Molecular Features of HPV-Independent Cervical Cancers
Abstract
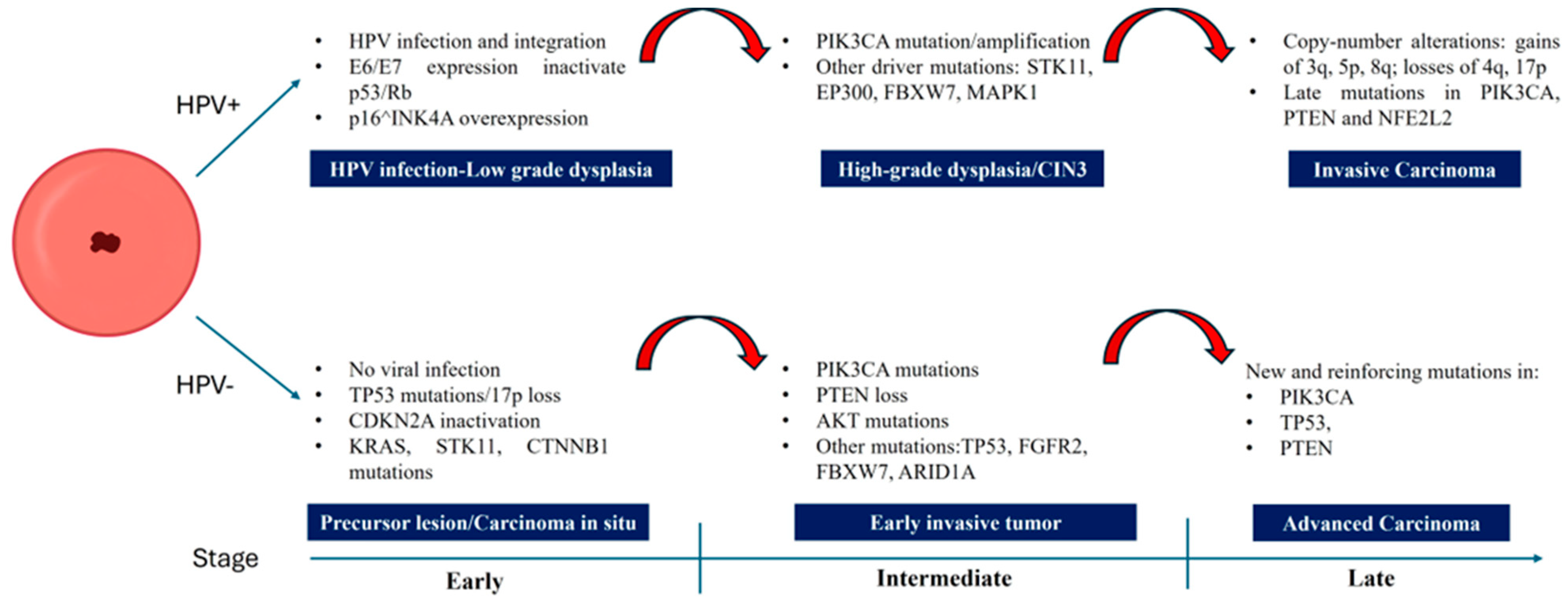
1. Introduction
2. Critical Issues in Identifying HPV-Independent Cervical Cancers: False Negatives
3. Main Histological Histotypes
4. Significant Mutations in HPV-Negative Cervical Cancers
5. Molecular Signaling Pathways
6. Molecular Differences Compared to HPV-Positive Tumors
7. Distinctive Immunohistochemical Markers
8. Diagnostic Implications and Targeted Therapies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| APOBEC | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide |
| ARID1A | AT-rich interactive domain-containing protein 1A |
| ARID1B | AT-rich interactive domain-containing protein 1B |
| BCOR | BCL6 corepressor |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| BRCA2 | Breast cancer gene 2 |
| CAIX | Carbonic anhydrase IX |
| CCC | Clear cell adenocarcinoma |
| CDKN2A | Cyclin dependent kinase inhibitor 2A |
| CEA | Carcinoembryonic antigen |
| CK7 | Cytokeratin 7 |
| CTNNB1 | Catenin Beta 1 |
| DES | Diethylstilbestrol |
| DNA | Deoxyribonucleic acid |
| DOAJ | Directory of open access journals |
| EAC | Endometroid adenocarcinoma |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| ERBB2 | Erb-B2 receptor tyrosine kinase 2 |
| ERBB3 | ERb-B2 receptor tyrosine kinase 3 |
| ERK | Extracellular signal-related kinase |
| EZH2 | Enhancer of Zeste Homolog 2 |
| FGFR | Fibroblast growth factor receptor |
| FGFR2 | Fibroblast growth factor receptor 2 |
| FIGO | International Federation of Gynecology and Obstetrics |
| GAS | Gastric (gastric-type) adenocarcinoma |
| HER2 | Human epidermal growth factor receptor 2 |
| HNF1β | Hepatocyte nuclear factor 1β |
| HPV | Human papillomavirus |
| HPV+ | Human papillomavirus positive |
| HPVA | HPV-Associated |
| IECC | International endocervical criteria and classification |
| IHC | immunohistochemistry |
| KRAS | Kirsten rat sarcoma |
| LD | Linear dichroism |
| LKB1 | Liver kinase B1 |
| lncRNA | Long non coding RNA |
| MAPK | Mitogen-activated protein kinase |
| MDA | Minimally differentiated mucinous carcinoma |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MEK | MAPK/ERK kinase |
| MMR | Mismatch repair |
| mTOR | Mammalian target of rapamycin |
| MUC6 | MUCIN 6 |
| NEF | Neighboring Enhancer of FOXA2 (lncRNA NEF) |
| NHPVA | Non-HPV-Associated |
| NRAS | Neuroblastoma RAS viral oncogene homolog |
| NTRK | Neurotrophic receptor tyrosine kinase |
| NTRK3 | Neurotrophic receptor tyrosine kinase 3 |
| P16 | P16INKA4a |
| Pap test | Papanicolau test |
| PAX8 | Paired box gene 8 |
| PD-L1 | Programmed death ligand 1 |
| PI3K | Phosphatidylinositol-3-kinase |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| POLE | DNA Polymerase epsilon |
| PR | Progesterone receptor |
| pRb | Retinoblastoma protein |
| PTEN | Phosphatase and tensin homolog |
| RAF | Rapidly accelerated fibrosarcoma kinase |
