; p. 67.
- Fotakis, E.A.; Mavridis, K.; Kampouraki, A.; Balaska, S.; Tanti, F.; Vlachos, G.; Gewehr, S.; Mourelatos, S.; Papadakis, A.; Kavalou, M.; et al. Mosquito Population Structure, Pathogen Surveillance and Insecticide Resistance Monitoring in Urban Regions of Crete, Greece. PLoS Negl. Trop. Dis. 2022, 16, e0010186. [Google Scholar] [CrossRef]
- Ateutchia-Ngouanet, S.; Nanfack-Minkeu, F.; Mavridis, K.; Wanji, S.; Demanou, M.; Vontas, J.; Djouaka, R. Monitoring Aedes Populations for Arboviruses, Wolbachia, Insecticide Resistance and Its Mechanisms in Various Agroecosystems in Benin. Acta Trop. 2024, 253, 107178. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue Virus Type 2: Replication and Tropisms in Orally Infected Aedes aegypti Mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Franz, A.; Kantor, A.; Passarelli, A.; Clem, R. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Lewis, J.; Gallichotte, E.N.; Randall, J.; Glass, A.; Foy, B.D.; Ebel, G.D.; Kading, R.C. Intrinsic Factors Driving Mosquito Vector Competence and Viral Evolution: A Review. Front. Cell. Infect. Microbiol. 2023, 13, 1330600. [Google Scholar] [CrossRef]
- Tabachnick, W.J. Ecological Effects on Arbovirus-Mosquito Cycles of Transmission. Curr. Opin. Virol. 2016, 21, 124–131. [Google Scholar] [CrossRef]
- Wu, P.; Yu, X.; Wang, P.; Cheng, G. Arbovirus Lifecycle in Mosquito: Acquisition, Propagation and Transmission. Expert Rev. Mol. Med. 2019, 21, e1. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced Competence to Arboviruses Following the Sustainable Invasion of Wolbachia into Native Aedes aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 10039. [Google Scholar] [CrossRef]
- Black, W.C.; Bennett, K.E.; Gorrochótegui-Escalante, N.; Barillas-Mury, C.V.; Fernández-Salas, I.; De Lourdes Muñoz, M.; Farfán-Alé, J.A.; Olson, K.E.; Beaty, B.J. Flavivirus Susceptibility in Aedes aegypti. Arch. Med. Res. 2002, 33, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bugallo, G.; Boullis, A.; Martinez, Y.; Hery, L.; Rodríguez, M.; Bisset, J.A.; Vega-Rúa, A. Vector Competence of Aedes aegypti from Havana, Cuba, for Dengue Virus Type 1, Chikungunya, and Zika Viruses. PLoS Negl. Trop. Dis. 2020, 14, e0008941. [Google Scholar] [CrossRef] [PubMed]
- Morales-Vargas, R.E.; Missé, D.; Chavez, I.F.; Kittayapong, P. Vector Competence for Dengue-2 Viruses Isolated from Patients with Different Disease Severity. Pathogens 2020, 9, 859. [Google Scholar] [CrossRef]
- Amoa-Bosompem, M.; Kobayashi, D.; Itokawa, K.; Murota, K.; Faizah, A.N.; Azerigyik, F.A.; Hayashi, T.; Ohashi, M.; Bonney, J.H.K.; Dadzie, S.; et al. Determining Vector Competence of Aedes aegypti from Ghana in Transmitting Dengue Virus Serotypes 1 and 2. Parasit. Vectors 2021, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Ehrlich, H.Y.; Magalhaes, T.; Miller, M.R.; Conway, P.J.; Bransfield, A.; Misencik, M.J.; Gloria-Soria, A.; Warren, J.L.; Andreadis, T.G.; et al. Successive Blood Meals Enhance Virus Dissemination within Mosquitoes and Increase Transmission Potential. Nat. Microbiol. 2019, 5, 239–247. [Google Scholar] [CrossRef]
