Current Trends in Approaches to Prevent and Control Antimicrobial Resistance in Aquatic Veterinary Medicine
Abstract
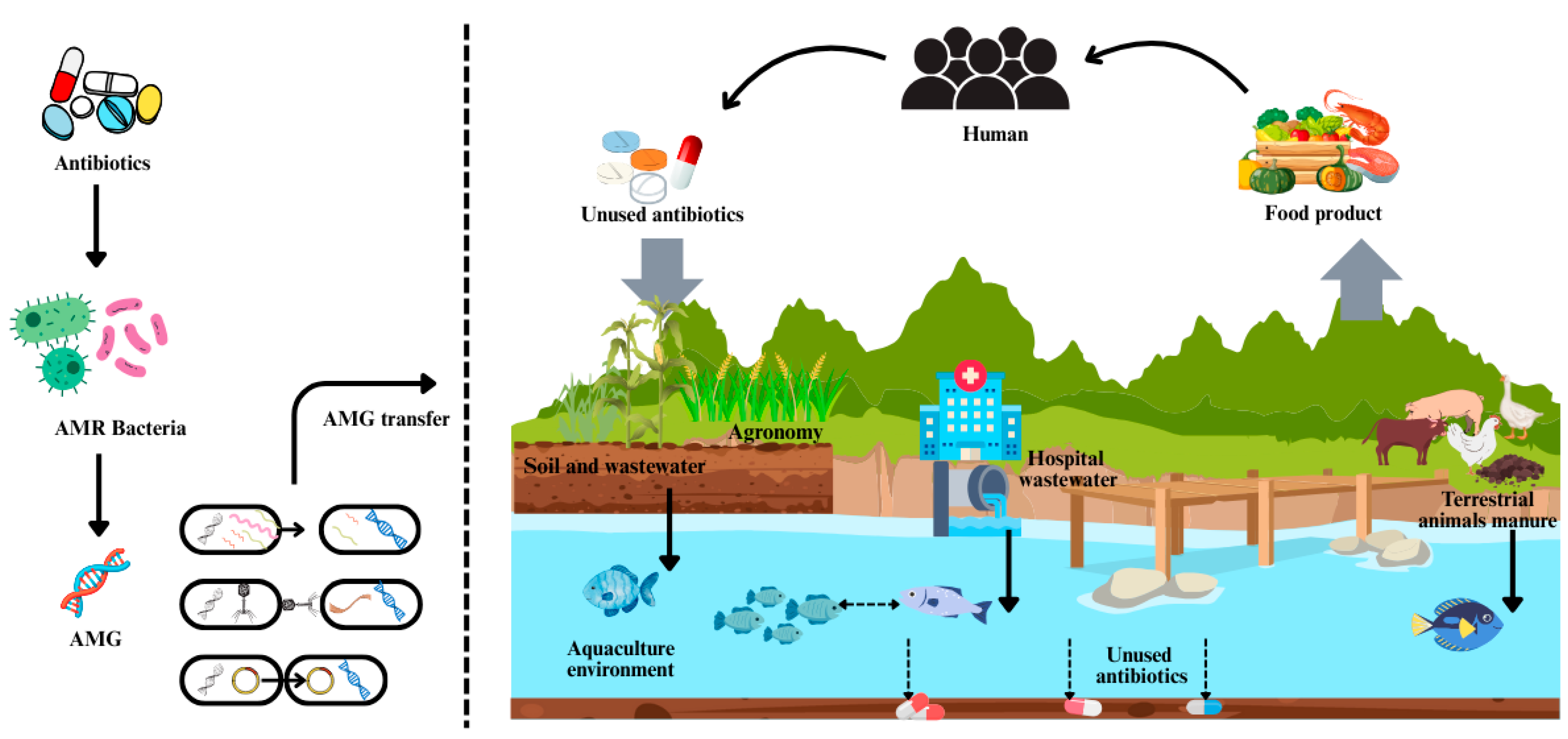
1. Introduction
2. Spread of Antimicrobial Resistance in Aquatic Environments
3. Prevalence of AMR and AMGs in Fish
3.1. Aeromonas
3.2. Vibrio
3.3. Streptococcus
3.4. Pseudomonas
3.5. Flavobacterium
3.6. Acinetobacter
3.7. Edwardsiella
3.8. Other Clinical Strains
4. Alternative Strategies
4.1. Vaccines
4.2. Bacteriophages
4.3. Probiotics
4.4. Biosurfactants
4.5. Bacteriocin
4.6. Antimicrobial Peptides
5. Current Considerations and Way Forward
- Scalable vaccine and phage delivery systems;
- Improved phage QC processes;
- Biosafety research for probiotics and bacteriocins;
- Adaptive regulatory frameworks tailored to aquaculture’s unique needs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMU | Antimicrobial use |
| AMR | Antimicrobial resistance |
| AMG | Antimicrobial resistance gene |
| ARB | Antibiotic-resistant bacteria |
| MDR | Multi-drug resistance |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| ESBL | Extended-spectrum β-lactamase |
References
- FAO. The State of World Fisheries and Aquaculture–2024, Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Nussbaum, W. Annual Farmed Finfish Production Survey: A Modest Supply Decline for 2023 and a Predicted Return to Growth in 2024. Available online: https://www.globalseafood.org/advocate/annual-farmed-finfish-production-survey-a-modest-supply-decline-for-2023-and-a-predicted-return-to-growth-in-2024/ (accessed on 22 May 2025).
- Peeler, E.J.; Gilbert, W.; Huntington, B.; Brun, E.; Misund, B.; Reantaso, M.; Walde, C.; Kennerley, A. The application of Global Burden of Animal Diseases methodology to aquatic animal production. Rev. Sci. Tech. OIE 2024, 43, 159–167. [Google Scholar] [CrossRef]
- Subasinghe, R.; Alday-Sanz, V.; Bondad-Reantaso, M.G.; Jie, H.; Shinn, A.P.; Sorgeloos, P. Biosecurity: Reducing the burden of disease. J. World Aquac. Soc. 2023, 54, 397–426. [Google Scholar] [CrossRef]
- Mkulo, E.M.; Wang, B.; Amoah, K.; Huang, Y.; Cai, J.; Jin, X.; Wang, Z. The current status and development forecasts of vaccines for aquaculture and its effects on bacterial and viral diseases. Microb. Pathog. 2024, 196, 106971. [Google Scholar] [CrossRef] [PubMed]
- Magouz, F.I.; Moustafa, E.M.; Abo-Remela, E.M.; Halawa, M.R.; Barakaat, P.M.; Omar, A.A. Summer mortality syndrome bacterial pathogens in farmed Nile tilapia (Oreochromis niloticus). Open Vet. J. 2024, 14, 53. [Google Scholar] [CrossRef]
- Irshath, A.A.; Rajan, A.P.; Vimal, S.; Prabhakaran, V.-S.; Ganesan, R. Bacterial Pathogenesis in Various Fish Diseases: Recent Advances and Specific Challenges in Vaccine Development. Vaccines 2023, 11, 470. [Google Scholar] [CrossRef]
- Milijasevic, M.; Veskovic-Moracanin, S.; Milijasevic, J.B.; Petrovic, J.; Nastasijevic, I. Antimicrobial Resistance in Aquaculture: Risk Mitigation within the One Health Context. Foods 2024, 13, 2448. [Google Scholar] [CrossRef]
- Caputo, A.; Bondad-Reantaso, M.G.; Karunasagar, I.; Hao, B.; Gaunt, P.; Verner-Jeffreys, D.; Fridman, S.; Dorado-Garcia, A. Antimicrobial resistance in aquaculture: A global analysis of literature and national action plans. Rev. Aquac. 2023, 15, 568–578. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Boeckel, T.P.V. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- de la Rocque, S.; Errecaborde, K.M.M.; Belot, G.; Brand, T.; Shadomy, S.; von Dobschuetz, S.; Aguanno, R.; Carron, M.; Caya, F.; Ding, S.; et al. One health systems strengthening in countries: Tripartite tools and approaches at the human-animal-environment interface. BMJ Glob. Health 2023, 8, e011236. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human Health Consequences of Use of Antimicrobial Agents in Aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef]
- Fuentes, M.D.; Gutierrez, S.; Sahagun, D.; Gomez, J.; Mendoza, J.; Ellis, C.C.; Bauer, S.; Blattner, J.; Lee, W.-Y.; Alvarez, M.; et al. Assessment of Antibiotic Levels, Multi-Drug-Resistant Bacteria and Genetic Biomarkers in the Waters of the Rio Grande River Between the United States-Mexico Border. J. Health Pollut. 2019, 9, 190912. [Google Scholar] [CrossRef]
- Lendo-Hernández, H.M.; Gutiérrez-Meza, J.J.; Bueno-Durán, A.; Márquez-González, A.R.; Velázquez-Meza, M.E.; Vargas Maya, N.I.; Franco, B.; Mondragón-Jaimes, V.A. Pollution and health hazards: Environmental isolates of Enterococcus faecalis and Enterococcus faecium characterized for virulence genes, antibiotic and heavy metal resistance. J. Glob. Ecol. Environ. 2017, 6, 182–189. [Google Scholar]
- Fu, S.; Wang, Q.; Wang, R.; Zhang, Y.; Lan, R.; He, F.; Yang, Q. Horizontal transfer of antibiotic resistance genes within the bacterial communities in aquacultural environment. Sci. Total Environ. 2022, 820, 153286. [Google Scholar] [CrossRef] [PubMed]
- Raharjo, H.M.; Budiyansah, H.; Mursalim, M.F.; Chokmangmeepisarn, P.; Sakulworakan, R.; Debnath, P.P.; Sivaramasamy, E.; Intan, S.T.; Chuanchuen, R.; Dong, H.T.; et al. The first evidence of blaCTX-M-55, QnrVC5, and novel insight into the genome of MDR Vibrio vulnificus isolated from Asian sea bass (Lates calcarifer) identified by resistome analysis. Aquaculture 2023, 571, 739500. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Han, Q.; Yu, Q.; Wanyan, R.; Li, H. Bibliometric analysis of papers on antibiotic resistance genes in aquatic environments on a global scale from 2012 to 2022: Evidence from universality, development and harmfulness. Sci. Total Environ. 2024, 909, 168597. [Google Scholar] [CrossRef] [PubMed]
- Girijan, S.K.; Paul, R.; Rejish Kumar, V.J.; Pillai, D. Investigating the impact of hospital antibiotic usage on aquatic environment and aquaculture systems: A molecular study of quinolone resistance in Escherichia coli. Sci. Total Environ. 2020, 748, 141538. [Google Scholar] [CrossRef]
- Duarte, D.J.; Zillien, C.; Kox, M.; Oldenkamp, R.; van der Zaan, B.; Roex, E.; Ragas, A.M.J. Characterization of urban sources of antibiotics and antibiotic-resistance genes in a Dutch sewer catchment. Sci. Total Environ. 2023, 905, 167439. [Google Scholar] [CrossRef]
- Ir, A.; Ce, O.; Ka, K. Physicochemical Properties, Bacteriological Quality and Antimicrobial Resistance Profile of Isolates from Groundwater Sources in Ile-Ife Suburbs, Southwest Nigeria. J. Environ. Sci. 2019, 13, 58–65. [Google Scholar]