| RAS | Rat sarcoma |
| RB | Retinoblastoma |
| SMARCA4 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4 |
| STK11 | Serine / threonine kinase 11 |
| SWI/SNF | Switch / sucrose non-fermenting |
| TGF-β | Transforming growth factor beta |
| TP53 | Tumor protein p53 |
| TRK inhibitors | Tropomyosin receptor kinase inhibitors |
| WHO | World Health Organization |
| WNT | Wingless-related integration site |
References
- Fernandes, A.; Viveros-Carreño, D.; Hoegl, J.; Ávila, M.; Pareja, R. Human Papillomavirus-Independent Cervical Cancer. Int. J. Gynecol. Cancer 2022, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.F.; Clifford, G.M. Human Papillomavirus Type Distribution in 30,848 Invasive Cervical Cancers Worldwide: Variation by Geographical Region, Histological Type and Year of Publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Petry, K.U.; Liebrich, C.; Luyten, A.; Zander, M.; Iftner, T. Surgical Staging Identified False HPV-Negative Cases in a Large Series of Invasive Cervical Cancers. Papillomavirus Res. 2017, 4, 85–89. [Google Scholar] [CrossRef]
- Burk, R.; Chen, Z.; Saller, C. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Blatt, A.; Kennedy, R.; Luff, R.; Austin, R.; Rabin, D. Comparison of Cervical Cancer Screening Results among 256,648 Women in Multiple Clinical Practices. Cancer Cytopathol. 2016, 124, 362–363. [Google Scholar] [CrossRef]
- Lu, S.; Shen, D.; Zhao, Y.; Kang, N.; Wang, X. Primary Endocervical Gastric-Type Adenocarcinoma: A Clinicopathologic and Immunohistochemical Analysis of 23 Cases. Diagn. Pathol. 2019, 14, 72. [Google Scholar] [CrossRef]
- Giannella, L.; Delli Carpini, G.; Di Giuseppe, J.; Grelloni, C.; Bogani, G.; Dri, M.; Sopracordevole, F.; Clemente, N.; Giorda, G.; De Vincenzo, R.; et al. Long-Term Follow-Up Outcomes in Women with In Situ/Microinvasive Adenocarcinoma of the Uterine Cervix Undergoing Conservative Treatment—Cervical Adenocarcinoma Study Group Italian Society of Colposcopy and Cervico-Vaginal Pathology. Cancers 2024, 16, 1241. [Google Scholar] [CrossRef] [PubMed]
- Giannella, L.; Delli Carpini, G.; Di Giuseppe, J.; Bogani, G.; Sopracordevole, F.; Clemente, N.; Giorda, G.; De Vincenzo, R.P.; Evangelista, M.T.; Gardella, B.; et al. In Situ/Microinvasive Adenocarcinoma of the Uterine Cervix and HPV-Type Impact: Pathologic Features, Treatment Options, and Follow-Up Outcomes—Cervical Adenocarcinoma Study Group (CAS-Group). Cancers 2023, 15, 2876. [Google Scholar] [CrossRef]
- Giannella, L.; Di Giuseppe, J.; Delli Carpini, G.; Grelloni, C.; Fichera, M.; Sartini, G.; Caimmi, S.; Natalini, L.; Ciavattini, A. HPV-Negative Adenocarcinomas of the Uterine Cervix: From Molecular Characterization to Clinical Implications. Int. J. Mol. Sci. 2022, 23, 15022. [Google Scholar] [CrossRef]
