Occurrence and Molecular Characterization of Cryptosporidium spp. in Swine Farms in Northeastern Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Selection
2.2. Sample Collection and Questionnaire
2.3. Fecal Oocyst Concentration and Examination by Immunofluorescence
2.4. DNA Extraction, Detection and Sequencing
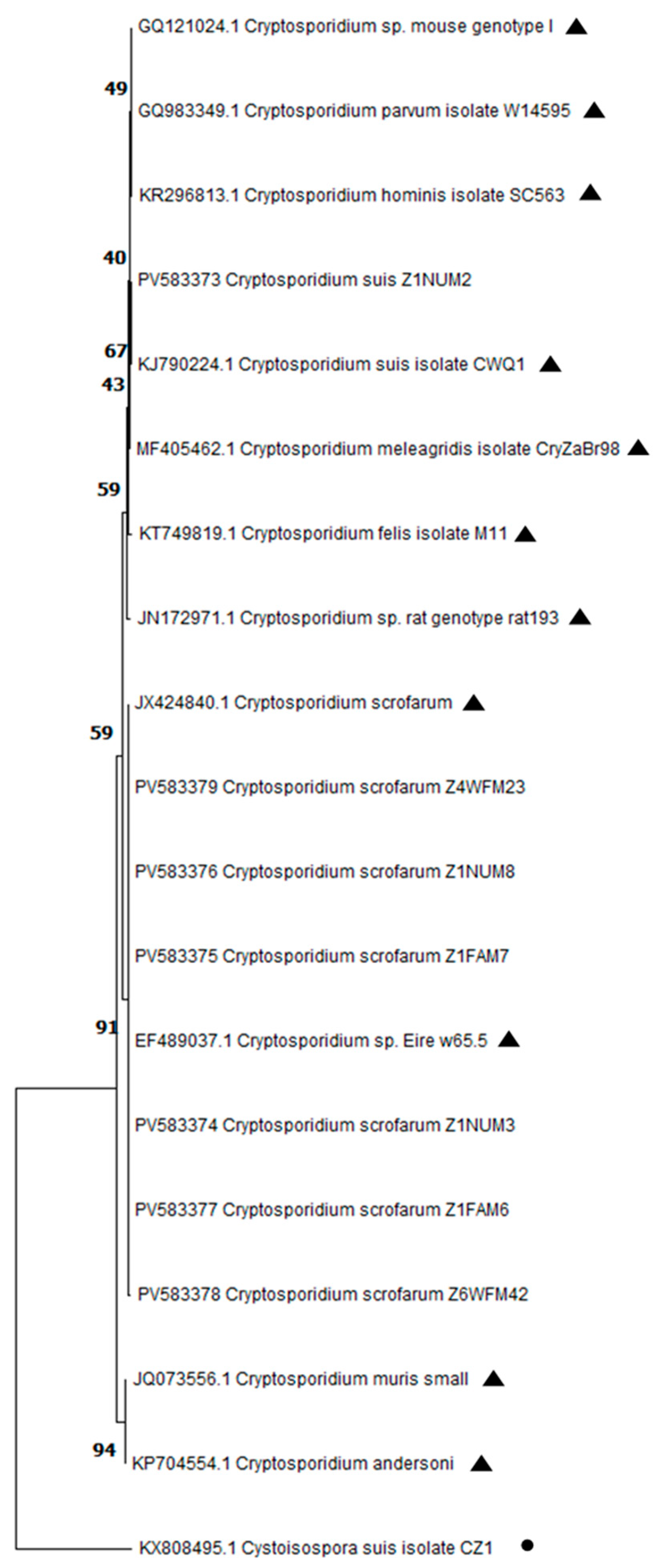
2.5. DNA Sequence and Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Positivity of Cryptosporidium spp. by Immunofluorescence and EOC
3.2. Detection of Cryptosporidium spp. by Nested PCR and Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef]
- Baldursson, S.; Karanis, P. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks—An update 2004–2010. Water Res. 2011, 45, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhang, Q.; Xu, C.; Zhang, Y.; Xing, J.; Tao, D.; Li, J.; Zhang, L. Prevalence and molecular characterization of Cryptosporidium spp. in pigs in Xinjiang, China. Acta Trop. 2020, 209, 105551. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, X.Y.; Chen, J.W.; Ding, L.; Zhao, G.H. Cryptosporidium suis infection in post-weaned and adult pigs in Shaanxi province, Northwestern China. Korean J. Parasitol. 2015, 53, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jia, T.; Huang, J.; Fan, Y.; Chang, H.; Han, S.; He, H. Identification of Enterocytozoon bieneusi and Cryptosporidium spp. in farmed wild boars (Sus scrofa) in Beijing, China. Infect. Genet. Evol. 2020, 80, 104231. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, H.; Wu, Y.; Xu, H.; Huang, J.; Li, J.; Zhang, L. Global prevalence of Cryptosporidium spp. in pigs: A systematic review and meta-analysis. Parasitology 2023, 150, 531–544. [Google Scholar] [CrossRef]
- Quílez, J.; Sánchez-Acedo, C.; Clavel, A.; del Cacho, E.; López-Bernad, F. Prevalence of Cryptosporidium infections in pigs in Aragón (northeastern Spain). Vet. Parasitol. 1996, 67, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Luengas, L.; Clavel, A.; Quílez, J.; Goñi-Cepero, M.P.; Torres, E.; Sánchez-Acedo, C.; del Cacho, E. Molecular characterization of Cryptosporidium isolates from pigs in Zaragoza (northeastern Spain). Vet. Parasitol. 2007, 148, 231–235. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Dashti, A.; López-López, P.; Muadica, A.S.; Risalde, M.L.A.; Köster, P.C.; Machuca, I.; Bailo, B.; de Mingo, M.H.; Dacal, E.; et al. Protist enteroparasites in wild boar (Sus scrofa ferus) and black Iberian pig (Sus scrofa domesticus) in southern Spain: A protective effect on hepatitis E acquisition? Parasites Vectors 2020, 13, 281. [Google Scholar] [CrossRef]