- Ahmed, N.; Thompson, S. The blue dimensions of aquaculture: A global synthesis. Sci. Total Environ. 2019, 652, 851–861. [Google Scholar] [CrossRef]
- Dželalija, M.; Fredotović, Ž.; Udiković-Kolić, N.; Kalinić, H.; Jozić, S.; Šamanić, I.; Ordulj, M.; Maravić, A. Large-Scale Biogeographical Shifts of Abundance of Antibiotic Resistance Genes and Marine Bacterial Communities as Their Carriers along a Trophic Gradient. Int. J. Mol. Sci. 2024, 25, 654. [Google Scholar] [CrossRef]
- Liang, H.; de Haan, W.P.; Cerdà-Domènech, M.; Méndez, J.; Lucena, F.; García-Aljaro, C.; Sanchez-Vidal, A.; Ballesté, E. Detection of faecal bacteria and antibiotic resistance genes in biofilms attached to plastics from human-impacted coastal areas. Environ. Pollut. 2023, 319, 120983. [Google Scholar] [CrossRef]
- Kühn, I.; Iversen, A.; Finn, M.; Greko, C.; Burman, L.G.; Blanch, A.R.; Vilanova, X.; Manero, A.; Taylor, H.; Caplin, J.; et al. Occurrence and Relatedness of Vancomycin-Resistant Enterococci in Animals, Humans, and the Environment in Different European Regions. Appl. Environ. Microbiol. 2005, 71, 5383. [Google Scholar] [CrossRef] [PubMed]
- Winkworth-Lawrence, C.; Lange, K. Antibiotic Resistance Genes in Freshwater Biofilms May Reflect Influences from High-Intensity Agriculture. Microb. Ecol. 2016, 72, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.M.; Lee, J.Y.H.; Gorrie, C.L.; Howden, B.P.; Carter, G.P. Genomic Insights Into Last-Line Antimicrobial Resistance in Multidrug-Resistant Staphylococcus and Vancomycin-Resistant Enterococcus. Front. Microbiol. 2021, 12, 637656. [Google Scholar] [CrossRef] [PubMed]
- Klase, G.; Lee, S.; Liang, S.; Kim, J.; Zo, Y.-G.; Lee, J. The microbiome and antibiotic resistance in integrated fishfarm water: Implications of environmental public health. Sci. Total Environ. 2019, 649, 1491–1501. [Google Scholar] [CrossRef]
- Lei, L.; Chen, N.; Chen, Z.; Zhao, Y.; Lin, H.; Li, X.; Hu, W.; Zhang, H.; Shi, J.; Luo, Y. Dissemination of antibiotic resistance genes from aboveground sources to groundwater in livestock farms. Water Res. 2024, 256, 121584. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef]
- Lopes, E.d.S.; Ferreira Santaren, K.C.; Araujo de Souza, L.C.; Parente, C.E.T.; Picão, R.C.; Jurelevicius, D. de A.; Seldin, L. Cross-environmental cycling of antimicrobial resistance in agricultural areas fertilized with poultry litter: A one health approach. Environ. Pollut. 2024, 363, 125177. [Google Scholar] [CrossRef]
- Varela, A.R.; Nunes, O.C.; Manaia, C.M. Quinolone resistant Aeromonas spp. as carriers and potential tracers of acquired antibiotic resistance in hospital and municipal wastewater. Sci. Total Environ. 2016, 542, 665–671. [Google Scholar] [CrossRef]
- Araújo, S.; Azenha, S.R.; Henriques, I.; Tacão, M. qnrA gene diversity in Shewanella spp. Microbiol. Read. Engl. 2021, 167, 001118. [Google Scholar]
- Balzer, F.; Zühlke, S.; Hannappel, S. Antibiotics in groundwater under locations with high livestock density in Germany. Water Supply 2016, 16, 1361–1369. [Google Scholar] [CrossRef]
- Singh, A.; Pratap, S.G.; Raj, A. Occurrence and dissemination of antibiotics and antibiotic resistance in aquatic environment and its ecological implications: A review. Environ. Sci. Pollut. Res. 2024, 31, 47505–47529. [Google Scholar] [CrossRef] [PubMed]
- Olymon, K.; Kumari, A.; Kinoo, N.; Teronpi, V.; Yella, V.R.; Kumar, A. Comparative genomic analysis reveals distinct virulence and resistance mechanisms in 21 bacterial fish pathogens. Microb. Pathog. 2024, 197, 107099. [Google Scholar] [CrossRef]
- Tong, L.; Qin, L.; Guan, C.; Wilson, M.E.; Li, X.; Cheng, D.; Ma, J.; Liu, H.; Gong, F. Antibiotic resistance gene profiling in response to antibiotic usage and environmental factors in the surface water and groundwater of Honghu Lake, China. Environ. Sci. Pollut. Res. 2020, 27, 31995–32005. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Lastauskienė, E.; Valskys, V.; Stankevičiūtė, J.; Kalcienė, V.; Gėgžna, V.; Kavoliūnas, J.; Ružauskas, M.; Armalytė, J. The Impact of Intensive Fish Farming on Pond Sediment Microbiome and Antibiotic Resistance Gene Composition. Front. Vet. Sci. 2021, 8, 673756. [Google Scholar] [CrossRef]
- Wanyan, R.; Pan, M.; Mai, Z.; Xiong, X.; Wang, S.; Han, Q.; Yu, Q.; Wang, G.; Wu, S.; Li, H. Fate of high-risk antibiotic resistance genes in large-scale aquaculture sediments: Geographical differentiation and corresponding drivers. Sci. Total Environ. 2023, 905, 167068. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Chen, W.; Bao, Y.; Zheng, Y.; Huang, B.; Mu, Q.; Wen, D.; Feng, C. Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening. Mar. Pollut. Bull. 2020, 151, 110810. [Google Scholar] [CrossRef]
- Sargenti, M.; Bartolacci, S.; Luciani, A.; Di Biagio, K.; Baldini, M.; Galarini, R.; Giusepponi, D.; Capuccella, M. Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy. Sustainability 2020, 12, 5176. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, J.; Chen, L.; Li, R.; Han, Y.; Di, Z.; Ling, B.; Ahmad, A.; Yang, N.; Fan, L.; et al. Prevalence, virulence-related genes and antimicrobial resistance of Aeromonas spp. from loach Misgurnus anguillicaudatus with skin ulcer and healthy controls in Southern China. Aquaculture 2022, 552, 738040. [Google Scholar] [CrossRef]
- Menanteau-Ledouble, S.; Kumar, G.; Saleh, M.; El-Matbouli, M. Aeromonas salmonicida: Updates on an old acquaintance. Dis. Aquat. Organ. 2016, 120, 49–68. [Google Scholar] [CrossRef]
- El-Bahar, H.M.; Ali, N.G.; Aboyadak, I.M.; Khalil, S.A.E.S.; Ibrahim, M.S. Virulence genes contributing to Aeromonas hydrophila pathogenicity in Oreochromis niloticus. Int. Microbiol. 2019, 22, 479–490. [Google Scholar] [CrossRef]
- El-Hossary, D.; Mahdy, A.; Elariny, E.Y.T.; Askora, A.; Merwad, A.M.A.; Saber, T.; Dahshan, H.; Hakami, N.Y.; Ibrahim, R.A. Antibiotic Resistance, Virulence Gene Detection, and Biofilm Formation in Aeromonas spp. Isolated from Fish and Humans in Egypt. Biology 2023, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.H.; Kassab, A.S. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol. 2023, 23, 80. [Google Scholar] [CrossRef]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016, 7, 1337. [Google Scholar] [CrossRef]