- Selenica, P.; Alemar, B.; Matrai, C.; Talia, K.L.; Veras, E.; Hussein, Y.; Oliva, E.; Beets-Tan, R.G.H.; Mikami, Y.; McCluggage, W.G.; et al. Massively Parallel Sequencing Analysis of 68 Gastric-Type Cervical Adenocarcinomas Reveals Mutations in Cell Cycle-Related Genes and Potentially Targetable Mutations. Mod. Pathol. 2021, 34, 1213–1225. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. WHO Classification of Tumours Editorial Board Female Genital Tumours, 5th ed.; WHO: Lyon, France, 2020; Volume 4. [Google Scholar]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Pike, M.C.; et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am. J. Surg. Pathol. 2018, 42, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Shao, N. Research Progress on Human Papillomavirus-Negative Cervical Cancer: A Review. Medicine 2024, 103, e39957. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; del Pino, M.; et al. HPV-Negative Tumors of the Uterine Cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef]
- Li, P.; Tan, Y.; Zhu, L.-X.; Zhou, L.-N.; Zeng, P.; Liu, Q.; Chen, M.-B.; Tian, Y. Prognostic Value of HPV DNA Status in Cervical Cancer before Treatment: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 66352–66359. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Catão Zampronha, R.; Freitas-Junior, R.; Murta, E.F.C.; Michelin, M.A.; Barbaresco, A.A.; Adad, S.J.; de Oliveira, A.M.; Rassi, A.B.; Oton, G.J.B. Human Papillomavirus Types 16 and 18 and the Prognosis of Patients with Stage I Cervical Cancer. Clinics 2013, 68, 809–814. [Google Scholar] [CrossRef]
- Tommasino, M.; Accardi, R.; Caldeira, S.; Dong, W.; Malanchi, I.; Smet, A.; Zehbe, I. The Role of TP53 in Cervical Carcinogenesis. Hum. Mutat. 2003, 21, 307–312. [Google Scholar] [CrossRef]
- Lu, S.; Shi, J.; Zhang, X.; Kong, F.; Liu, L.; Dong, X.; Wang, K.; Shen, D. Comprehensive Genomic Profiling and Prognostic Analysis of Cervical Gastric-Type Mucinous Adenocarcinoma. Virchows Arch. 2021, 479, 893–903. [Google Scholar] [CrossRef]
- Yoshida, H.; Shiraishi, K.; Kato, T. Molecular Pathology of Human Papilloma Virus-Negative Cervical Cancers. Cancers 2021, 13, 6351. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Sheng, Y.; Wu, G.; Zhao, Y. Human Papillomavirus-Negative Cervical Cancer: A Comprehensive Review. Front. Oncol. 2021, 10, 606335. [Google Scholar] [CrossRef]
- Pirog, E.C.; Lloveras, B.; Molijn, A.; Tous, S.; Guimerà, N.; Alejo, M.; Clavero, O.; Klaustermeier, J.; Jenkins, D.; Quint, W.G.; et al. HPV Prevalence and Genotypes in Different Histological Subtypes of Cervical Adenocarcinoma, a Worldwide Analysis of 760 Cases. Mod. Pathol. 2014, 27, 1559–1567. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Ronco, G.; Dillner, J.; Elfström, K.M.; Snijders, P.J.F.; Arbyn, M.; Berkhof, J.; Carozzi, F.; Del Mistro, A.; De Sanjosè, S.; et al. Why Follow-back Studies Should Be Interpreted Cautiously: The Case of an HPV-negative Cervical Lesion. Cancer Cytopathol. 