- Johnson, R.M.; Cozens, D.W.; Ferdous, Z.; Armstrong, P.M.; Brackney, D.E. Increased Blood Meal Size and Feeding Frequency Compromise Aedes aegypti Midgut Integrity and Enhance Dengue Virus Dissemination. PLoS Negl. Trop. Dis. 2023, 17, e0011703. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Dieme, C.; Sproch, H.; Kramer, L.D.; Ciota, A.T.; Brackney, D.E.; Armstrong, P.M. Multiple Bloodmeals Enhance Dissemination of Arboviruses in Three Medically Relevant Mosquito Genera. Parasit. Vectors 2024, 17, 432. [Google Scholar] [CrossRef]
- Shaw, W.R.; Holmdahl, I.E.; Itoe, M.A.; Werling, K.; Marquette, M.; Paton, D.G.; Singh, N.; Buckee, C.O.; Childs, L.M.; Catteruccia, F. Multiple Blood Feeding in Mosquitoes Shortens the Plasmodium falciparum Incubation Period and Increases Malaria Transmission Potential. PLoS Pathog. 2020, 16, e1009131. [Google Scholar] [CrossRef]
- Brackney, D.E.; LaReau, J.C.; Smith, R.C. Frequency Matters: How Successive Feeding Episodes by Blood-Feeding Insect Vectors Influences Disease Transmission. PLoS Pathog. 2021, 17, e1009590. [Google Scholar] [CrossRef]
- Vasilakis, N.; Tesh, R.B. Insect-Specific Viruses and Their Potential Impact on Arbovirus Transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef]
- Carvalho, V.L.; Long, M.T. Insect-Specific Viruses: An Overview and Their Relationship to Arboviruses of Concern to Humans and Animals. Virology 2021, 557, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Cansado-Utrilla, C.; Zhao, S.Y.; McCall, P.J.; Coon, K.L.; Hughes, G.L. The Microbiome and Mosquito Vectorial Capacity: Rich Potential for Discovery and Translation. Microbiome 2021, 9, 111. [Google Scholar] [CrossRef]
- Laureti, M.; Paradkar, P.N.; Fazakerley, J.K.; Rodriguez-Andres, J. Superinfection Exclusion in Mosquitoes and Its Potential as an Arbovirus Control Strategy. Viruses 2020, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Minwuyelet, A.; Petronio, G.P.; Yewhalaw, D.; Sciarretta, A.; Magnifico, I.; Nicolosi, D.; Di Marco, R.; Atenafu, G. Symbiotic Wolbachia in Mosquitoes and Its Role in Reducing the Transmission of Mosquito-Borne Diseases: Updates and Prospects. Front. Microbiol. 2023, 14, 1267832. [Google Scholar] [CrossRef]
- Lima, E.P.; Paiva, M.H.S.; De Araújo, A.P.; Da Silva, É.V.G.; Da Silva, U.M.; De Oliveira, L.N.; Santana, A.E.G.; Barbosa, C.N.; De Paiva Neto, C.C.; Goulart, M.O.; et al. Insecticide Resistance in Aedes aegypti Populations from Ceará, Brazil. Parasit. Vectors 2011, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary Status of Insecticide Resistance in the Major Aedes Vectors of Arboviruses Infecting Humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.N.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Vulule, J.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Characteristics of Aedes aegypti Adult Mosquitoes in Rural and Urban Areas of Western and Coastal Kenya. PLoS ONE 2017, 12, e0189971. [Google Scholar] [CrossRef]
- Chandrasegaran, K.; Lahondère, C.; Escobar, L.E.; Vinauger, C. Linking Mosquito Ecology, Traits, Behavior, and Disease Transmission. Trends Parasitol. 2020, 36, 393–403. [Google Scholar] [CrossRef]
- Harrington, L.C.; Edman, J.D.; Scott, T.W. Why Do Female Aedes aegypti (Diptera: Culicidae) Feed Preferentially and Frequently on Human Blood? J. Med. Entomol. 2001, 38, 411–422. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal Biology of Mosquito-borne Disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef]