- Chong, R.S.-M. Chapter 30-Furunculosis. In Aquaculture Patho-Physiology; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.-M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 395–406. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Múzquiz, J.L. Effect of Lactococcus lactis CLFP 100 and Leuconostoc mesenteroides CLFP 196 on Aeromonas salmonicida Infection in Brown Trout (Salmo trutta). J. Mol. Microbiol. Biotechnol. 2009, 17, 153–157. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Cholewińska, P.; Steinbauer, P.; Lautwein, T.; Hussein, W.; Streb, L.-M.; Palić, D. Genomic Analysis of Aeromonas salmonicida ssp. salmonicida Isolates Collected During Multiple Clinical Outbreaks Supports Association with a Single Epidemiological Unit . Pathogens 2024, 13, 908. [Google Scholar]
- Deng, Y.; Wu, Y.; Jiang, L.; Tan, A.; Zhang, R.; Luo, L. Multi-Drug Resistance Mediated by Class 1 Integrons in Aeromonas Isolated from Farmed Freshwater Animals. Front. Microbiol. 2016, 7, 935. [Google Scholar] [CrossRef]
- Vásquez-Ponce, F.; Higuera-Llantén, S.; Parás-Silva, J.; Gamboa-Acuña, N.; Cortés, J.; Opazo-Capurro, A.; Ugalde, J.A.; Alcalde-Rico, M.; Olivares-Pacheco, J. Genetic characterization of clinically relevant class 1 integrons carried by multidrug resistant bacteria (MDRB) isolated from the gut microbiota of highly antibiotic treated Salmo salar. J. Glob. Antimicrob. Resist. 2022, 29, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Elkenany, R.; Younis, G. Virulent and Multiple Anti-microbial Resistance Aeromonas hydrophila Isolated from Diseased Nile Tilapia Fish (Oreochromis niloticus) in Egypt with Sequencing of Some Virulence-Associated Genes. Biocontrol Sci. 2021, 26, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, N.N.F.N.M.; Hamdan, R.H.; Mohamed, M.; Ismail, A.; Zin, A.A.M.; Mohamad, N.F.A. Prevalence, antibiotic susceptibility, and presence of drug resistance genes in Aeromonas spp. isolated from freshwater fish in Kelantan and Terengganu states, Malaysia. Vet. World 2021, 14, 2064. [Google Scholar] [CrossRef]
- Adah, D.A.; Saidu, L.; Oniye, S.J.; Adah, A.S.; Daoudu, O.B.; Ola-Fadunsin, S.D. Molecular characterization and antibiotics resistance of Aeromonas species isolated from farmed African catfish Clarias gariepinus Burchell, 1822. BMC Vet. Res. 2024, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qin, L.; Zhu, Y.; Qian, Q.; Gao, X.; Jiang, Q.; Wang, J.; Liu, G.; Zhang, X. Characteristics and Complete Genome Analysis of a Pathogenic Aeromonas veronii SJ4 from Diseased Siniperca chuatsi. Mar. Biotechnol. 2023, 25, 966–982. [Google Scholar] [CrossRef]
- Villa, Y.C.; Triga, A.; Katharios, P. Polyinfection in Fish Aeromoniasis: A Study of Co-Isolated Aeromonas Species in Aeromonas veronii Outbreaks. Pathogens 2023, 12, 1337. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, S.; Guo, M.; Wang, Z.; Hu, B.; Zhou, B.; Chen, S. The dynamic immune response of the liver and spleen in leopard coral grouper (Plectropomus leopardus) to Vibrio harveyi infection based on transcriptome analysis. Front. Immunol. 2024, 15, 1457745. [Google Scholar] [CrossRef]
- Urku, C.; Secer, F.S.; Onalan, S.; Akayli, T. Investigation of vibriosis caused by Vibrio anguillarum in rainbow trout (Oncorhynchus mykiss). Cell. Mol. Biol. 2024, 70, 32–38. [Google Scholar] [CrossRef]
- Chuang, P.-Y.; Yang, T.-Y.; Huang, T.-W.; Tsai, Y.-H.; Huang, K.-C.; Weng, H.-H. Hepatic disease and the risk of mortality of Vibrio vulnificus necrotizing skin and soft tissue infections: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0223513. [Google Scholar] [CrossRef]
- Mesa, C.A.D.; Mendoza, R.M.; Penir, S.M.U.; de la Peña, L.D.; Amar, E.C.; Saloma, C.P. Genomic analysis of Vibrio harveyi strain PH1009, a potential multi-drug resistant pathogen due to acquisition of toxin genes. Heliyon 2023, 9, e14926. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, B.; Dong, Y.; Li, X.; Zhang, J. Coinfection of Cage-Cultured Spotted Sea Bass (Lateolabrax maculatus) with Vibrio harveyi and Photobacterium damselae subsp. piscicida Associated with Skin Ulcer. Microorganisms 2024, 12, 503. [Google Scholar]
- Li, Z.; Sun, Y.; Tan, R.; Gao, Y. Identification, characterization and complete genome analysis of a Vibrio anguillarum isolated from Sebastes schlegelii. Microb. Pathog. 2024, 190, 106611. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-H.; Ab Mutalib, N.-S.; Law, J.W.-F.; Wong, S.H.; Letchumanan, V. Discovery on Antibiotic Resistance Patterns of Vibrio parahaemolyticus in Selangor Reveals Carbapenemase Producing Vibrio parahaemolyticus in Marine and Freshwater Fish. Front. Microbiol. 2018, 9, 2513. [Google Scholar] [CrossRef]
- Kumarage, P.M.; Majeed, S.; Silva, L.A.D.S.D.; Heo, G.-J. Detection of virulence, antimicrobial resistance, and heavy metal resistance properties in Vibrio anguillarum isolated from mullet (Mugil cephalus) cultured in Korea. Braz. J. Microbiol. 2023, 54, 415. [Google Scholar] [CrossRef] [PubMed]
- Thaotumpitak, V.; Sripradite, J.; Atwill, E.R.; Jeamsripong, S. Emergence of colistin resistance and characterization of antimicrobial resistance and virulence factors of Aeromonas hydrophila, Salmonella spp., and Vibrio cholerae isolated from hybrid red tilapia cage culture. PeerJ 2023, 11, e14896. [Google Scholar] [CrossRef]
- Fu, H.; Yu, P.; Liang, W.; Kan, B.; Peng, X.; Chen, L. Virulence, Resistance, and Genomic Fingerprint Traits of Vibrio cholerae Isolated from 12 Species of Aquatic Products in Shanghai, China. Microb. Drug Resist. 2020, 26, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Cortés, M.Z.; Vázquez, L.E.C.; Díaz, S.F.M.; Félix, C.S.C. Streptococcus iniae in aquaculture: A review of pathogenesis, virulence, and antibiotic resistance. Int. J. Vet. Sci. Med. 2024, 12, 25. [Google Scholar] [CrossRef]
- Agnew, W.; Barnes, A.C. Streptococcus iniae: An aquatic pathogen of global veterinary significance and a challenging candidate for reliable vaccination. Vet. Microbiol. 2007, 122, 1–15. [Google Scholar] [CrossRef]
- El Tawab, A.A.; El Hofy, F.; Ali, N.; Saad, W.; El-Mougy, E.; Mohammed, A. Antibiotic resistance genes in Streptococcus iniae isolated from diseased Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2022, 26, 413–428. [Google Scholar] [CrossRef]
- Heckman, T.I.; Soto, E. Streptococcus iniae biofilm formation enhances environmental persistence and resistance to antimicrobials and disinfectants. Aquaculture 2021, 540, 736739. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Geng, Y.; Zheng, L.; Rehman, T.; Zhao, R.; Wang, K.; OuYang, P.; Chen, D.; Huang, X.; et al. Molecular serotyping and antimicrobial susceptibility of Streptococcus agalactiae isolated from fish in China. Aquaculture 2019, 510, 84–89. [Google Scholar] [CrossRef]
