Evaluating the Antiviral Activity of Termin-8 and Finio Against a Surrogate ASFV-like Algal Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and EhV-86 Stock
2.2. Standard qPCR and Viability qPCR Assays
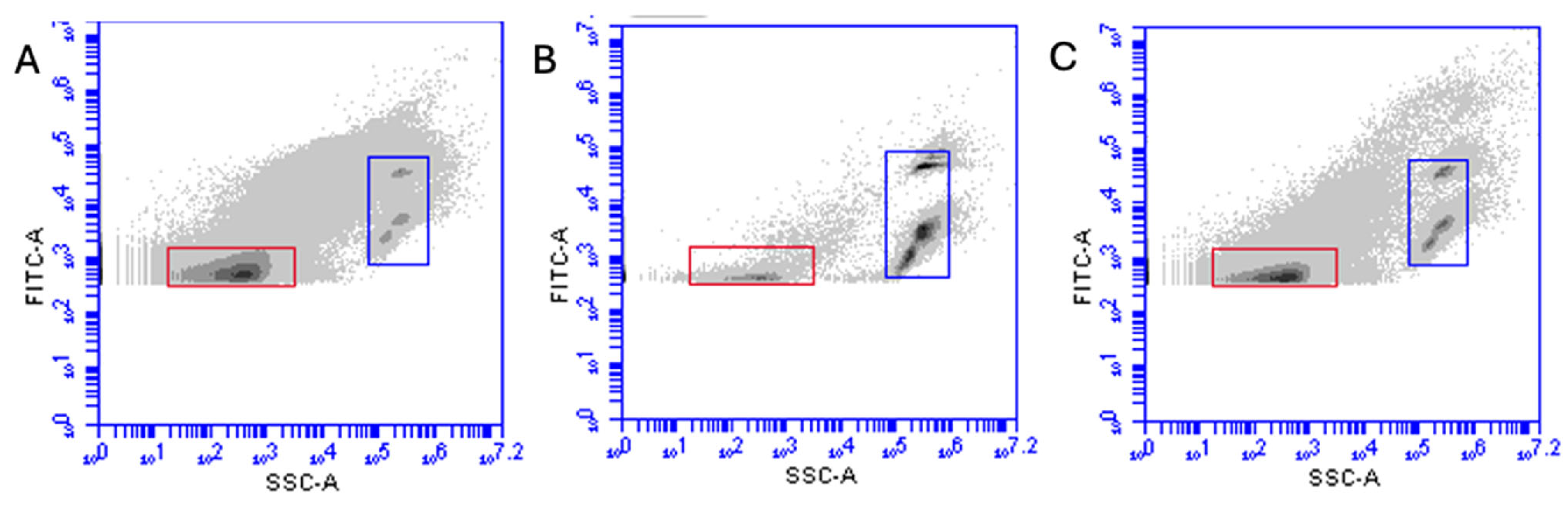
2.3. Flow Cytometry
2.4. Statistical Analysis
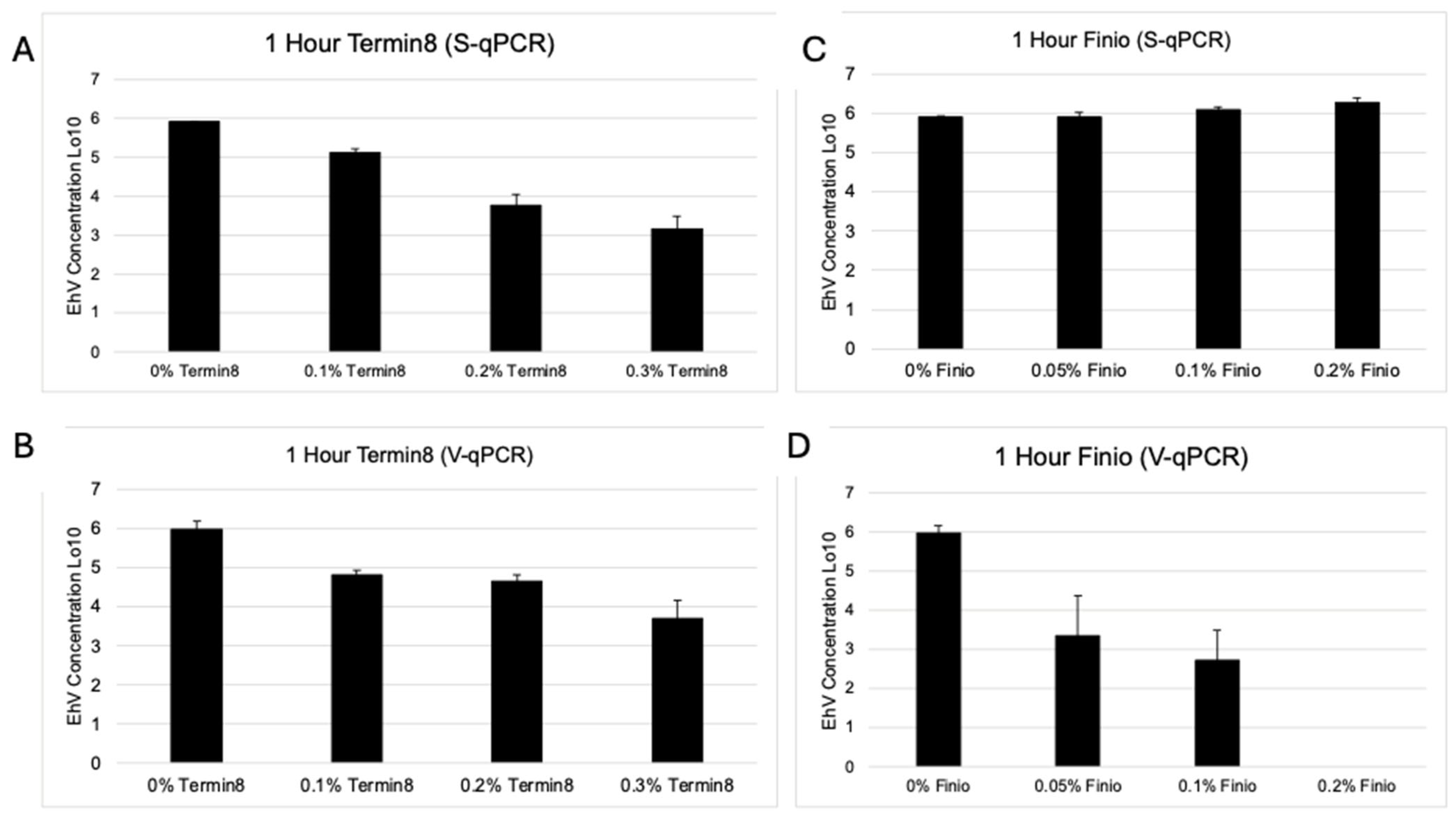
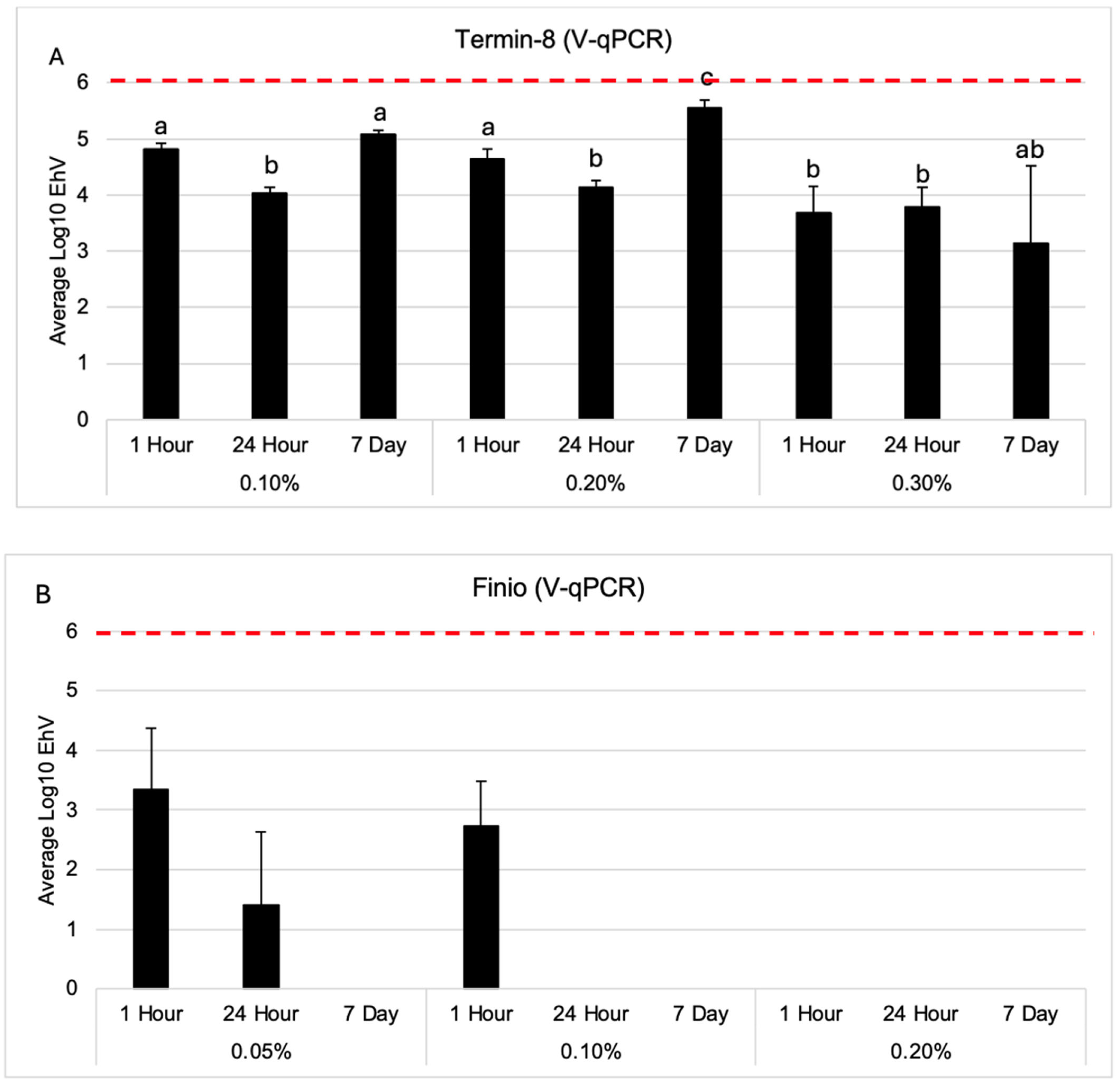
3. Results
Viability of EhV After Exposure to Temin-8 and Finio
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palowski:, A.; Balestreri, C.; Urriola, P.E.; van de Ligt, J.L.G.; Sampedro, F.; Dee, S.; Shah, A.; Yancy, H.F.; Shurson, G.C.; Schroeder, D.C. Survival of a surrogate African swine fever virus-like algal virus in feed matrices using a 23-day commercial United States truck transport model. Front. Microbiol. 2022, 13, 1059118. [Google Scholar] [CrossRef] [PubMed]
- Shurson, G.C.; Ramirez-Camba, C.D.; Urriola, P.E.; Schroeder, D.C. Stability of a surrogate African swine fever-like algal virus in corn- and soybean-based feed ingredients during extended storage and in vitro digestion processes. Front. Vet. Sci. 2024, 11, 1498977. [Google Scholar] [CrossRef] [PubMed]
- Balestreri, C.; Schroeder, D.C.; Sampedro, F.; Marqués, G.; Palowski, A.; Urriola, P.E.; van de Ligt, J.L.G.; Yancy, H.F.; Shurson, G.C. Unexpected thermal stability of two enveloped megaviruses Emiliania huxleyi virus African swine fever virus as measured by viability PCR. Virol. J. 2024, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Shah, A.; Jones, C.; Singrey, A.; Hanson, D.; Edler, R.; Spronk, G.; Niederwerder, M.; Nelson, E. Evidence