- Lahondère, C.; Bonizzoni, M. Thermal Biology of Invasive Aedes Mosquitoes in the Context of Climate Change. Curr. Opin. Insect Sci. 2022, 51, 100920. [Google Scholar] [CrossRef] [PubMed]
- Alencar, J.; De Mello, C.F.; Leite, P.J.; Bastos, A.Q.; Freitas Silva, S.O.; Serdeiro, M.; Dos Santos Silva, J.; Müller, G.A. Oviposition Activity of Haemagogus leucocelaenus (Diptera: Culicidae) during the Rainy and Dry Seasons, in Areas with Yellow Fever Virus Circulation in the Atlantic Forest, Rio de Janeiro, Brazil. PLoS ONE 2021, 16, e0261283. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Insulin Signaling and the Regulation of Insect Diapause. Front. Physiol. 2013, 4, 189. [Google Scholar] [CrossRef]
- Silva, H.H.G.D.; Silva, I.G.D. Influência do período de quiescência dos ovos sobre o ciclo de vida de Aedes aegypti (Linnaeus, 1762) (Diptera, Culicidae) em condições de laboratório. Rev. Soc. Bras. Med. Trop. 1999, 32, 349–355. [Google Scholar] [CrossRef]
- Prasad, A.; Sreedharan, S.; Bakthavachalu, B.; Laxman, S. Eggs of the Mosquito Aedes aegypti Survive Desiccation by Rewiring Their Polyamine and Lipid Metabolism. PLoS Biol. 2023, 21, e3002342. [Google Scholar] [CrossRef] [PubMed]
- Multini, L.C.; Oliveira-Christe, R.; Medeiros-Sousa, A.R.; Evangelista, E.; Barrio-Nuevo, K.M.; Mucci, L.F.; Ceretti-Junior, W.; Camargo, A.A.; Wilke, A.B.B.; Marrelli, M.T. The Influence of the pH and Salinity of Water in Breeding Sites on the Occurrence and Community Composition of Immature Mosquitoes in the Green Belt of the City of São Paulo, Brazil. Insects 2021, 12, 797. [Google Scholar] [CrossRef]
- de Brito Arduino, M.; Mucci, L.; Serpa, L.N.; Rodrigues, M.d.M. Effect of Salinity on the Behavior of Aedes aegypti Populations from the Coast and Plateau of Southeastern Brazil. J. Vector Borne Dis. 2015, 52, 79–87. [Google Scholar] [CrossRef]
- Ratnasari, A.; Jabal, A.R.; Syahribulan, S.; Idris, I.; Rahma, N.; Rustam, S.N.R.N.; Karmila, M.; Hasan, H.; Wahid, I. Salinity Tolerance of Larvae Aedes aegypti Inland and Coastal Habitats in Pasangkayu, West Sulawesi, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22. [Google Scholar] [CrossRef]
- Consoli, R.A.G.B.; Oliveira, R.L.D. Principais Mosquitos de Importância Sanitária No Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994; ISBN 978-85-7541-290-9. [Google Scholar]
- Lequime, S.; Paul, R.E.; Lambrechts, L. Determinants of Arbovirus Vertical Transmission in Mosquitoes. PLoS Pathog. 2016, 12, e1005548. [Google Scholar] [CrossRef]
- Dahiya, N.; Yadav, M.; Yadav, A.; Sehrawat, N. Zika Virus Vertical Transmission in Mosquitoes: A Less Understood Mechanism. J. Vector Borne Dis. 2022, 22, 37–44. [Google Scholar] [CrossRef]
