Investigation of Leishmania infantum Infection and Feeding Preferences of Lutzomyia longipalpis During Deltamethrin (4%) Dog Collar Intervention
Abstract
1. Introduction
2. Materials and Methods
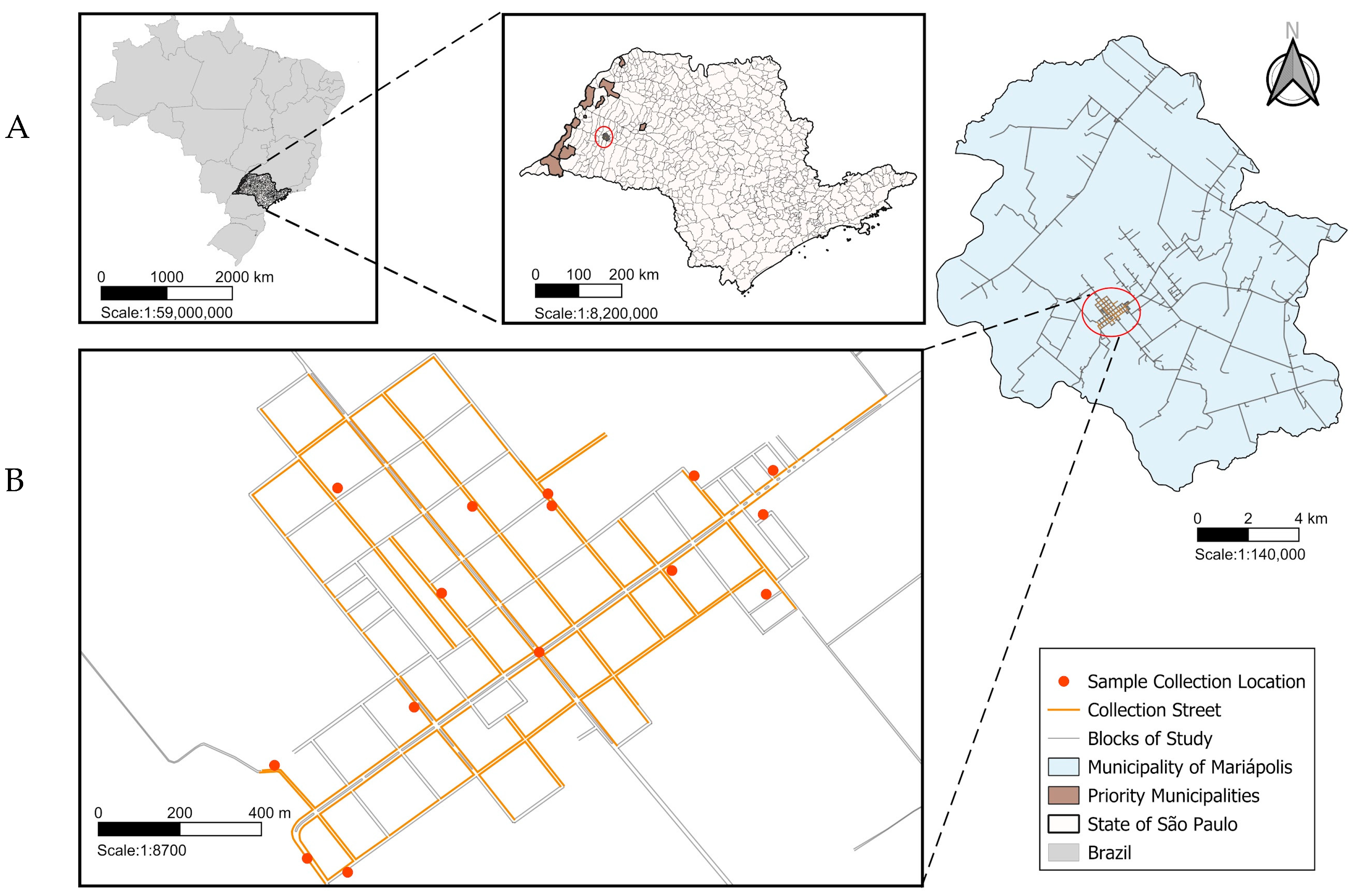
2.1. Study Area
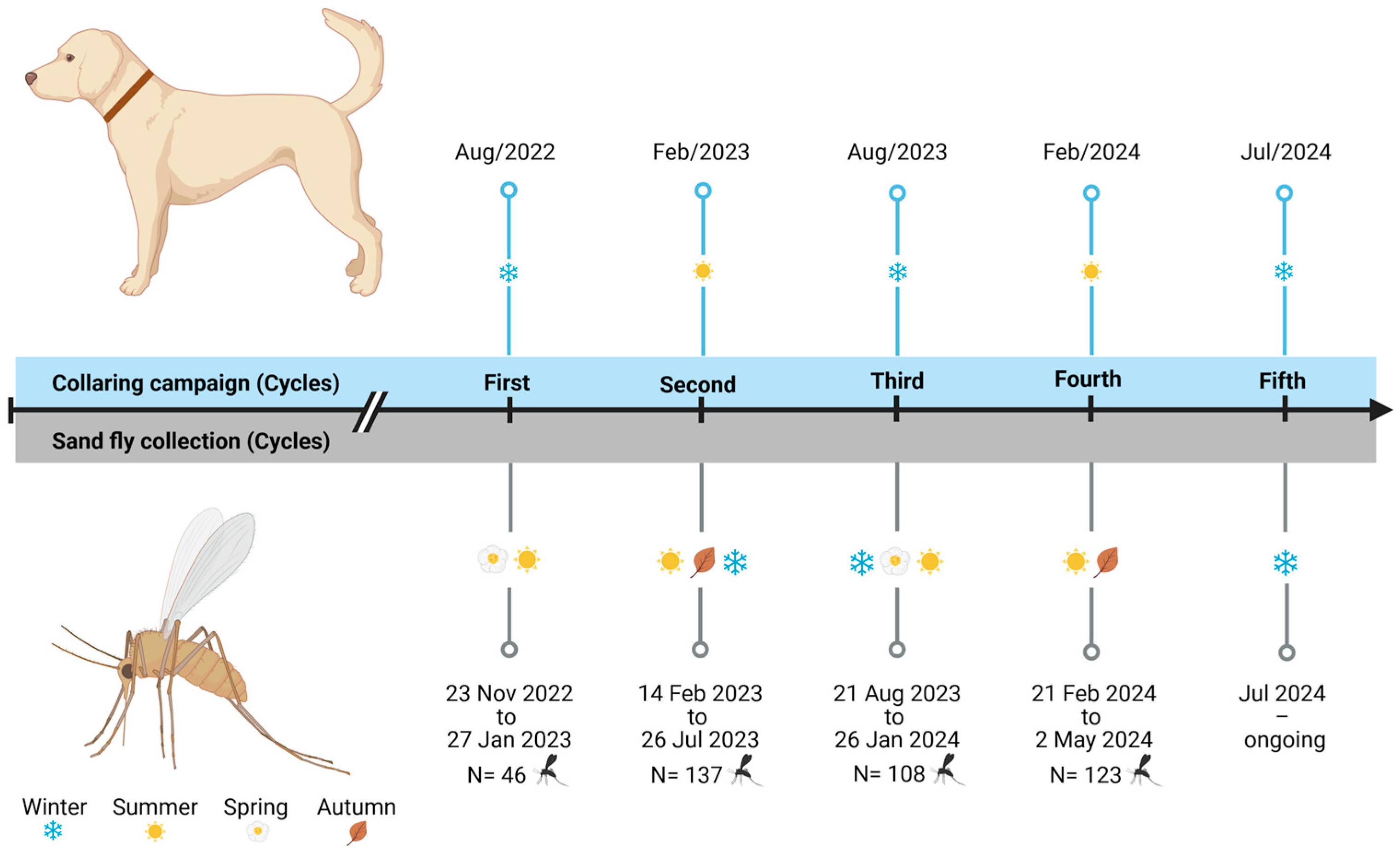
2.2. Entomological Capture and Sand Fly Identification
2.3. DNA Extraction
2.4. PCR for Detection of Leishmania spp.
2.5. Sequencing
2.6. Analysis of Sand Fly Blood-Feeding Sources
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kato, H. Epidemiology of Leishmaniasis: Risk factors for its pathology and infection. Parasitol. Int. 2025, 105, 102999. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Ruiz-Postigo, J.A.; Grout, L.; Jain, S. Global leishmaniasis surveillance, 2017–2018, and first report on 5 additional indicators. Wkly. Epidemiol. Rec. 2020, 25, 165–280. Available online: https://www.who.int/publications/i/item/who-wer9525 (accessed on 30 May 2025).