2016, 124, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Katki, H.A.; Kinney, W.K.; Fetterman, B.; Lorey, T.; Poitras, N.E.; Cheung, L.; Demuth, F.; Schiffman, M.; Wacholder, S.; Castle, P.E. Cervical Cancer Risk for Women Undergoing Concurrent Testing for Human Papillomavirus and Cervical Cytology: A Population-Based Study in Routine Clinical Practice. Lancet Oncol. 2011, 12, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Houghton, O.; Jamison, J.; Wilson, R.; Carson, J.; McCluggage, W.G. P16 Immunoreactivity in Unusual Types of Cervical Adenocarcinoma Does Not Reflect Human Papillomavirus Infection. Histopathology 2010, 57, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Luo, X.; Zhang, N. LncRNA NEF Inhibits Migration and Invasion of HPV-Negative Cervical Squamous Cell Carcinoma by Inhibiting TGF-β Pathway. Biosci. Rep. 2019, 39, BSR20180878. [Google Scholar] [CrossRef]
- Mirkovic, J.; Sholl, L.M.; Garcia, E.; Lindeman, N.; MacConaill, L.; Hirsch, M.; Dal Cin, P.; Gorman, M.; Barletta, J.A.; Nucci, M.R.; et al. Targeted Genomic Profiling Reveals Recurrent KRAS Mutations and Gain of Chromosome 1q in Mesonephric Carcinomas of the Female Genital Tract. Mod. Pathol. 2015, 28, 1504–1514. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Sheng, B.; Pan, S.; Wang, Z.; Zhu, X. The Functions of LncRNAs in the HPV-Negative Cervical Cancer Compared with HPV-Positive Cervical Cancer. Apoptosis 2022, 27, 685–696. [Google Scholar] [CrossRef]
- Bonin, L.; Devouassoux-Shisheboran, M.; Golfier, F. Clinicopathological Characteristics of Patients with Mucinous Adenocarcinoma of the Uterine Cervix: A Retrospective Study of 21 Cases. J. Gynecol. Obs. Hum. Reprod. 2019, 48, 319–327. [Google Scholar] [CrossRef]
- Garg, S.; Nagaria, T.S.; Clarke, B.; Freedman, O.; Khan, Z.; Schwock, J.; Bernardini, M.Q.; Oza, A.M.; Han, K.; Smith, A.C.; et al. Molecular Characterization of Gastric-Type Endocervical Adenocarcinoma Using next-Generation Sequencing. Mod. Pathol. 2019, 32, 1823–1833. [Google Scholar] [CrossRef]
- Park, K.J. Cervical Adenocarcinoma: Integration of HPV Status, Pattern of Invasion, Morphology and Molecular Markers into Classification. Histopathology 2020, 76, 112–127. [Google Scholar] [CrossRef]
- Kenny, S.L.; McBride, H.A.; Jamison, J.; McCluggage, W.G. Mesonephric Adenocarcinomas of the Uterine Cervix and Corpus. Am. J. Surg. Pathol. 2012, 36, 799–807. [Google Scholar] [CrossRef]
- Silver, S.A.; Devouassoux-Shisheboran, M.; Mezzetti, T.P.; Tavassoli, F.A. Mesonephric Adenocarcinomas of the Uterine Cervix. Am. J. Surg. Pathol. 2001, 25, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Devarashetty, S.; Chennapragada, S.S.; Mansour, R. Not Your Typical Adenocarcinoma: A Case of Mesonephric Adenocarcinoma of the Cervix With Fibroblast Growth Factor Receptor 2 (FGFR2) Mutation. Cureus 2022, 14, e25098. [Google Scholar] [CrossRef]