of viral survival in representative volumes of feed and feed ingredients during long-distance commercial transport across the continental United States. Transbound. Emerg. Dis. 2021, 69, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Palowski, A.; Blestreri, C.; Urriola, P.E.; van de Ligt, J.L.G.; Ozer, R.; Shurson, G.C.; Schroeder, D.C. Evaluating the inactivation of a surrogate ASFV-like algal virus in a pilot solvent extraction soybean processing facility. Front. Anim. Sci. 2025, 6, 1521492. [Google Scholar] [CrossRef]
- Homola, M.; Büttner, C.R.; Füzik, T.; Křepelka, P.; Holbová, R.; Nováček, J.; Chaillet, M.L.; Žák, J.; Grybchuk, D.; Förster, F.; et al. Structure and replication cycle of a virus infecting climate-modulating alga Emiliania huxleyi. Sci. Adv. 2024, 10, eadk1954. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C.; Oke, J.; Malin, G.; Wilson, W.H. Coccolithovirus (Phycodnaviridae): Characterisation of a new large dsDNA algal virus that infects Emiliania huxleyi. Arch. Virol. 2002, 147, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Schambow, R.A.; Sampedro, F.; Urriola, P.E.; van de Ligt, J.L.G.; Perez, A.; Shurson, G.C. Rethinking the uncertainty of African swine fever virus contamination in feed ingredients and risk of introduction into the United States. Transbound. Emerg. Dis. 2021, 69, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; De Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Survival of viral pathogens in animal feed ingredients under transboundary 85 shipping models. PLoS ONE 2018, 13, e0194509. [Google Scholar] [CrossRef]