- Pan American Health Organization. Leishmaniasis: Epidemiological report on the Region of the Americas. No. 13, December 2024. Washington, D.C.: PAHO. 2024. Available online: https://iris.paho.org/handle/10665.2/51742 (accessed on 30 May 2025).
- Madeira, M.D.F.; Schubach, A.D.O.; Schubach, T.M.P.; Leal, C.A.; Marzochi, M.C.D.A. Identification of Leishmania (Leishmania) chagasi Isolated from Healthy Skin of Symptomatic and Asymptomatic Dogs Seropositive for Leishmaniasis in the Municipality of Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2004, 8, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Manual de Vigilância e Controle da Leishmaniose Visceral. 2006. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral.pdf (accessed on 25 February 2025).
- Monteiro, F.M.; Machado, A.S.; Rocha-Silva, F.; Assunção, C.B.; Graciele-Melo, C.; Costa, L.E.; Portela, A.S.; Ferraz Coelho, E.A.; Maria De Figueiredo, S.; Caligiorne, R.B. Canine Visceral Leishmaniasis: Detection of Leishmania spp. Genome in Peripheral Blood of Seropositive Dogs by Real-Time Polymerase Chain Reaction (Rt-PCR). Microb. Pathog. 2019, 126, 263–268. [Google Scholar] [CrossRef] [PubMed]
- de Camargo-Neves, V.L.F.; Calemes, E.B.; Rodas, L.A.C.; Galvis-Ovallos, F.; Silva, L.J.D. Control of Canine Visceral Leishmaniasis: A Success Case Based on Deltamethrin 4% Collars. Epidemiologia 2021, 2, 502–518. [Google Scholar] [CrossRef]
- Oliveira, S.S.; Hiramoto, R.M.; Rangel, O.; Henriques, L.F.; Viviani, A., Jr.; Taniguchi, H.H.; Barbosa, J.E.R.; Casanova, C.; Sampaio, S.M.P.; Spinola, R.; et al. Classificação Epidemiológica Dos Municípios Do Estado de São Paulo Segundo o Programa de Vigilância e Controle Da Leishmaniose Visceral, 2018. Bepa 2019, 16, 29–46. Available online: https://pdfs.semanticscholar.org/ed1b/7bd2b3c4287b852940d39cc4e950b444d79f.pdf (accessed on 30 May 2025).
- Ministry of Health. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/leishmaniose-visceral/estratificacao-de-risco/estratificacao-lv-2019-a-2021.pdf/view (accessed on 15 March 2025).
- CVE Centro de Vigilância Epidemiológica “Prof. Alexandre Vranjac”. Available online: https://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/dados/leish/lv1725_lpi.pdf (accessed on 30 May 2025).
- Alcover, M.M.; Gramiccia, M.; Di Muccio, T.; Ballart, C.; Castillejo, S.; Picado, A.; Portús, M.; Gállego, M. Application of Molecular Techniques in the Study of Natural Infection of Leishmania infantum Vectors and Utility of Sandfly Blood Meal Digestion for Epidemiological Surveys of Leishmaniasis. Parasitol. Res. 2012, 111, 515–523. [Google Scholar] [CrossRef]
- Sudia, W.D.; Chamberlain, R.W. Battery-Operated Light Trap, an Improved Model. By W. D. Sudia and R. W. Chamberlain, 1962. J. Am. Mosq. Control Assoc. 1988, 4, 536–538. [Google Scholar]
- Nasci, R.S. A Lightweight Battery-Powered Aspirator for Collecting Resting Mosquitoes in the Field. Mosq. News 1981, 41, 808–811. [Google Scholar]
- Shannon, R.C. Methods for collecting feeding mosquitoes in jungle yellow fever studies. Am. J. Trop. Med. 1939, 19, 131–140. [Google Scholar]
- Galati, E.A.B. Phlebotominae (Diptera. Psychodidae): Classificação, Morfologia, Terminologia e Identificação de Adultos. Apostila da Disciplina HEP 5752-Bioecologia e Identificação de Phlebotominae; Faculdade de Saúde Pública. Universidade de São Paulo: São Paulo, Brazil, 2014; pp. 1–120. [Google Scholar]