- Domeénech, A.; Derenaáandez-Garayzábal, J.F.; Pascual, C.; Garcia, J.A.; Cutuli, M.T.; Moreno, M.A.; Collins, M.D.; Dominguez, L. Streptococcosis in cultured turbot, Scopthalmus maximus (L.), associated with Streptococcus parauberis. J. Fish Dis. 1996, 19, 33–38. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, N.; Roh, H.; Ho, D.T.; Park, J.; Lee, J.Y.; Kim, Y.-J.; Kang, H.-Y.; Lee, J.; Song, J.-Y.; et al. Serotype distribution and antibiogram of Streptococcus parauberis isolated from fish in South Korea. Microbiol. Spectr. 2023, 11, e04400-22. [Google Scholar] [CrossRef]
- Luo, X.; Fu, X.; Liao, G.; Chang, O.; Huang, Z.; Li, N. Isolation, pathogenicity and characterization of a novel bacterial pathogen Streptococcus uberis from diseased mandarin fish Siniperca chuatsi. Microb. Pathog. 2017, 107, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Jayarao, B.M.; Gillespie, B.E.; Lewis, M.J.; Dowlen, H.H.; Oliver, S.P. Epidemiology of Streptococcus uberis Intramammary Infections in a Dairy Herd. J. Vet. Med. Ser. B 1999, 46, 433–442. [Google Scholar] [CrossRef]
- Elez, R.M.M.A.; Zahra, E.M.F.; Gharieb, R.M.A.; Mohamed, M.E.M.; Samir, M.; Saad, A.M.; Merwad, A.M.A. Resistance patterns, virulence determinants, and biofilm genes of multidrug-resistant Pseudomonas aeruginosa isolated from fish and fish handlers. Sci. Rep. 2024, 14, 24063. [Google Scholar]
- Bakry, K.A.; Nasr, M.; Al-Amgad, Z.; Kondos, E.; Kondos, M.K.N.; Mehanny, P.E.; Alghamdi, A.A.A.; Khormi, M.A.; Abd-ElHafeez, H.H.; Emeish, W.F.A. Resistance of Nile tilapia fed with Padina boergesenii extract to Pseudomonas putida infection. BMC Vet. Res. 2024, 20, 281. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.A.H.M.O.; Safa, M. Imran. Study of some Bacterial Pathogens that Infect Common Carp (Cyprinus carpio) in the Diwaniya River and their Relationship to Water Characteristics. Adv. Anim. Vet. Sci. 2024, 12, 1961–1968. [Google Scholar] [CrossRef]
- Urku, C. Isolation and characterization of Pseudomonas putida caused granulomas in cultured sea bass (Dicentrarchus labrax) in Turkey. J. Hell. Vet. Med. Soc. 2021, 72, 2661–2668. [Google Scholar] [CrossRef]
- Oh, W.T.; Kim, J.H.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; et al. Genetic Characterization and Pathological Analysis of a Novel Bacterial Pathogen, Pseudomonas tructae, in Rainbow Trout (Oncorhynchus mykiss). Microorganisms 2019, 7, 432. [Google Scholar] [CrossRef]
- Suresh, K.; Pillai, D.; Soman, M.; Sreenivas, A.; Paul, R. Isolation and identification of antimicrobial susceptibility, biofilm formation, efflux pump activity, and virulence determinants in multi-drug resistant Pseudomonas aeruginosa isolated from freshwater fishes. J. Water Health 2023, 21, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Mabrok, M.; Sivaramasamy, E.; Youssef, F.M.; Atwa, M.H.; El-kholy, A.W.; Hetta, H.F.; Hozzein, W.N. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and blaTEM, blaCTX-M, and tetA antibiotic-resistance genes. Sci. Rep. 2020, 10, 15961. [Google Scholar] [CrossRef] [PubMed]
- Shabana, B.M.; Elkenany, R.M.; Younis, G. Sequencing and multiple antimicrobial resistance of Pseudomonas fluorescens isolated from Nile tilapia fish in Egypt. Braz. J. Biol. 2022, 84, e257144. [Google Scholar] [CrossRef] [PubMed]
- López, J.R.; Diéguez, A.L.; Doce, A.; De la Roca, E.; De la Herran, R.; Navas, J.I.; Toranzo, A.E.; Romalde, J.L. Pseudomonas baetica sp. nov., a fish pathogen isolated from wedge sole, Dicologlossa cuneata (Moreau). Int. J. Syst. Evol. Microbiol. 2012, 62, 874–882. [Google Scholar] [CrossRef]
- Russell Danner, G.; Merrill, P. Disinfectants, Disinfection, and Biosecurity in Aquaculture. In Aquaculture Biosecurity; John Wiley, Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 91–128. [Google Scholar] [CrossRef]
- Viel, A.; Rostang, A.; Morvan, M.-L.; Fournel, C.; Daniel, P.; Thorin, C.; Baron, S.; Sanders, P.; Calvez, S. Population pharmacokinetics/pharmacodynamics modelling of enrofloxacin for the three major trout pathogens Aeromonas salmonicida, Flavobacterium psychrophilum and Yersinia ruckeri. Aquaculture 2021, 545, 737119. [Google Scholar] [CrossRef]
- Kumru, S.; Tekedar, H.C.; Blom, J.; Lawrence, M.L.; Karsi, A. Genomic diversity in flavobacterial pathogens of aquatic origin. Microb. Pathog. 2020, 142, 104053. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, I.; Arana, L.; Ugarte-Uribe, B.; Gómez-Rubio, E.; Martín-Santamaría, S.; Garbisu, C.; Alkorta, I. Type IV Coupling Proteins as Potential Targets to Control the Dissemination of Antibiotic Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Declercq, A.M.; Tilleman, L.; Gansemans, Y.; Witte, C.D.; Haesebrouck, F.; Nieuwerburgh, F.V.; Smet, A.; Decostere, A. Comparative genomics of Flavobacterium columnare unveils novel insights in virulence and antimicrobial resistance mechanisms. Vet. Res. 2021, 52, 18. [Google Scholar] [CrossRef]
- Colclough, A.L.; Alav, I.; Whittle, E.E.; Pugh, H.L.; Darby, E.M.; Legood, S.W.; McNeil, H.E.; Blair, J.M. RND Efflux Pumps in Gram-Negative Bacteria; Regulation, Structure and Role in Antibiotic Resistance. Future Microbiol. 2020, 15, 143–157. [Google Scholar] [CrossRef]
- Bi, B.; Yuan, Y.; Jia, D.; Jiang, W.; Yan, H.; Yuan, G.; Gao, Y. Identification and Pathogenicity of Emerging Fish Pathogen Acinetobacter johnsonii from a Disease Outbreak in Rainbow Trout (Oncorhynchus mykiss). Aquac. Res. 2023, 2023, 1995494. [Google Scholar] [CrossRef]
- Bingöl, A.; Er, A.; İpek, Z.Z.; Kayış, Ş. Bacterial Examination of Wild and Cultured Fish Present in the Same Aquatic Ecosystem, and the Antibiotic Resistance of the Isolated Bacteria. Genet. Aquat. Org. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Kayansamruaj, P.; Srisapoome, P. Aci-netobacter Strain KUO11TH, a Unique Organism Related to Acinetobacter pittii and Isolated from the Skin Mucus of Healthy Bighead Catfish and Its Efficacy Against Several Fish Pathogens. Microorganisms 2019, 7, 549. [Google Scholar] [CrossRef]
- Brahmi, S.; Touati, A.; Cadière, A.; Djahmi, N.; Pantel, A.; Sotto, A.; Lavigne, J.-P.; Dunyach-Remy, C. First Description of Two Sequence Type 2 Acinetobacter baumannii Isolates Carrying OXA-23 Carbapenemase in Pagellus acarne Fished from the Mediterranean Sea near Bejaia, Algeria. Antimicrob. Agents Chemother. 2016, 60, 2513–2515. [Google Scholar] [CrossRef] [PubMed]