- Stoian, A.M.M.; Zimmerman, J.; Ji, J.; Hefley, T.J.; Dee, S.; Diel, D.G.; Rowland, R.R.R.; Niederwerder, M.C. Half-life of African swine fever virus in shipped feed. Emerg. Infect. Dis. 2019, 25, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C. Long-Term Surrogate Virus Assays and Methods. U.S. Patent PCT/US2020/039072, 21 November 2024. [Google Scholar]
- Ramires, J. Susceptibility of Salmonella Strains in Feed to a Formaldehyde-Based Product; 98 (E-Supplement 1), 108th Annual Meeting Abstracts; Poultry Science Association: Champaign, IL, USA, 2019. [Google Scholar]
- Formaldehyde. 21 CFR § 573.460. 2003. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-E/part-573/subpart-B/section-573.460 (accessed on 2 January 2025).
- Niederwerder, M.C.; Dee, S.; Diel, D.G.; Stoian, A.M.M.; Constance, L.A.; Olcha, M.; Petrovan, V.; Patterson, G.; Cino-Ozuna, A.G.; Rowland, R.R.R. Mitigating the risk of African swine fever virus in feed with anti-viral chemical additives. Transbound. Emerg. Dis. 2020, 68, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Harrison, O.L.; Bai, J.; Larson, M.; Pograninchniy, R.M.; Domingues, F.; Holcombe, N.; Lopez, O.; Jones, C.K. Evaluation of formaldehyde when complete feed and soybean meal were inoculated with porcine epidemic diarrhea virus, porcine reproductive and respiratory syndrome virus, and Seneca Valley virus 1. Transl. Anim. Sci. 2024, 8, txae121. [Google Scholar] [CrossRef] [PubMed]