- Kirstein, O.D.; Talavera, G.A.; Wei, Z.; Ciau-Carrilo, K.J.; Koyoc-Cardeña, E.; Puerta-Guardo, H.; Rodríguez-Martín, E.; Medina-Barreiro, A.; Mendoza, A.C.; Piantadosi, A.L.; et al. Natural Aedes-Borne Virus Infection Detected in Male Adult Aedes aegypti (Diptera: Culicidae) Collected From Urban Settings in Mérida, Yucatán, México. J. Med. Entomol. 2022, 59, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Alencar, J.; Ferreira De Mello, C.; Brisola Marcondes, C.; Érico Guimarães, A.; Toma, H.K.; Queiroz Bastos, A.; Olsson Freitas Silva, S.; Lisboa Machado, S. Natural Infection and Vertical Transmission of Zika Virus in Sylvatic Mosquitoes Aedes albopictus and Haemagogus leucocelaenus from Rio de Janeiro, Brazil. Trop. Med. Infect. Dis. 2021, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Bakran-Lebl, K.; Camp, J.V.; Kolodziejek, J.; Weidinger, P.; Hufnagl, P.; Cabal Rosel, A.; Zwickelstorfer, A.; Allerberger, F.; Nowotny, N. Diversity of West Nile and Usutu Virus Strains in Mosquitoes at an International Airport in Austria. Transbound. Emerg. Dis. 2022, 69, 2096–2109. [Google Scholar] [CrossRef]
- Pereira-Silva, J.W.; Nascimento, V.A.D.; Belchior, H.C.M.; Almeida, J.F.; Pessoa, F.A.C.; Naveca, F.G.; Ríos-Velásquez, C.M. First Evidence of Zika Virus Venereal Transmission in Aedes aegypti Mosquitoes. Mem. Inst. Oswaldo Cruz 2017, 113, 56–61. [Google Scholar] [CrossRef]
- Campos, S.S.; Fernandes, R.S.; Dos Santos, A.A.C.; De Miranda, R.M.; Telleria, E.L.; Ferreira-de-Brito, A.; De Castro, M.G.; Failloux, A.-B.; Bonaldo, M.C.; Lourenço-de-Oliveira, R. Zika Virus Can Be Venereally Transmitted between Aedes aegypti Mosquitoes. Parasit. Vectors 2017, 10, 605. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Bozic, J.; Mathias, D.; Smartt, C.T. Immune-Related Transcripts, Microbiota and Vector Competence Differ in Dengue-2 Virus-Infected Geographically Distinct Aedes aegypti Populations. Parasit. Vectors 2023, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Bamou, R.; Dao, A.; Yaro, A.; Kouam, C.; Ergunay, K.; Bourke, B.; Diallo, M.; Sanogo, Z.; Samake, D.; Afrane, Y.; et al. Pathogens Spread by High-Altitude Windborne Mosquitoes. bioRxiv 2024. [Google Scholar] [CrossRef]
- Torres, M.G.; Weakley, A.M.; Hibbert, J.D.; Kirstein, O.D.; Lanzaro, G.C.; Lee, Y. Ethanol as a Potential Mosquito Sample Storage Medium for RNA Preservation. F1000Research 2019, 8, 1431. [Google Scholar] [CrossRef]
- Kai, I.; Kobayashi, D.; Itokawa, K.; Sanjoba, C.; Itoyama, K.; Isawa, H. Evaluation of Long-Term Preservation Methods for Viral RNA in Mosquitoes at Room Temperature. J. Virol. Methods 2024, 325, 114887. [Google Scholar] [CrossRef]
- Jain, J.; Kushwah, R.B.S.; Singh, S.S.; Sharma, A.; Adak, T.; Singh, O.P.; Bhatnagar, R.K.; Subbarao, S.K.; Sunil, S. Evidence for Natural Vertical Transmission of Chikungunya Viruses in Field Populations of Aedes aegypti in Delhi and Haryana States in India—A Preliminary Report. Acta Trop. 2016, 162, 46–55. [Google Scholar] [CrossRef]
- Lustig, Y.; Hindiyeh, M.; Orshan, L.; Weiss, L.; Koren, R.; Katz-Likvornik, S.; Zadka, H.; Glatman-freedman, A.; Mendelson, E.; Shulman, L.M. Mosquito Surveillance for 15 Years Reveals High Genetic Diversity Among West Nile Viruses in Israel. J. Infect. Dis. 2016, 213, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Altan, E.; Deng, X.; Barker, C.M.; Fang, Y.; Coffey, L.L.; Delwart, E. Virome of >12 Thousand Culex Mosquitoes from throughout California. Virology 2018, 523, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Gewehr, S.; Tsioka, K.; Kalaitzopoulou, S.; Pappa, S.; Mourelatos, S. Detection of Flaviviruses and Alphaviruses in Mosquitoes in Central Macedonia, Greece, 2018. Acta Trop. 2020, 202, 105278. [Google Scholar] [CrossRef]