- Malick, R.C.; Bera, A.K.; Chowdhury, H.; Bhattacharya, M.; Abdulla, T.; Swain, H.S.; Baitha, R.; Kumar, V.; Das, B.K. Identification and pathogenicity study of emerging fish pathogens Acinetobacter junii and Acinetobacter pittii recovered from a disease outbreak in Labeo catla (Hamilton, 1822) and Hypophthalmichthys molitrix (Valenciennes, 1844) of freshwater wetland in West Bengal, India. Aquac. Res. 2020, 51, 2410–2420. [Google Scholar]
- Diao, H.; Lu, G.; Zhang, Y.; Wang, Z.; Liu, X.; Ma, Q.; Yu, H.; Li, Y. Risk factors for multidrug-resistant and extensively drug-resistant Acinetobacter baumannii infection of patients admitted in intensive care unit: A systematic review and meta-analysis. J. Hosp. Infect. 2024, 149, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hasiri, Z.; Rahimi, E.; Momtaz, H.; Shakerian, A. Prevalence and Phenotypic and Genotypic Patterns of Antibiotic Resistance of Acinetobacter baumannii Strains Isolated from Fish, Shrimp, and Lobster Samples. J. Food Process. Preserv. 2023, 2023, 6245225. [Google Scholar] [CrossRef]
- Laltlanmawia, C.; Ghosh, L.; Saha, R.K.; Parhi, J.; Pal, P.; Dhar, B.; Saha, H. Isolation, identification and patho-genicity study of emerging multi-drug resistant fish pathogen Acinetobacter pittii from diseased rohu (Labeo rohita) in India. Aquac. Rep. 2023, 31, 101629. [Google Scholar] [CrossRef]
- Darby, E.M.; Moran, R.A.; Holden, E.; Morris, T.; Harrison, F.; Clough, B.; McInnes, R.S.; Schneider, L.; Frickel, E.M.; Webber, M.A.; et al. Differential development of antibiotic resistance and virulence between Acinetobacter species. mSphere 2024, 9, e0010924. [Google Scholar] [CrossRef]
- Oke, M.T.; Martz, K.; Mocăniță, M.; Knezevic, S.; D’Costa, V.M. Analysis of Acinetobacter P-type type IV secretion system-encoding plasmid diversity uncovers extensive secretion system conservation and diverse antibiotic resistance determinants. Antimicrob. Agents Chemother. 2024, 68, e0103824. [Google Scholar] [CrossRef]
- Hawke, J.P.; Mcwhorter, A.C.; Steigerwalt, A.G.; Brenner, D.J. Edwardsiella ictaluri sp. nov., the Causative Agent of Enteric Septicemia of Catfish. Int. J. Syst. Evol. Microbiol. 1981, 31, 396–400. [Google Scholar] [CrossRef]
- Armwood, A.R.; Griffin, M.J.; Richardson, B.M.; Wise, D.J.; Ware, C.; Camus, A.C. Pathology and virulence of Edwardsiella tarda, Edwardsiella piscicida, and Edwardsiella anguillarum in channel (Ic-talurus punctatus), blue (Ictalurus furcatus), and channel × blue hybrid catfish. J. Fish Dis. 2022, 45, 1683–1698. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Lai, Q.; Liu, Q.; Wu, H.; Xiao, J.; Shao, Z.; Wang, Q.; Zhang, Y. Phylogenomics characterization of a highly virulent Edwardsiella strain ET080813T encoding two distinct T3SS and three T6SS gene clusters: Propose a novel species as Edwardsiella anguillarum sp. nov. Syst. Appl. Microbiol. 2015, 38, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Duman, M. Expanding the Spectrum of Diseases and Disease Associations Caused by Edwardsiella tarda and Related Species. Microorganisms 2024, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Riman, M.M.; Mannan, S.; Lawrence, M.L.; Abdelhamed, H. Characterisation and mobilisation of IncA/C plasmid-mediated antibiotic resistance in Edwardsiella ictalurid. J. Glob. Antimicrob. Resist. 2023, 33, 177–185. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. The A to Z of A/C plasmidsA/C. Plasmid 2015, 80, 63–82. [Google Scholar] [CrossRef]
- Fernández-Alarcón, C.; Singer, R.S.; Johnson, T.J. Comparative Genomics of Multidrug Resistance-Encoding IncA/C Plasmids from Commensal and Pathogenic Escherichia coli from Multiple Animal Sources. PLoS ONE 2011, 6, e23415. [Google Scholar] [CrossRef]
- Abdelhamed, H.; Ramachandran, R.; Ozdemir, O.; Waldbieser, G.; Lawrence, M.L. Characterization of a Novel Conjugative Plasmid in Edwardsiella piscicida Strain MS-18-199. Front. Cell. Infect. Microbiol. 2019, 9, 404. [Google Scholar] [CrossRef]
- Rahmawaty, A.; Cheng, L.-W.; Wang, P.-C.; Chen, S.-C. Comparative pathogenicity and histopathological analysis of Edwardsiella anguillarum intraperitoneal infection in milkfish (Chanos chanos), Nile tilapia (Oreochromis niloticus) and Asian seabass (Lates calcarifer). J. Fish Dis. 2024, 47, e13982. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, S.; Wan, Q.; Xu, M.; Chen, M.; Guo, S. Pathogenicity of Edwardsiella anguillarum to American eels (Anguilla rostrata) and RNA-seq analysis of host immune response to the E. anguillarum infection. Fish Shellfish Immunol. 2023, 141, 109042. [Google Scholar] [CrossRef]
- Byadgi, O.V.; Rahmawaty, A.; Wang, P.-C.; Chen, S.-C. Comparative genomics of Edwardsiella anguillarum and Edwardsiella piscicida isolated in Taiwan enables the identification of distinctive features and potential virulence factors using Oxford-Nanopore MinION® sequencing. J. Fish Dis. 2023, 46, 287–297. [Google Scholar] [CrossRef]
- Ewing, W.H.; Mcwhorter, A.C.; Escobar, M.R.; Lubin, A.H. Edwardsiella, a new genus of Enterobacteriaceae based on a new species, E. tarda. Int. J. Syst. Evol. Microbiol. 1965, 15, 33–38. [Google Scholar] [CrossRef]
- Aoki, T.; Arai, T.; Egusa, S. Detection of R Plasmids in Naturally Occurring Fish-Pathogenic Bacteria, Edwardsiella tarda. Microbiol. Immunol. 1977, 21, 77–83. [Google Scholar] [CrossRef]
- Algammal, A.M.; Mabrok, M.; Ezzat, M.; Alfifi, K.J.; Esawy, A.M.; Elmasry, N.; El-Tarabili, R.M. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture 2022, 548, 737643. [Google Scholar] [CrossRef]
- Fri, J.; Njom, H.A.; Ateba, C.N.; Ndip, R.N. Antibiotic Resistance and Virulence Gene Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolated from Healthy Edible Marine Fish. Int. J. Microbiol. 2020, 2020, 9803903. [Google Scholar] [CrossRef]
- Jinnai, M.; Yamaguchi, T.; Minh, D.T.N.; Hoang, O.N.; Thi, H.L.; Thanh, P.N.; Hoai, P.H.; Do, P.N.; Van, C.D.; Kumeda, Y.; et al. Edible river fish-derived extended-spectrum β-lactamase (ESBL)-producing Enterobacterales harboring transferable plasmids encoding blaCTX-M-15, blaCTX-M-27, and blaCTX-M-55. One Health 2024, 18, 100685. [Google Scholar] [CrossRef]
- Abd El-Tawab, A.; El-Hofy, F.; EL-Gamal, R.; Awad, S.; El-Mougy, E.; Mohamed, S. Phenotypic and genotypic characterization of Antibiotic resistant strains of Flavobacterium columnare isolated from Oreochromis niloticus (Nile tilapia). Benha Vet. Med. J. 2020, 38, 141–145. [Google Scholar] [CrossRef]
- Zhang, M.; Dou, Y.; Xiao, Z.; Xue, M.; Jiang, N.; Liu, W.; Xu, C.; Fan, Y.; Zhang, Q.; Zhou, Y. Identification of an Acinetobacter lwoffii strain isolated from diseased hybrid sturgeon (Acipenser baerii♀ × Acipenser schrenckii♂). Aquaculture 2023, 574, 739649. [Google Scholar] [CrossRef]