- Savani, E.S.M.M.; De Oliveira Camargo, M.C.G.; De Carvalho, M.R.; Zampieri, R.A.; Dos Santos, M.G.; D’Áuria, S.R.N.; Shaw, J.J.; Floeter-Winter, L.M. The First Record in the Americas of an Autochthonous Case of Leishmania (Leishmania) infantum chagasi in a Domestic Cat (Felix catus) from Cotia County, São Paulo State, Brazil. Vet. Parasitol. 2004, 120, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Uliana, S.R.B.; Affonso, M.H.T.; Camargo, E.P.; Floeter-Winter, L.M. Leishmania: Genus Identification Based on a Specific Sequence of the 18S Ribosomal RNA Sequence. Exp. Parasitol. 1991, 72, 157–163. [Google Scholar] [CrossRef]
- Parodi, B.; Aresu, O.; Bini, D.; Lorenzini, R.; Schena, F.; Visconti, P.; Cesaro, M.; Ferrera, D.; Andreotti, V.; Ruzzon, T. Species identification and confirmation of human and animal cell lines: A PCR-based method. Biotechniques 2002, 32, 432–440. [Google Scholar] [CrossRef]
- Chang, M.C.; Teng, H.J.; Chen, C.F.; Chen, Y.C.; Jeng, C.R. The Resting Sites and Blood-Meal Sources of Anopheles minimus in Taiwan. Malar. J. 2008, 7, 105. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of Mitochondrial DNA Evolution in Animals: Amplification and Sequencing with Conserved Primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Ratzlaff, F.R.; Fernandes, F.D.; Osmari, V.; Silva, D.; de Paula Vasconcellos, J.S.; Braunig, P.; Vogel, F.S.F.; de Ávila Botton, S.; Dos Santos, H.F.; Cargnelutti, J.F.; et al. Prevalence and molecular detection of Leishmania spp. in bats from Rio Grande do Sul state, Brazil. Parasitol. Res. 2022, 121, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R.; Killick-Kendrick, M.; Focheux, C.; Dereure, J.; Puech, M.P.; Cadiergues, M.C. Protection of dogs from bites of phlebotomine sandflies by deltamethrin collars for control of canine leishmaniasis. Med. Vet. Entomol. 1997, 11, 105–111. [Google Scholar] [CrossRef]
- David, J.R.; Stamm, L.M.; Bezerra, H.S.; Souza, R.N.; Killick-Kendrick, R.; Lima, J.W.O. Deltamethrin-Impregnated Dog Collars Have a Potent Anti-Feeding and Insecticidal Effect on Lutzomyia longipalpis and Lutzomyia migonei. Mem. Inst. Oswaldo Cruz 2001, 96, 839–847. [Google Scholar] [CrossRef]
- Ferroglio, E.; Poggi, M.; Trisciuoglio, A. Evaluation of 65% permethrin spot-on and deltamethrin-impregnated collars for canine Leishmania infantum infection prevention. Zoonoses Public Health 2008, 55, 145–148. [Google Scholar] [CrossRef]
- Galvis-Ovallos, F.; Casanova, C.; Sevá, A.D.P.; Galati, E.A.B. Ecological parameters of the (S)-9-methylgermacrene-B population of the Lutzomyia longipalpis complex in a visceral leishmaniasis area in São Paulo state, Brazil. Parasit. Vectors 2017, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.D.; Galvis-Ovallos, F.; Casanova, C.; Silva, V.G.D.; Leonel, J.A.F.; Oliveira, T.M.F.S.; Galati, E.A.B. Natural infection of Lutzomyia longipalpis (Cembrene-1 population) with Leishmania infantum in a new visceral leishmaniasis focus in the eastern region of São Paulo State, Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e05862020. [Google Scholar] [CrossRef] [PubMed]
- Leonel, J.A.F.; Vioti, G.; Alves, M.L.; Spada, J.C.P.; Yamaguchi, A.K.; Pereira, N.W.B.; da Silva, D.T.; Benassi, J.C.; Galvis-Ovallos, F.; Galati, E.A.B.; et al. Species, Natural Leishmania spp. Detection and Blood Meal Sources of Phlebotomine Sandflies (Diptera: Psychodidae: Phlebotominae) in Peridomiciles from a Leishmaniases Endemic Area of Brazil. Transbound. Emerg. Dis. 2024, 2024, 9932530. [Google Scholar] [CrossRef]