- Pereira-Silva, J.W.; Ríos-Velásquez, C.M.; Lima, G.R.D.; Marialva Dos Santos, E.F.; Belchior, H.C.M.; Luz, S.L.B.; Naveca, F.G.; Pessoa, F.A.C. Distribution and Diversity of Mosquitoes and Oropouche-like Virus Infection Rates in an Amazonian Rural Settlement. PLoS ONE 2021, 16, e0246932. [Google Scholar] [CrossRef]
- Rothman, S.E.; Jones, J.A.; LaDeau, S.L.; Leisnham, P.T. Higher West Nile Virus Infection in Aedes albopictus (Diptera: Culicidae) and Culex (Diptera: Culicidae) Mosquitoes From Lower Income Neighborhoods in Urban Baltimore, MD. J. Med. Entomol. 2021, 58, 1424–1428. [Google Scholar] [CrossRef]
- Seo, M.-G.; Lee, H.S.; Yang, S.-C.; Noh, B.-E.; Kim, T.-K.; Lee, W.-G.; Lee, H.I. National Monitoring of Mosquito Populations and Molecular Analysis of Flavivirus in the Republic of Korea in 2020. Microorganisms 2021, 9, 2085. [Google Scholar] [CrossRef]
- Chan, A.; Chiang, L.-P.; Hapuarachchi, H.C.; Tan, C.-H.; Pang, S.-C.; Lee, R.; Lee, K.-S.; Ng, L.-C.; Lam-Phua, S.-G. DNA Barcoding: Complementing Morphological Identification of Mosquito Species in Singapore. Parasites Vectors 2014, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- McDermott, E.G.; Lysyk, T.J. Sampling Considerations for Adult and Immature Culicoides (Diptera: Ceratopogonidae). J. Insect Sci. 2020, 20, 2. [Google Scholar] [CrossRef]
- Manzi, S.; Nelli, L.; Fortuna, C.; Severini, F.; Toma, L.; Di Luca, M.; Michelutti, A.; Bertola, M.; Gradoni, F.; Toniolo, F.; et al. A Modified BG-Sentinel Trap Equipped with FTA Card as a Novel Tool for Mosquito-Borne Disease Surveillance: A Field Test for Flavivirus Detection. Sci. Rep. 2023, 13, 12840. [Google Scholar] [CrossRef]
- L’Ambert, G.; Gendrot, M.; Briolant, S.; Nguyen, A.; Pages, S.; Bosio, L.; Palomo, V.; Gomez, N.; Benoit, N.; Savini, H.; et al. Analysis of Trapped Mosquito Excreta as a Noninvasive Method to Reveal Biodiversity and Arbovirus Circulation. Mol. Ecol. Resour. 2023, 23, 410–423. [Google Scholar] [CrossRef]
- Kittichai, V.; Kaewthamasorn, M.; Samung, Y.; Jomtarak, R.; Naing, K.M.; Tongloy, T.; Chuwongin, S.; Boonsang, S. Automatic Identification of Medically Important Mosquitoes Using Embedded Learning Approach-Based Image-Retrieval System. Sci. Rep. 2023, 13, 10609. [Google Scholar] [CrossRef] [PubMed]
- Batovska, J.; Lynch, S.E.; Cogan, N.O.I.; Brown, K.; Darbro, J.M.; Kho, E.A.; Blacket, M.J. Effective Mosquito and Arbovirus Surveillance Using Metabarcoding. Mol. Ecol. Resour. 2018, 18, 32–40. [Google Scholar] [CrossRef]
- Moraes Zenker, M.; Portella, T.P.; Pessoa, F.A.C.; Bengtsson-Palme, J.; Galetti, P.M. Low Coverage of Species Constrains the Use of DNA Barcoding to Assess Mosquito Biodiversity. Sci. Rep. 2024, 14, 7432. [Google Scholar] [CrossRef]
- Oliveira, T.M.P.; Saraiva, J.F.; Da Silva, H.; Sallum, M.A.M. Molecular Identification of Mosquitoes (Diptera: Culicidae) Using COI Barcode and D2 Expansion of 28S Gene. DNA 2024, 4, 507–518. [Google Scholar] [CrossRef]