- Elabd, H.; Abd El-latif, A.; Shaheen, A.; Matter, A. Identification of emerging Acinetobacter johnsonii virulence and antibiotic resistance genes associated with high mortality in cultured Oreochromis niloticus. Egypt. J. Aquac. 2020, 10, 19–33. [Google Scholar] [CrossRef]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Barnes, A.C.; Rudenko, O.; Landos, M.; Dong, H.T.; Lusiastuti, A.; Phuoc, L.H.; Delamare-Deboutteville, J. Autogenous vaccination in aquaculture: A locally enabled solution towards reduction of the global antimicrobial resistance problem. Rev. Aquac. 2022, 14, 907–918. [Google Scholar] [CrossRef]
- Klaus-Peter, B.; Chairman, E.M.A.V.; Gerfried Zeller, M.D.E. EMAV_Manual-Autogenous-Vaccines_Munich_082023; EMAV: Munich, Germany, 2023. [Google Scholar]
- Moreau, E.; Marie, S.; Bachelet, F.; Pineau, L.; Calvez, S. Comparative safety and efficacy of autogenous vaccine administrated by different routes against furunculosis caused by Aeromonas salmonicida sub. salmonicida in large Rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2024, 152, 109757. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Krpetic, Z.; Martínez, M.M.; Garcia-Ordoñez, M.; Roher, N.; Palić, D. Self-assembling ferritin nanoplatform for the development of infectious hematopoietic necrosis virus vaccine. Front. Immunol. 2024, 15, 1346512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Middha, S.K.; Menon, S.V.; Paital, B.; Gokarn, S.; Nelli, M.; Rajanikanth, R.B.; Chandra, H.M.; Mugunthan, S.P.; Kantwa, S.M.; et al. Current Challenges of Vaccination in Fish Health Management. Animal 2024, 14, 2692. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.; Xu, Y.; Xu, G.; Chen, J.; Wang, Y.; Kang, Y.; Shan, X.; Kong, L.; Ma, H. An effective live attenuated vaccine against Aeromonas veronii infection in the loach (Misgurnus anguillicaudatus). Fish Shellfish Immunol. 2020, 104, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Bio-technological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Maiti, B.; Dubey, S.; Munang’andu, H.M.; Karunasagar, I.; Karunasagar, I.; Evensen, Ø. Application of Outer Membrane Protein-Based Vaccines Against Major Bacterial Fish Pathogens in India. Front. Immunol. 2020, 11, 1362. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, S.; He, M.; Liu, A.; Long, H.; Guo, W.; Cao, Z.; Xie, Z.; Zhou, Y. Construction and analysis of the immune effect of Vibrio harveyi subunit vaccine and DNA vaccine encoding TssJ antigen. Fish Shellfish Immunol. 2020, 98, 45–51. [Google Scholar] [CrossRef]
- Rosa, D.S.; Ribeiro, S.P.; Almeida, R.R.; Mairena, E.C.; Postól, E.; Kalil, J.; Cunha-Neto, E. A DNA Vaccine Encoding Multiple HIV CD4 Epitopes Elicits Vigorous Polyfunctional, Long-Lived CD4+ and CD8+ T Cell Re-sponses. PLoS ONE 2011, 6, e16921. [Google Scholar] [CrossRef]
- Ahangarzadeh, M.; Houshmand, H.; Torfi Mozanzadeh, M.; Kakoolaki, S.; Nazemroaya, S.; Sepahdari, A.; Peyghan, R.; Ajdari, A.; Sadr, A.S. Effect of killed autogenous polyvalent vaccines against Vibrio harveyi, V. alginolyticus and Streptococcus iniae on survival and immunogenicity of Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2023, 143, 109226. [Google Scholar] [CrossRef]
- Su, H.; Yakovlev, I.A.; van Eerde, A.; Su, J.; Clarke, J.L. Plant-Produced Vaccines: Future Applications in Aquaculture. Front. Plant Sci. 2021, 12, 718775. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.-E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef]
- Foged, C. Subunit Vaccines of the Future: The Need for safe, Customized and Optimized Particulate Delivery Systems. Ther. Deliv. 2011, 2, 1057–1077. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Christiansen, R.H.; Dalsgaard, I.; Madsen, L.; Middelboe, M. Bacteriophage Resistance Mechanisms in the Fish Pathogen Flavobacterium psychrophilum: Linking Genomic Mutations to Changes in Bacterial Virulence Factors. Appl. Environ. Microbiol. 2015, 81, 1157–1167. [Google Scholar] [CrossRef]
- Ninawe, A.S.; Sivasankari, S.; Ramasamy, P.; Kiran, G.S.; Selvin, J. Bacteriophages for aquaculture disease control. Aquac. Int. 2020, 28, 1925–1938. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage Therapy as an Approach to Prevent Vibrio anguillarum Infections in Fish Larvae Production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cong, C.; Wang, L.; Li, X.; Li, J.; Yang, H.; Li, S.; Xu, Y. Protective effectiveness of feeding phage cocktails in controlling Vibrio harveyi infection of turbot Scophthalmus maximus. Aquaculture 2021, 535, 736390. [Google Scholar] [CrossRef]
- Silva, Y.J.; Moreirinha, C.; Pereira, C.; Costa, L.; Rocha, R.J.M.; Cunha, Â.; Gomes, N.C.M.; Calado, R.; Almeida, A. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS-A. Aquaculture 2016, 450, 225–233. [Google Scholar] [CrossRef]
- Castillo, D.; Højsting, A.R.; Roosvall, A.; Smyrlis, G.; Jørgensen, J.; Middelboe, M. In Vitro Evolution of Specific Phages Infecting the Fish Pathogen Flavobacterium psychrophilum. PHAGE Ther. Appl. Res. 2022, 3, 28–37. [Google Scholar] [CrossRef]
- Kalatzis, P.G.; Castillo, D.; Katharios, P.; Middelboe, M. Bacteriophage Interactions with Marine Pathogenic Vibrios: Implications for Phage Therapy. Antibiotics 2018, 7, 15. [Google Scholar] [CrossRef]
- Antoine, C.; Mackay, D.; Midtlyng, P.; Kleppen, H.P.; Palić, D.; Pirnay, J.-P.; Thiry, D.; Thiry, E. Bacteriophage-based veterinary products: Aligning regulatory framework and development challenges for market integration. Biologicals 2025, 91, 101847. [Google Scholar] [CrossRef]
- Duarte, J.; Pereira, C.; Moreirinha, C.; Salvio, R.; Lopes, A.; Wang, D.; Almeida, A. New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: An in vitro preliminary study. Aquaculture 2018, 495, 970–982. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, H.; Kaur, B.; Naveen Kumar, B.T.; Tyagi, A.; Singh, P.; Tanuj; Dubey, S.; Munang’andu, H.M. Isolating pathogenic multidrug-resistant Aeromonas hydrophila from diseased fish and assessing the effectiveness of a novel lytic Aeromonas veronii bacteriophage (AVP3) for biocontrol. Microb. Pathog. 2024, 196, 106914. [Google Scholar] [CrossRef]