- Dierickx, A.; Göker, M.; Braems, G.; Tummers, P.; Van den Broecke, R. Mesonephric Adenocarcinoma of the Cervix: Case Report and Literature Review. Gynecol. Oncol. Rep. 2016, 17, 7–11. [Google Scholar] [CrossRef]
- Park, K.J.; Kiyokawa, T.; Soslow, R.A.; Lamb, C.A.; Oliva, E.; Zivanovic, O.; Juretzka, M.M.; Pirog, E.C. Unusual Endocervical Adenocarcinomas. Am. J. Surg. Pathol. 2011, 35, 633–646. [Google Scholar] [CrossRef]
- Pirog, E.C. Cervical Adenocarcinoma: Diagnosis of Human Papillomavirus–Positive and Human Papillomavirus–Negative Tumors. Arch. Pathol. Lab. Med. 2017, 141, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jin, Y.; Li, Y.; Huang, H.-F.; Wu, M.; Shen, K.; Pan, L.-Y. Clear Cell Carcinoma of the Uterine Cervix: Clinical Characteristics and Feasibility of Fertility-Preserving Treatment. Onco Targets Ther. 2014, 7, 111. [Google Scholar] [CrossRef]
- Tantitamit, T.; Hamontri, S.; Rangsiratanakul, L. Clear Cell Adenocarcinoma of the Cervix in Second Generation Young Women Who Are without Maternal Exposure to Diethylstilbestrol: A Case Report. Gynecol. Oncol. Rep. 2017, 20, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Cue, L.; Martingano, D.J.; Mahdy, H. Clear Cell Carcinoma of the Cervix. In StatPearls. Treasure Island; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hanselaar, A.; van Loosbroek, M.; Schuurbiers, O.; Helmerhorst, T.; Bulten, J.; Bernheim, J. Clear Cell Adenocarcinoma of the Vagina and Cervix. Cancer 1997, 79, 2229–2236. [Google Scholar] [CrossRef]
- Ahrens, W.A.; Barrón-Rodriguez, L.P.; McKee, M.; Rivkees, S.; Reyes-Múgica, M. Clear Cell Adenocarcinoma of the Cervix in a Child without in Utero Exposure to Diethylstilbestrol: A Case Report and Review of the Literature. Pediatr. Dev. Pathol. 2005, 8, 690–695. [Google Scholar] [CrossRef]
- Liebrich, C.; Brummer, O.; Von Wasielewski, R.; Wegener, G.; Meijer, C.; Iftner, T.; Petry, K.U. Primary Cervical Cancer Truly Negative for High-Risk Human Papillomavirus Is a Rare but Distinct Entity That Can Affect Virgins and Young Adolescents. Eur. J. Gynaecol. Oncol. 2009, 30, 45–48. [Google Scholar]
- Lee, E.K.; Lindeman, N.I.; Matulonis, U.A.; Konstantinopoulos, P.A. POLE-Mutated Clear Cell Cervical Cancer Associated with in-Utero Diethylstilbestrol Exposure. Gynecol. Oncol. Rep. 2019, 28, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Murakami, N.; Takahashi, K.; Kuno, I.; Takayanagi, D.; Asami, Y.; Matsuda, M.; Shimada, Y.; Yamano, S.; Sunami, K.; et al. Genomic Alterations in STK11 Can Predict Clinical Outcomes in Cervical Cancer Patients. Gynecol. Oncol. 2020, 156, 203–210. [Google Scholar] [CrossRef]
- Hodgson, A.; Howitt, B.E.; Park, K.J.; Lindeman, N.; Nucci, M.R.; Parra-Herran, C. Genomic Characterization of HPV-Related and Gastric-Type Endocervical Adenocarcinoma: Correlation With Subtype and Clinical Behavior. Int. J. Gynecol. Pathol. 2020, 39, 578–586. [Google Scholar] [CrossRef]