- Savani, E.S.M.M.; Nunes, V.L.B.; Galati, E.A.B.; Castilho, T.M.; Zampieri, R.A.; Floeter-Winter, L.M. The finding of Lutzomyia almerioi and Lutzomyia longipalpis naturally infected by Leishmania spp. in a cutaneous and canine visceral leishmaniases focus in Serra da Bodoquena, Brazil. Vet. Parasitol. 2009, 160, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Missawa, N.A.; Michalsky, E.M.; Fortes-Dias, C.L.; Santos Dias, E. Lutzomyia longipalpis naturally infected by Leishmania (L.) chagasi in Várzea Grande, Mato Grosso State, Brazil, an area of intense transmission of visceral leishmaniasis. Cad. Saude Publica 2010, 26, 2414–2419. [Google Scholar] [CrossRef]
- Soares, M.R.; Carvalho, C.C.; Silva, L.A.; Lima, M.S.; Barral, A.M.; Rebêlo, J.M.; Pereira, S.R. Análise molecular da infecção natural de Lutzomyia longipalpis em área endêmica de leishmaniose visceral no Brasil [Molecular analysis of natural infection of Lutzomyia longipalpis in an endemic area for visceral leishmaniasis in Brazil]. Cad. Saude Publica 2010, 26, 2409–2413, In Portuguese. [Google Scholar] [CrossRef]
- Michalsky, E.M.; Guedes, K.S.; Lara e Silva, F.O.; França-Silva, J.C.; Dias, C.L.; Barata, R.A.; Dias, E.S. Infecção natural de Lutzomyia (Lutzomyia) longipalpis (Diptera: Psychodidae) por Leishmania infantum chagasi em flebotomíneos capturados no município de Janaúba, Estado de Minas Gerais, Brasil [Natural infection with Leishmania infantum chagasi in Lutzomyia (Lutzomyia) longipalpis (Diptera: Psychodidae) sandflies captured in the municipality of Janaúba, State of Minas Gerais, Brazil]. Rev. Soc. Bras. Med. Trop. 2011, 44, 58–62, In Portuguese. [Google Scholar] [CrossRef]
- Kazimoto, T.A.; Amora, S.S.A.; Figueiredo, F.B.; Magalhães, J.M.E.; Freitas, Y.B.N.; Sousa, M.L.R.; Melo, A.E.C.D.S.; Campos, M.P.; Alves, N.D.; Werneck, G.L. Impact of 4% Deltamethrin-Impregnated Dog Collars on the Prevalence and Incidence of Canine Visceral Leishmaniasis. Vector Borne Zoonotic Dis. 2018, 18, 356–363. [Google Scholar] [CrossRef]
- Clements, A.N. The Biology of Mosquitoes, Volume 2: Sensory Reception and Behaviour; CABI Publishing: Wallingford, UK, 1999; pp. 480–503. [Google Scholar]
- Yuval, B. The other habit: Sugar feeding by mosquitoes. Bull. Soc. Vector Ecol. 1992, 17, 150–156. [Google Scholar]
- Coura-Vital, W.; Leal, G.G.D.A.; Marques, L.A.; Pinheiro, A.D.C.; Carneiro, M.; Reis, A.B. Effectiveness of deltamethrin-impregnated dog collars on the incidence of canine infection by Leishmania infantum: A large scale intervention study in an endemic area in Brazil. PLoS ONE 2018, 13, e0208613. [Google Scholar] [CrossRef]
- Yimam, Y.; Mohebali, M. Effectiveness of Insecticide-Impregnated Dog Collars in Reducing Incidence Rate of Canine Visceral Leishmaniasis: A Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0238601. [Google Scholar] [CrossRef]
- Sales, K.G.D.S.; Costa, P.L.; De Morais, R.C.S.; Otranto, D.; Brandão-Filho, S.P.; Cavalcanti, M.D.P.; Dantas-Torres, F. Identification of phlebotomine sand fly blood meals by real-time PCR. Parasit. Vectors 2015, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Dutra-Rêgo, F.; Binder, C.; Capucci, D.C.; Vaz, T.P.; Andrade Filho, J.D.; Fontes, G.; Gontijo, C.M.F. Diversity, Leishmania detection, and blood meal sources of sand flies from Iguatama, Minas Gerais, Brazil. PLoS ONE 2024, 19, e0302567. [Google Scholar] [CrossRef]