- Castillo, D.; Rørbo, N.; Jørgensen, J.; Lange, J.; Tan, D.; Kalatzis, P.G.; Svenningsen, S.L.; Middelboe, M. Phage defense mechanisms and their genomic and phenotypic implications in the fish pathogen Vibrio anguillarum. FEMS Microbiol. Ecol. 2019, 95, fiz004. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Shi, X.; Yang, K.; Xu, Q.; Wang, F.; Chen, S.; Xu, T.; Liu, J.; Wen, W.; Chen, R.; et al. Phage-antibiotic synergy suppresses resistance emergence of Klebsiella pneumoniae by altering the evolutionary fitness. mBio 2024, 15, e01393-24. [Google Scholar] [CrossRef]
- Bulssico, J.; PapukashvilI, I.; Espinosa, L.; Gandon, S.; Ansaldi, M. Phage-antibiotic synergy: Cell filamentation is a key driver of successful phage predation. PLoS Pathog. 2023, 19, e1011602. [Google Scholar] [CrossRef] [PubMed]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture 2019, 513, 734423. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquac. 2021, 13, 862–906. [Google Scholar] [CrossRef]
- Shokrak, N.M.; Khairi, N.; Hazrin-Chong, N.H.; Mohamed, R.A.; Abdella, B. Isolation, characterization, and assessment of Bacillus rugosus potential as a new probiotic for aquaculture applications. Sci. Rep. 2024, 14, 25019. [Google Scholar] [CrossRef] [PubMed]
- Say, P.; Nimitkul, S.; Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Effects of the combination of chitosan and Acinetobacter KU011TH on the growth and health performances and disease resistance of juvenile hybrid catfish (Clarias gariepinus × C. macrocephalus). Fish Shellfish Immunol. 2023, 142, 109177. [Google Scholar] [CrossRef]
- Anokyewaa, M.A.; Amoah, K.; Li, Y.; Lu, Y.; Kuebutornye, F.K.A.; Asiedu, B.; Seidu, I. Prevalence of virulence genes and an-tibiotic susceptibility of Bacillus used in commercial aquaculture probiotics in China. Aquac. Rep. 2021, 21, 100784. [Google Scholar] [CrossRef]
- Mouafo, H.T.; Sokamte, A.T.; Manet, L.; Mbarga, A.J.M.; Nadezdha, S.; Devappa, S.; Mbawala, A. Biofilm Inhibition, Antibacterial and Antiadhesive Properties of a Novel Biosurfactant from Lactobacillus paracasei N2 against Multi-Antibiotics-Resistant Pathogens Isolated from Braised Fish. Fermentation 2023, 9, 646. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Park, S.C. Immunomodulatory Role of Microbial Surfactants, with Special Emphasis on Fish. Int. J. Mol. Sci. 2020, 21, 7004. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Role of Bacillus subtilis VSG4-derived biosurfactant in mediating immune responses in Labeo rohita. Fish Shellfish Immunol. 2016, 54, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.R.; Borges, A.C. Biodegradability of bacterial surfactants. Biodegradation 2011, 22, 585–592. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, B.; Cai, Q.; Ling, J.; Lee, K.; Chen, B. Fish Waste Based Lipopeptide Production and the Potential Application as a Bio-Dispersant for Oil Spill Control. Front. Bioeng. Biotechnol. 2020, 8, 734. [Google Scholar] [CrossRef]
- Ismail, R.; Baaity, Z.; Csóka, I. Regulatory status quo and prospects for biosurfactants in pharmaceutical applications. Drug Discov. Today 2021, 26, 1929–1935. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimi-crobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Liu, C.-H.; Hu, S.-Y. Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 84, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Biron, E.; Ben Said, L.; Subirade, M.; Fliss, I. Bacteriocin-Based Synergetic Consortia: A Promising Strategy to Enhance Antimicrobial Activity and Broaden the Spectrum of Inhibition. Microbiol. Spectr. 2022, 10, e00406-21. [Google Scholar] [CrossRef]
- Anyairo, C.; Unban, K.; Shetty, K.; Khanongnuch, C. Bacteriocin producing Bacillus and their potential applications in fish farming. Int. Aquat. Res. 2024, 16, 17–37. [Google Scholar] [CrossRef]
- Huttner, K.M.; Bevins, C.L. Antimicrobial Peptides as Mediators of Epithelial Host Defense. Pediatr. Res. 1999, 45, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Batool, A.I.; Rehman, M.F.U.; Naz, S. Evaluation of the antibacterial activity and protein profiling of Nile tilapia (Oreochromis niloticus) epidermal mucus under different feeds and culture systems (biofloc technology and earthen pond). J. Fish Dis. 2024, 47, e13884. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Li, H.; Kong, X.; Zhou, D.; Chen, Q.; Zhang, Q.; Yu, H. Characterization of biochemical properties of the skin mucus of turbot (Scophthalmus maximu) and differential proteomic analysis of skin mucus in response to Vibrio anguillarum. Aquaculture 2023, 577, 739960. [Google Scholar] [CrossRef]
- Xiao, X.; Lu, H.; Zhu, W.; Zhang, Y.; Huo, X.; Yang, C.; Xiao, S.; Zhang, Y.; Su, J. A Novel Antimicrobial Peptide Derived from Bony Fish IFN1 Exerts Potent Antimicrobial and Anti-Inflammatory Activity in Mammals. Microbiol. Spectr. 2022, 10, e02013-21. [Google Scholar] [CrossRef] [PubMed]
- Squitieri, D.; Massaro, F.; Graziano, M.M.; Borocci, S.; Cacaci, M.; Di Vito, M.; Porcelli, F.; Rosato, R.; Ceccacci, F.; Sanguinetti, M.; et al. Trematocine-derived antimicrobial peptides from the Antarctic fish Trematomus bernacchaii: Potent antibacterial agents against ESKAPE pathogens. Front. Microbiol. 2024, 15, 1447301. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhang, H.; Du, X.; Cao, Z.; Wu, Y.; Liu, C.; Sun, Y. Antibacterial Activity and Mechanisms of TroHepc2-22, a Derived Peptide of Hepcidin2 from Golden Pompano (Trachinotus ovatus). Int. J. Mol. Sci. 2023, 24, 9251. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; et al. Expression of the Antimicrobial Peptide Piscidin 1 and Neuropeptides in Fish Gill and Skin: A Potential Participation in Neuro-Immune Interaction. Mar. Drugs 2022, 20, 145. [Google Scholar] [CrossRef]
- Katzenback, B.A. Antimicrobial Peptides as Mediators of Innate Immunity in Teleosts. Biology 2015, 4, 607–639. [Google Scholar] [CrossRef]
- Bedekar, M.K.; Kole, S. Fundamentals of Fish Vaccination. In Vaccine Design: Methods and Protocols, Volume 2. Vaccines for Veterinary Diseases; Thomas, S., Ed.; Springer: New York, NY, USA, 2022; pp. 147–173. [Google Scholar] [CrossRef]
- Yamasaki, M.; Araki, K.; Maruyoshi, K.; Matsumoto, M.; Nakayasu, C.; Moritomo, T.; Nakanishi, T.; Yamamoto, A. Comparative analysis of adaptive immune response after vaccine trials using live attenuated and formalin-killed cells of Edwardsiella tarda in ginbuna crucian carp (Carassius auratus langsdorfii). Fish Shellfish Immunol. 2015, 45, 437–442. [Google Scholar] [CrossRef]
- Ram Bhajan, M.; Shrestha, M.K.; Pant, J.; Jha, D.; Live Attenuated Bacterial Vaccines in Aquaculture. ISTA9 Publications. Available online: https://www.researchgate.net/publication/280326803_ISTA9_Publications (accessed on 8 June 2025).