- Ueno, S.; Sudo, T.; Oka, N.; Wakahashi, S.; Yamaguchi, S.; Fujiwara, K.; Mikami, Y.; Nishimura, R. Absence of Human Papillomavirus Infection and Activation of PI3K-AKT Pathway in Cervical Clear Cell Carcinoma. Int. J. Gynecol. Cancer 2013, 23, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shao, Y.; Lu, W.; Lu, B. An Analysis of HER2 Amplification in Cervical Adenocarcinoma: Correlation with Clinical Outcomes and the International Endocervical Adenocarcinoma Criteria and Classification. J. Pathol. Clin. Res. 2021, 7, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Verlaat, W.; Snijders, P.J.; van Moorsel, M.I.; Bleeker, M.; Rozendaal, L.; Sie, D.; Ylstra, B.; Meijer, C.J.; Steenbergen, R.D.; Heideman, D.A. Somatic Mutation in PIK3CA Is a Late Event in Cervical Carcinogenesis. J. Pathol. Clin. Res. 2015, 1, 207–211. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Ling, M.T.; Zhao, L.; Zhao, K.-N. The Role of the PI3K/Akt/MTOR Signalling Pathway in Human Cancers Induced by Infection with Human Papillomaviruses. Mol. Cancer 2015, 14, 87. [Google Scholar] [CrossRef]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of Genomic Alterations in Cervical Carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Spaans, V.M.; Trietsch, M.D.; Peters, A.A.W.; Osse, M.; ter Haar, N.; Fleuren, G.J.; Jordanova, E.S. Precise Classification of Cervical Carcinomas Combined with Somatic Mutation Profiling Contributes to Predicting Disease Outcome. PLoS ONE 2015, 10, e0133670. [Google Scholar] [CrossRef]
- Chung, T.K.H.; Van Hummelen, P.; Chan, P.K.S.; Cheung, T.H.; Yim, S.F.; Yu, M.Y.; Ducar, M.D.; Thorner, A.R.; MacConaill, L.E.; Doran, G.; et al. Genomic Aberrations in Cervical Adenocarcinomas in Hong Kong Chinese Women. Int. J. Cancer 2015, 137, 776–783. [Google Scholar] [CrossRef]
- Wright, A.A.; Howitt, B.E.; Myers, A.P.; Dahlberg, S.E.; Palescandolo, E.; Van Hummelen, P.; MacConaill, L.E.; Shoni, M.; Wagle, N.; Jones, R.T.; et al. Oncogenic Mutations in Cervical Cancer. Cancer 2013, 119, 3776–3783. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Q. Targeting KRAS in Gynecological Malignancies. FASEB J. 2024, 38, e70089. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the P53 Network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Spencer, R.J. Recurrent Cervical Cancer Treated Successfully with Single-Agent PARP-Inhibitor, Olaparib. Case Rep. Obs. Gynecol. 2022, 2022, 6579715. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.R.; Raza, S.T.; Sharma, R.; Pratap, P.; Eba, A.; Singh, M. Association of DNA Repair Genes XRCC1 and APE-1 with the Risk of Cervical Cancer in North Indian Population. Asian Pac. J. Cancer Prev. 2020, 21, 2061–2065. [Google Scholar] [CrossRef]
- Mann, M.; Singh, V.P.; Kumar, L. Cervical Cancer: A Tale from HPV Infection to PARP Inhibitors. Genes Dis. 2023, 10, 1445–1456. [Google Scholar] [CrossRef]