- García-Presedo, I.; Pedraza-Díaz, S.; González-Warleta, M.; Mezo, M.; Gómez-Bautista, M.; Ortega-Mora, L.M.; Castro-Hermida, J.A. Presence of Cryptosporidium scrofarum, C. suis and C. parvum subtypes IIaA16G2R1 and IIaA13G1R1 in Eurasian wild boars (Sus scrofa). Vet. Parasitol. 2013, 196, 497–502. [Google Scholar] [CrossRef]
- Ryan, U.M.; Samarasinghe, B.; Read, C.; Buddle, J.R.; Robertson, I.D.; Thompson, R.C. Identification of a novel Cryptosporidium genotype in pigs. Appl. Environ. Microbiol. 2003, 69, 3970–3974. [Google Scholar] [CrossRef]
- Vítovec, J.; Hamadejová, K.; Landová, L.; Kvác, M.; Kvetonová, D.; Sak, B. Prevalence and pathogenicity of Cryptosporidium suis in pre- and post-weaned pigs. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 239–243. [Google Scholar] [CrossRef]
- Enemark, H.L.; Ahrens, P.; Bille-Hansen, V.; Heegaard, P.M.; Vigre, H.; Thamsborg, S.M.; Lind, P. Cryptosporidium parvum: Infectivity and pathogenicity of the ‘porcine’ genotype. Parasitology 2003, 126, 407–416. [Google Scholar] [CrossRef]
- Hamnes, I.S.; Gjerde, B.K.; Forberg, T.; Robertson, L.J. Occurrence of Cryptosporidium and Giardia in suckling piglets in Norway. Vet. Parasitol. 2007, 144, 222–233. [Google Scholar] [CrossRef]
- Schubnell, F.; von Ah, S.; Graage, R.; Sydler, T.; Sidler, X.; Hadorn, D.; Basso, W. Occurrence, clinical involvement and zoonotic potential of endoparasites infecting Swiss pigs. Parasitol. Int. 2016, 65, 618–624. [Google Scholar] [CrossRef]
- Maddox-Hyttel, C.; Langkjaer, R.B.; Enemark, H.L.; Vigre, H. Cryptosporidium and Giardia in different age groups of Danish cattle and pigs: Occurrence and management-associated risk factors. Vet. Parasitol. 2006, 141, 48–59. [Google Scholar] [CrossRef]
- Němejc, K.; Sak, B.; Květoňová, D.; Hanzal, V.; Janiszewski, P.; Forejtek, P.; Rajský, D.; Ravaszová, P.; McEvoy, J.; Kváč, M. Cryptosporidium suis and Cryptosporidium scrofarum in Eurasian wild boars (Sus scrofa) in Central Europe. Vet. Parasitol. 2013, 197, 504–508. [Google Scholar] [CrossRef]
- Kvác, M.; Kvetonová, D.; Sak, B.; Ditrich, O. Cryptosporidium pig genotype II in immunocompetent man. Emerg. Infect. Dis. 2009, 15, 982–983. [Google Scholar] [CrossRef]