- Hoyos-López, R.; Suaza-Vasco, J.; Rúa-Uribe, G.; Uribe, S.; Gallego-Gómez, J.C. Molecular Detection of Flaviviruses and Alphaviruses in Mosquitoes (Diptera: Culicidae) from Coastal Ecosystems in the Colombian Caribbean. Mem. Inst. Oswaldo Cruz 2016, 111, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Guarido, M.M.; Govender, K.; Riddin, M.A.; Schrama, M.; Gorsich, E.E.; Brooke, B.D.; Almeida, A.P.G.; Venter, M. Detection of Insect-Specific Flaviviruses in Mosquitoes (Diptera: Culicidae) in Northeastern Regions of South Africa. Viruses 2021, 13, 2148. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Wahaab, A.; Shan, T.; Wang, X.; Khan, S.; Di, D.; Xiqian, L.; Zhang, J.-J.; Anwar, M.N.; Nawaz, M.; et al. A Metagenomic Analysis of Mosquito Virome Collected From Different Animal Farms at Yunnan–Myanmar Border of China. Front. Microbiol. 2021, 11, 591478. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Garza-Hernández, J.A.; Ortega Morales, A.I.; Prosser, S.W.J.; Hebert, P.D.N.; Nikolova, N.I.; Barrero, E.; De Luna-Santillana, E.D.J.; González-Alvarez, V.H.; Mendez-López, R.; et al. An Integrated Molecular Approach to Untangling Host–Vector–Pathogen Interactions in Mosquitoes (Diptera: Culicidae) From Sylvan Communities in Mexico. Front. Vet. Sci. 2021, 7, 564791. [Google Scholar] [CrossRef]
- Beebe, N.W. DNA Barcoding Mosquitoes: Advice for Potential Prospectors. Parasitology 2018, 145, 622–633. [Google Scholar] [CrossRef]
- Main, B.J.; Nicholson, J.; Winokur, O.C.; Steiner, C.; Riemersma, K.K.; Stuart, J.; Takeshita, R.; Krasnec, M.; Barker, C.M.; Coffey, L.L. Vector Competence of Aedes aegypti, Culex tarsalis, and Culex quinquefasciatus from California for Zika Virus. PLoS Negl. Trop. Dis. 2018, 12, e0006524. [Google Scholar] [CrossRef]
- Moonen, J.P.; Schinkel, M.; Van Der Most, T.; Miesen, P.; Van Rij, R.P. Composition and Global Distribution of the Mosquito Virome—A Comprehensive Database of Insect-Specific Viruses. One Health 2023, 16, 100490. [Google Scholar] [CrossRef]
- Gómez, M.; Martínez, D.; Páez-Triana, L.; Luna, N.; De Las Salas, J.L.; Hernández, C.; Flórez, A.Z.; Muñoz, M.; Ramírez, J.D. Characterizing Viral Species in Mosquitoes (Culicidae) in the Colombian Orinoco: Insights from a Preliminary Metagenomic Study. Sci. Rep. 2023, 13, 22081. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, F.; Liu, X.; Fu, Y.; Fang, W.; Kang, X.; Lu, H.; Li, S.; Liu, B.; Guo, W.; et al. Association of Virome Dynamics with Mosquito Species and Environmental Factors. Microbiome 2023, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Condotta, S.A.; Hunter, F.F.; Bidochka, M.J. West Nile Virus Infection Rates in Pooled and Individual Mosquito Samples. Vector-Borne Zoonotic Dis. 2004, 4, 198–203. [Google Scholar] [CrossRef]
- Balingit, J.C.; Carvajal, T.M.; Saito-Obata, M.; Gamboa, M.; Nicolasora, A.D.; Sy, A.K.; Oshitani, H.; Watanabe, K. Surveillance of Dengue Virus in Individual Aedes aegypti Mosquitoes Collected Concurrently with Suspected Human Cases in Tarlac City, Philippines. Parasit. Vectors 2020, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.J.; Grossi-Soyster, E.N.; Ndenga, B.A.; Mutuku, F.M.; Sahoo, M.K.; Ngugi, H.N.; Mbakaya, J.O.; Siema, P.; Kitron, U.; Zahiri, N.; et al. Evidence of Transovarial Transmission of Chikungunya and Dengue Viruses in Field-Caught Mosquitoes in Kenya. PLoS Negl. Trop. Dis. 2020, 14, e0008362. [Google Scholar] [CrossRef]