- Nocker, A.; Sossa, K.E.; Camper, A.K. Molecular monitoring of disinfection efficacy using propidium monoazide in combination with quantitative PCR. J. Microbiol. Methods 2007, 70, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Elizaquivel, P.; Aznar, R.; Sanchez, G. Recent developments in the use of viability dyes and quantitative PCR in the food microbiology field. J. Appl. Microbiol. 2014, 116, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.W.; Toews, J.; Kast, J. Utility of formaldehyde cross-linking and mass spectrometry in the study of protein–protein interactions. J. Mass Spectrom. 2008, 43, 699–715. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palowski, A.; Domingues, F.; Lopez, O.; Holcombe, N.; Shurson, G.; Schroeder, D.C. Evaluating the Antiviral Activity of Termin-8 and Finio Against a Surrogate ASFV-like Algal Virus. Pathogens 2025, 14, 672. https://doi.org/10.3390/pathogens14070672
Palowski A, Domingues F, Lopez O, Holcombe N, Shurson G, Schroeder DC. Evaluating the Antiviral Activity of Termin-8 and Finio Against a Surrogate ASFV-like Algal Virus. Pathogens. 2025; 14(7):672. https://doi.org/10.3390/pathogens14070672
Chicago/Turabian StylePalowski, Amanda, Francisco Domingues, Othmar Lopez, Nicole Holcombe, Gerald Shurson, and Declan C. Schroeder. 2025. "Evaluating the Antiviral Activity of Termin-8 and Finio Against a Surrogate ASFV-like Algal Virus" Pathogens 14, no. 7: 672. https://doi.org/10.3390/pathogens14070672
APA StylePalowski, A., Domingues, F., Lopez, O., Holcombe, N., Shurson, G., & Schroeder, D. C. (2025). Evaluating the Antiviral Activity of Termin-8 and Finio Against a Surrogate ASFV-like Algal Virus. Pathogens, 14(7), 672. https://doi.org/10.3390/pathogens14070672