- Sant’Anna, M.R.; Jones, N.G.; Hindley, J.A.; Mendes-Sousa, A.F.; Dillon, R.J.; Cavalcante, R.R.; Alexander, B.; Bates, P.A. Blood meal identification and parasite detection in laboratory-fed and field-captured Lutzomyia longipalpis by PCR using FTA databasing paper. Acta Trop. 2008, 107, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tanure, A.; Peixoto, J.C.; Afonso, M.M.D.S.; Duarte, R.; Pinheiro, A.D.C.; Coelho, S.V.B.; Barata, R.A. Identification of sandflies (Diptera: Psychodidae: Phlebotominae) blood meals in an endemic leishmaniasis area in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef]
- Alexander, B.; de Carvalho, R.L.; McCallum, H.; Pereira, M.H. Role of the domestic chicken (Gallus gallus) in the epidemiology of urban visceral leishmaniasis in Brazil. Emerg Infect Dis. 2002, 8, 1480–1485. [Google Scholar] [CrossRef]

| Capture Method | Number of Sand Flies * | Frequency (%) | 95% CI ** |
|---|---|---|---|
| Manual aspiration | 378 | 91.3 | 88.06–93.76 |
| CDC electric traps | 32 | 7.73 | 5.43–10.85 |
| Shannon trap | 4 | 0.97 | 0.31–2.63 |
| Total | 414 |
| Blood Meal | Number of Sand Flies | (%) | Sources | Number of Sand Flies | (%) |
|---|---|---|---|---|---|
| Single | Human | 48/113 | 42.5 | ||
| 113 | 54.8 | Chicken | 50/113 | 44.2 | |
| Dog | 8/113 | 7.1 | |||
| Duck | 7/113 | 6.2 | |||
| Double | 76 | Human/chicken | 62/76 | 81.6 | |
| 36.9 | Chicken/dog | 3/76 | 3.9 | ||
| Human/dog | 11/76 | 14.5 | |||
| Triple | 17 | 8.3 | Human/chicken/dog | ||
| Total | 206 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, G.F.F.; Luz-Requena, K.A.M.; Mathias, B.S.; Suto, T.M.T.; Suto, R.; Rocha, L.T.R.; Rangel, O.; Bresciani, K.D.S.; Sampaio, S.M.P.; Rodas, L.A.C.; et al. Investigation of Leishmania infantum Infection and Feeding Preferences of Lutzomyia longipalpis During Deltamethrin (4%) Dog Collar Intervention. Pathogens 2025, 14, 671. https://doi.org/10.3390/pathogens14070671
Rodrigues GFF, Luz-Requena KAM, Mathias BS, Suto TMT, Suto R, Rocha LTR, Rangel O, Bresciani KDS, Sampaio SMP, Rodas LAC, et al. Investigation of Leishmania infantum Infection and Feeding Preferences of Lutzomyia longipalpis During Deltamethrin (4%) Dog Collar Intervention. Pathogens. 2025; 14(7):671. https://doi.org/10.3390/pathogens14070671
Chicago/Turabian StyleRodrigues, Gabriel F. F., Keuryn A. M. Luz-Requena, Bruno S. Mathias, Tania M. T. Suto, Rosemari Suto, Luciana T. R. Rocha, Osias Rangel, Katia D. S. Bresciani, Susy M. P. Sampaio, Lilian A. C. Rodas, and et al. 2025. "Investigation of Leishmania infantum Infection and Feeding Preferences of Lutzomyia longipalpis During Deltamethrin (4%) Dog Collar Intervention" Pathogens 14, no. 7: 671. https://doi.org/10.3390/pathogens14070671
APA StyleRodrigues, G. F. F., Luz-Requena, K. A. M., Mathias, B. S., Suto, T. M. T., Suto, R., Rocha, L. T. R., Rangel, O., Bresciani, K. D. S., Sampaio, S. M. P., Rodas, L. A. C., & Kirchgatter, K. (2025). Investigation of Leishmania infantum Infection and Feeding Preferences of Lutzomyia longipalpis During Deltamethrin (4%) Dog Collar Intervention. Pathogens, 14(7), 671. https://doi.org/10.3390/pathogens14070671