- Fish, D.; Tesh, R.B.; Guzman, H.; Travassos Da Rosa, A.P.A.; Balta, V.; Underwood, J.; Sither, C.; Vasilakis, N. Emergence Potential of Mosquito-Borne Arboviruses from the Florida Everglades. PLoS ONE 2021, 16, e0259419. [Google Scholar] [CrossRef]
- Gu, W.; Lampman, R.; Novak, R.J. Problems in Estimating Mosquito Infection Rates Using Minimum Infection Rate. J. Med. Entomol. 2003, 40, 595–596. [Google Scholar] [CrossRef]
- Varghese, J.; De Silva, I.; Millar, D.S. Latest Advances in Arbovirus Diagnostics. Microorganisms 2023, 11, 1159. [Google Scholar] [CrossRef]
- Gyawali, N.; Bradbury, R.S.; Aaskov, J.G.; Taylor-Robinson, A.W. Neglected Australian Arboviruses: Quam Gravis? Microbes Infect. 2017, 19, 388–401. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and Future Arboviral Threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Bianchi, F.P.; Frisicale, E.M.; Guicciardi, S.; Fiacchini, D.; Tafuri, S.; et al. (Re-)Emergence of Oropouche Virus (OROV) Infections: Systematic Review and Meta-Analysis of Observational Studies. Viruses 2024, 16, 1498. [Google Scholar] [CrossRef]
- BRAZIL. Ministry of Health. NOTA TÉCNICA No 6/2024-CGARB/DEDT/SVSA/MS 2024. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/notas-tecnicas/2024/nota-tecnica-no-6-2024-cgarb-dedt-svsa-ms/view (accessed on 23 December 2024).
- Naveca, F.G.; Almeida, T.A.P.D.; Souza, V.; Nascimento, V.; Silva, D.; Nascimento, F.; Mejía, M.; Oliveira, Y.S.D.; Rocha, L.; Xavier, N.; et al. Human Outbreaks of a Novel Reassortant Oropouche Virus in the Brazilian Amazon Region. Nat. Med. 2024, 30, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche Fever, an Emergent Disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef]
- De Mendonça, S.F.; Rocha, M.N.; Ferreira, F.V.; Leite, T.H.J.F.; Amadou, S.C.G.; Sucupira, P.H.F.; Marques, J.T.; Ferreira, A.G.A.; Moreira, L.A. Evaluation of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus Mosquitoes Competence to Oropouche Virus Infection. Viruses 2021, 13, 755. [Google Scholar] [CrossRef] [PubMed]
- Bonifay, T.; Le Turnier, P.; Epelboin, Y.; Carvalho, L.; De Thoisy, B.; Djossou, F.; Duchemin, J.-B.; Dussart, P.; Enfissi, A.; Lavergne, A.; et al. Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America. Viruses 2023, 15, 1268. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche Virus: A Neglected Global Arboviral Threat. Virus Res. 2024, 341, 199318. [Google Scholar] [CrossRef] [PubMed]
- Requena-Zúñiga, E.; Palomino-Salcedo, M.; García-Mendoza, M.P.; Figueroa-Romero, M.D.; Merino-Sarmiento, N.S.; Escalante-Maldonado, O.; Cornelio-Santos, A.L.; Cárdenas-Garcia, P.; Jiménez, C.A.; Cabezas-Sanchez, C. First Detection of Oropouche Virus in Culicoides insignis in the Ucayali Region, Peru: Evidence of a Possible New Vector. medRxiv 2024. [Google Scholar] [CrossRef]
- Carpenter, S.; Mellor, P.S.; Torr, S.J. Control Techniques for Culicoides Biting Midges and Their Application in the U.K. and Northwestern Palaearctic. Med. Vet. Entomol. 2008, 22, 175–187. [Google Scholar] [CrossRef]
- Santos-Melo, G.Z.; Andrade, S.R.D.; Rocha, Y.A.D.; Cosme, K.D.O.; Pereira, T.C.L.; Monteiro, A.X.; Ribeiro, G.M.D.A.; Passos, S.M.D.A. Importância e desafios da vigilância em saúde em uma região de fronteira internacional: Um estudo de caso. Saúde E Soc. 2023, 32, e220433pt. [Google Scholar] [CrossRef]