- Jenkins, D.; Molijn, A.; Kazem, S.; Pirog, E.C.; Alemany, L.; de Sanjosé, S.; Dinjens, W.; Quint, W. Molecular and Pathological Basis of HPV-negative Cervical Adenocarcinoma Seen in a Global Study. Int. J. Cancer 2020, 147, 2526–2536. [Google Scholar] [CrossRef]
- Carleton, C.; Hoang, L.; Sah, S.; Kiyokawa, T.; Karamurzin, Y.S.; Talia, K.L.; Park, K.J.; McCluggage, W.G. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-Type Adenocarcinomas. Am. J. Surg. Pathol. 2016, 40, 636–644. [Google Scholar] [CrossRef]
- Turashvili, G.; Morency, E.G.; Kracun, M.; DeLair, D.F.; Chiang, S.; Soslow, R.A.; Park, K.J.; Murali, R. Morphologic Features of Gastric-Type Cervical Adenocarcinoma in Small Surgical and Cytology Specimens. Int. J. Gynecol. Pathol. 2019, 38, 263–275. [Google Scholar] [CrossRef]
- Wada, T.; Ohishi, Y.; Kaku, T.; Aman, M.; Imamura, H.; Yasutake, N.; Sonoda, K.; Kato, K.; Oda, Y. Endocervical Adenocarcinoma with Morphologic Features of Both Usual and Gastric Types. Am. J. Surg. Pathol. 2017, 41, 696–705. [Google Scholar] [CrossRef]
- Stewart, C.J.R.; Frost, F.; Leake, R.; Raj Mohan, G.; Tan, J. Foamy Gland Changes in Gastric-Type Endocervical Neoplasia. Pathology 2015, 47, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xia, X.; Su, W.; Yan, H.; Ma, Y.; Xu, L.; Luo, H.; Liu, W.; Yin, D.; Zhang, W.-H.; et al. Association of Recurrent APOBEC3B Alterations with the Prognosis of Gastric-Type Cervical Adenocarcinoma. Gynecol. Oncol. 2022, 165, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Stolnicu, S.; Hoang, L.; Chiu, D.; Hanko-Bauer, O.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Oliva, E.; et al. Clinical Outcomes of HPV-Associated and Unassociated Endocervical Adenocarcinomas Categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am. J. Surg. Pathol. 2019, 43, 466–474. [Google Scholar] [CrossRef] [PubMed]
- de Cremoux, P.; de la Rochefordière, A.; Savignoni, A.; Kirova, Y.; Alran, S.; Fourchotte, V.; Plancher, C.; Thioux, M.; Salmon, R.J.; Cottu, P.; et al. Different Outcome of Invasive Cervical Cancer Associated with High-risk versus Intermediate-risk HPV Genotype. Int. J. Cancer 2009, 124, 778–782. [Google Scholar] [CrossRef]
- Fulmer, C.G.; Hoda, R.S.; Pirog, E.C.; Park, K.J.; Holcomb, K. Cytomorphology of Gastric-Type Cervical Adenocarcinoma on a ThinPrep Pap Test: Report of a P16-Positive Tumor Case. Diagn. Cytopathol. 2016, 44, 710–713. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Song, F.; Jia, M.; Yu, S.; Cao, L.; Sun, P.; Gao, H. PD-L1 Expression and Immune Stromal Features in HPV-independent Cervical Adenocarcinoma. Histopathology 2021, 79, 861–871. [Google Scholar] [CrossRef]
- Chen, L.; Lucas, E.; Zhang, X.; Liu, Q.; Zhuang, Y.; Lin, W.; Chen, H.; Zhou, F. Programmed Death-ligand 1 Expression in Human Papillomavirus-independent Cervical Adenocarcinoma and Its Prognostic Significance. Histopathology 2022, 80, 338–347. [Google Scholar] [CrossRef]


| IECC 2018/WHO 2020 | |
|---|---|