- Hu, T.; Wang, Y.; Wang, Y.; Cui, H.; Zhang, J.; Chen, H.; Wu, B.; Hao, S.; Chu, C.C.; Wu, Y.; et al. Production and evaluation of three kinds of vaccines against largemouth bass virus, and DNA vaccines show great application prospects. Fish Shellfish Immunol. 2024, 153, 109841. [Google Scholar] [CrossRef]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Jinendiran, S.; Archana, R.; Sathishkumar, R.; Kannan, R.; Selvakumar, G.; Sivakumar, N. Dietary Administration of Probiotic Aeromonas veronii V03 on the Modulation of Innate Immunity, Expression of Immune-Related Genes and Disease Resistance Against Aeromonas hydrophila Infection in Common Carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 13, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhang, R.; Wang, C.; Liu, Z.; Fei, M.; Tang, B.; Yang, H.; Sun, D. Engineering probiotic Escherichia coli Nissle 1917 to block transfer of multiple antibiotic resistance genes by exploiting a type I CRISPR-Cas system. Appl. Environ. Microbiol. 2024, 90, e00811-24. [Google Scholar] [CrossRef] [PubMed]
- Hamza, F.; Satpute, S.; Banpurkar, A.; Kumar, A.R.; Zinjarde, S. Biosurfactant from a marine bacterium disrupts biofilms of pathogenic bacteria in a tropical aquaculture system. FEMS Microbiol. Ecol. 2017, 93, fix140. [Google Scholar] [CrossRef] [PubMed]
- Baños, A.; Ariza, J.J.; Nuñez, C.; Gil-Martínez, L.; García-López, J.D.; Martínez-Bueno, M.; Valdivia, E. Effects of Enterococcus faecalis UGRA10 and the enterocin AS-48 against the fish pathogen Lactococcus garvieae. Studies in vitro and in vivo. Food Microbiol. 2019, 77, 69–77. [Google Scholar] [CrossRef]

| Bacteria | Resistance Genes | Antimicrobial Susceptibility Test | Fish Species | References |
|---|---|---|---|---|
| A. salmonicida | mcr-3, fox-2, cphA5, and OXA-427 | - | Brook trout and char | [52] |
| FOX-4, cphA5, blaOXA-427-like, APH[3″]-Ib, APH[6]-Id, floR, sul2 | Cefoxitin, chloramphenicol, florphenicol, tetracycline, oxytetracycline | Salmon | [54] | |
| A. hydrophila | act, aerA, alt | Chloramphenicol, amikacin, gentamicin | Nile tilapia fish | [55] |
| sul1, strA-strB, aadA, blaTEM, blaSHV, tetA-tetE, tetM | Ampicillin, streptomycin, kanamycin, nalidixic acid | African catfish and Pangasius catfish | [56] | |
| A. veronii | H-NS, bacA, mdtH, vatF, dfrA3, cphA3 | - | Mandarin fish | [58] |
| V. harveyi | - | Cephalosporin (vancomycin, cefoperazone, cefradine), aminoglycoside (piperacillin) | Spotted sea bass | [64] |
| V. anguillarum | carB-19, mecB, novA, QnrS2, erm, sul4, catB9, optrA, cfr | Penicillin, oxacillin, ampicillin, cefradine, neomycin, pipemidic acid, ofloxacin, norfloxacin | Rockfish (Sebastes schlegelii) | [65] |
| V. anguillarum | qnrS, qnrB, StrAB | Oxacillin, ticarcillin, streptomycin, ciprofloxacin | Korean mullets | [67] |
| V. parahaemolyticus | - | Ampicillin, amikacin, kanamycin | Yellowstripe scad, Indian mackerel, Black pomfret, catfish, Red tilapia | [66] |
| V. vulnificus | QnrVC1, QnrVC7, tetR, tetB, blaCTX-M-55, tet(59), sul2, QnrVC5, gyrA, gyrB, parC | Amoxicillin, Colistin sulfate, Metronidazole, Streptomycin, Clindamycin | Asian sea bass | [16] |
| V. cholerae | - | Streptomycin, ampicillin, rifampicin | Bighead carp, Goldfish, Grass carp, Channel catfish, Longface emperor, Northern snakehead, White Amur bream, Turbot | [69] |
| S. iniae | ermB, tetM, tetO | Erythromycin, tetracycline | Nile Tilapia | [72] |
| S. agalactiae | ermB, tetM, gyrA, parC | Erythromycin, tetracycline, enrofloxacin, penicillin | Schizothorax prenant, Schizopygopsis pylzovi | [74] |
| S. parauberis | serotype Ia: erm(B), tet(S), ANT (6)-Ia serotype II: tet(M), mef(J)-msr(I) | Amoxicillin, Oxytetracycline, Erythromycin | Olive flounder | [76] |
| P. putida | - | Piperacillin-tazobactam, Cefepime, Amikacin, Levofloxacin, Amoxycillin | Rainbow Trout | [83] |
| P. aeruginosa | tetA, tetD, tetM, sul1, blaCTX-M, blaTEM, blaSHV | Oxytetracycline, co-trimoxazole, doxycycline, enrofloxacin, ciprofloxacin, cefotaxime, ceftazidime, and ampicillin | Rohu, Catla, Pangasius | [84] |
| P. fluorescens | - | Piperacillin, ceftazidime and cefepime | Nile Tilapia | [86] |
| P. baetica | mexF, aac(6′)-31, qacH, blaOXA-2, qacEΔ1, sul1, tet(A) | Ceftriaxone, cefotaxime, cefoxitin, aztreonam, florphenicol, tetracycline, oxytetracycline | Salmon | [54] |
| F. columnare | blaTEM, blaSHV, tetA | Pencillin, cephalosporin, aminoglycoside, nitrofurans, polymyxin B and tetracycline | Nile Tilapia | [120] |
| F. psychrophilum | AGly, bla, col, flq, gly, mls, oxzln, phe, sul, tet, tmt | - | Ayu, Stickleback, Rainbow trout, Coho salmon, Atlantic salmon | [90] |
| A. baumanni | blaCITM, blaSHV, tetA, qnrA, blaVIM, aac(3)-IV, sul1, dfrA1, qnr | Tetracycline, ampicillin, gentamicin, erythromycin | Fish market | [100] |
| A. pittii | - | Cephalexin, cefoxitin, nitrofurantoin, ampicillin, oxacillin, penicillin-G, bacitracin, and trimethoprim | Rohu | [101] |
| A. lwoffii | - | Florfenicol, sulfadiazinum, penicillin, tetracycline | Hybrid sturgeons (Acipenser baerii♀ × Acipenser schrenckii♂) | [121] |
| A. johnsonii | qacED1, qnrS, sul1, dfrA, aadA1 | Ampicillin, gentamicin, lincomycin, nalidixic acid, tetracycline, oxytetracycline | Nile tilapia | [122] |
| E. ictaluri | floR, tetD, sul2 | - | Channel catfish | [108] |
| E. piscicida | floR, tetA, tetR, sul2, aph(6)-Id (strB), aph(3)-Ib (strA) | Florfenicol, chloramphenicol, oxytetracycline, doxycycline, erythromycin, tetracycline, azitromycin, spectinomycin, sulfonamide, and bacitracin | Hybrid catfish (channel catfish × blue catfish) | [111] |
| E. tarda | blaTEM, sul1, tetA, blaCTX-M, aad1, qnrS, qnrA | Ampicillin, amoxicillin, tetracycline, trimethoprim-sulphamethazole, cefotaxime, streptomycin, gentamycin, ciprofloxacinand, enrofloxacin | Nile tilapia and African catfish | [117] |
| Methicillin-resistant Staphylococcus aureus (MRSA) | femA, blaZ, tetA, tetM, ermB | Erythromycin, ampicillin, rifampicin, clindamycin | Marine aquaculture fish | [118] |
| Extended-spectrum β-Lactamase (ESBL)-producing Enterobacterales | blaCTX-M-15, blaCTX-M-27, blaCTX-M-55 | Ampicillin, tetracycline, trimthoprim-sulfamethoxazole, chloramphenicol, nalidixic acid, streptomycin, and ciprofloxacin | Edible river fish | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Wojnarowski, K.; Cholewińska, P.; Palić, D. Current Trends in Approaches to Prevent and Control Antimicrobial Resistance in Aquatic Veterinary Medicine. Pathogens 2025, 14, 681. https://doi.org/10.3390/pathogens14070681
Zhao D, Wojnarowski K, Cholewińska P, Palić D. Current Trends in Approaches to Prevent and Control Antimicrobial Resistance in Aquatic Veterinary Medicine. Pathogens. 2025; 14(7):681. https://doi.org/10.3390/pathogens14070681
Chicago/Turabian StyleZhao, Dongqing, Konrad Wojnarowski, Paulina Cholewińska, and Dušan Palić. 2025. "Current Trends in Approaches to Prevent and Control Antimicrobial Resistance in Aquatic Veterinary Medicine" Pathogens 14, no. 7: 681. https://doi.org/10.3390/pathogens14070681
APA StyleZhao, D., Wojnarowski, K., Cholewińska, P., & Palić, D. (2025). Current Trends in Approaches to Prevent and Control Antimicrobial Resistance in Aquatic Veterinary Medicine. Pathogens, 14(7), 681. https://doi.org/10.3390/pathogens14070681