- Benchimol, J.L. História Da Febre Amarela No Brasil. História Ciênc. Saúde-Manguinhos 1994, 1, 121–124. [Google Scholar] [CrossRef]
- Braga, I.A.; Valle, D. Aedes aegypti: Histórico do controle no Brasil. Epidemiol. Serviços Saúde 2007, 16, 113–118. [Google Scholar]
- Faria, N.R.; Quick, J.; Claro, I.M.; Thézé, J.; De Jesus, J.G.; Giovanetti, M.; Kraemer, M.U.G.; Hill, S.C.; Black, A.; Da Costa, A.C.; et al. Establishment and Cryptic Transmission of Zika Virus in Brazil and the Americas. Nature 2017, 546, 406–410. [Google Scholar] [CrossRef]
- Naveca, F.G.; Claro, I.; Giovanetti, M.; De Jesus, J.G.; Xavier, J.; Iani, F.C.D.M.; Do Nascimento, V.A.; De Souza, V.C.; Silveira, P.P.; Lourenço, J.; et al. Genomic, Epidemiological and Digital Surveillance of Chikungunya Virus in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2019, 13, e0007065. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.; Yu, M.A.; Sacks, J.; Barnadas, C.; Pereyaslov, D.; Cognat, S.; Briand, S.; Ryan, M.; Samaan, G. Global Genomic Surveillance Strategy for Pathogens with Pandemic and Epidemic Potential 2022–2032. Bull. World Health Organ. 2022, 100, 239-239A. [Google Scholar] [CrossRef]
- Wallau, G.L.; Abanda, N.N.; Abbud, A.; Abdella, S.; Abera, A.; Ahuka-Mundeke, S.; Falconi-Agapito, F.; Alagarasu, K.; Ariën, K.K.; Ayres, C.F.J.; et al. Arbovirus Researchers Unite: Expanding Genomic Surveillance for an Urgent Global Need. Lancet Glob. Health 2023, 11, e1501–e1502. [Google Scholar] [CrossRef]
- De Souza, W.M.; Weaver, S.C. Effects of Climate Change and Human Activities on Vector-Borne Diseases. Nat. Rev. Microbiol. 2024, 22, 476–491. [Google Scholar] [CrossRef]
- Pan American Health Organization. Effective Communication Strategies and Practices for Dengue and Other Arboviral Diseases: Systematic Review. Eff. Commun. 2024. Available online: https://medbox.org/pdf/67a08eb9e592850f3e37ff12 (accessed on 16 December 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemos, P.d.S.; Pacheco, M.M.M.; Nascimento, B.L.S.d.; Coelho, M.S.; Franco Filho, L.C.; Dias, D.D.; Sena, L.; Silva, S.P.d.; Sallum, M.A.M. The Frontier of Entomo-Virology: Applications and Tools for Virus and Vector Surveillance. Pathogens 2025, 14, 699. https://doi.org/10.3390/pathogens14070699
Lemos PdS, Pacheco MMM, Nascimento BLSd, Coelho MS, Franco Filho LC, Dias DD, Sena L, Silva SPd, Sallum MAM. The Frontier of Entomo-Virology: Applications and Tools for Virus and Vector Surveillance. Pathogens. 2025; 14(7):699. https://doi.org/10.3390/pathogens14070699
Chicago/Turabian StyleLemos, Poliana da Silva, Mayron Mielly Morais Pacheco, Bruna Laís Sena do Nascimento, Mônica Silva Coelho, Luciano Chaves Franco Filho, Daniel Damous Dias, Leonardo Sena, Sandro Patroca da Silva, and Maria Anice Mureb Sallum. 2025. "The Frontier of Entomo-Virology: Applications and Tools for Virus and Vector Surveillance" Pathogens 14, no. 7: 699. https://doi.org/10.3390/pathogens14070699
APA StyleLemos, P. d. S., Pacheco, M. M. M., Nascimento, B. L. S. d., Coelho, M. S., Franco Filho, L. C., Dias, D. D., Sena, L., Silva, S. P. d., & Sallum, M. A. M. (2025). The Frontier of Entomo-Virology: Applications and Tools for Virus and Vector Surveillance. Pathogens, 14(7), 699. https://doi.org/10.3390/pathogens14070699