| HPV-Associated (HPVA) | Non-HPV-Associated (NHPVA) |
| Usual type | Gastric Type |
| Villoglandular | Clear Cells |
| Mucinous, NOS | Mesonephric |
| Mucinous, intestinal | Endometrioid |
| Invasive stratified mucin-producing | |
| Micropapillary | |
| Serous-like | |
| Clinical Features | HPV-Independent Cervical Adenocarcinomas | ||
|---|---|---|---|
| Gastric Type | Mesonephric Type | Clear Cell Type | |
| HPV Testing | Negative | Negative | 70% negative |
| Frequency | 10% of adenocarcinomas | <1% of adenocarcinomas | 2–7% of adenocarcinomas |
| Age of Incidence (Average) | 50–52 years | 52 years | 19 years (DES-exposed), variable (non-DES-exposed) |
| Clinical Features |
|
|
|
| Pathology Features |
|
|
|
| Main Genetic Mutations | TP53, STK11, KRAS, PTEN, ARID1A, BRCA2, CDKN2A, ERRB2ampl | KRAS, NRAS, PIK3CA, PTEN | PI3K/AKT/mTOR, MMR, POLE |
| p16^INK4a | p16 protein; expression blocked in HPV+ tumors; absent or focal in HPV-negative tumors. |
| p53 | p53 protein; wild-type pattern in HPV+ tumors; in mutated HPV-negative tumors appears with nuclear overexpression (or absent) |
| MUC6/HIK1083 | Gastric mucins; markers of gastric differentiation, expressed in ~60–80% of HPV-negative GASs. |
| Ki-67 | Variable; lower in well-differentiated HPV negative. High and diffuse in HPV positive lesions. |
| ER, PR, Vimentin | Hormone receptors and vimentin; generally negative in HPV-negative gastric/mesonephric carcinomas (on the contrary positive in endometrial neoplasms). |
| CD10 | Marker of endometrial origin; negative in HPV-negative cervical carcinomas (useful in the differential diagnosis with uterine metastases). |
| CEA | Often positive in gastric-type ADC. Variable in HPV positive tumors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannella, L.; Grelloni, C.; Natalini, L.; Sartini, G.; Bordini, M.; Delli Carpini, G.; Di Giuseppe, J.; Dugo, E.; Piva, F.; Ciavattini, A. Molecular Features of HPV-Independent Cervical Cancers. Pathogens 2025, 14, 668. https://doi.org/10.3390/pathogens14070668
Giannella L, Grelloni C, Natalini L, Sartini G, Bordini M, Delli Carpini G, Di Giuseppe J, Dugo E, Piva F, Ciavattini A. Molecular Features of HPV-Independent Cervical Cancers. Pathogens. 2025; 14(7):668. https://doi.org/10.3390/pathogens14070668
Chicago/Turabian StyleGiannella, Luca, Camilla Grelloni, Leonardo Natalini, Gianmarco Sartini, Mila Bordini, Giovanni Delli Carpini, Jacopo Di Giuseppe, Erica Dugo, Francesco Piva, and Andrea Ciavattini. 2025. "Molecular Features of HPV-Independent Cervical Cancers" Pathogens 14, no. 7: 668. https://doi.org/10.3390/pathogens14070668
APA StyleGiannella, L., Grelloni, C., Natalini, L., Sartini, G., Bordini, M., Delli Carpini, G., Di Giuseppe, J., Dugo, E., Piva, F., & Ciavattini, A. (2025). Molecular Features of HPV-Independent Cervical Cancers. Pathogens, 14(7), 668. https://doi.org/10.3390/pathogens14070668