- Sone, B.; Ambe, L.A.; Ampama, M.N.; Ajohkoh, C.; Che, D.; Nguinkal, J.A.; Taubert, A.; Hermosilla, C.; Kamena, F. Prevalence and molecular characterization of Cryptosporidium species in diarrheic children in Cameroon. Pathogens 2025, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, E.; Safarpour, H.; Xiao, L.; Zarean, M.; Hatam-Nahavandi, K.; Barac, A.; Picot, S.; Rahimi, M.T.; Rubino, S.; Mahami-Oskouei, M.; et al. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite 2020, 27, 27. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.V.; Matthews, E.; Inns, T.; Roberts, C.; Matizanadzo, J.; Cleary, P.; Elson, R.; Williams, C.J.; Jarratt, R.; Chalmers, R.M.; et al. Retrospective case-case study investigation of a significant increase in Cryptosporidium spp. in England and Wales, August to September 2023. Euro Surveill. 2025, 30, 2400493. [Google Scholar] [CrossRef]
- Okhuysen, P.C.; Chappell, C.L.; Crabb, J.H.; Sterling, C.R.; DuPont, H.L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 1999, 180, 1275–1281. [Google Scholar] [CrossRef]
- Chappell, C.L.; Okhuysen, P.C.; Langer-Curry, R.; Widmer, G.; Akiyoshi, D.E.; Tanriverdi, S.; Tzipori, S. Cryptosporidium hominis: Experimental challenge of healthy adults. Am. J. Trop. Med. Hyg. 2006, 75, 851–857. [Google Scholar] [CrossRef]
- Alum, A.; Absar, I.M.; Asaad, H.; Rubino, J.R.; Ijaz, M.K. Impact of environmental conditions on the survival of Cryptosporidium and Giardia on environmental surfaces. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 210385. [Google Scholar] [CrossRef]
- Zahedi, A.; Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020, 132, 500–512. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Resultados De Las Encuestas De Ganado Porcino; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2023; Catálogo de Publicaciones de la Administración General del Estado; NIPO: 003-22-137-5.
- Young, K.H.; Bullock, S.L.; Melvin, D.M.; Spruill, C.L. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 1979, 10, 852–853. [Google Scholar] [CrossRef]
- Xiao, L.; Escalante, L.; Yang, C.; Sulaiman, I.; Escalante, A.A.; Montali, R.J.; Fayer, R.; Lal, A.A. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 1999, 65, 1578–1583. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Izumiyama, S.; Furukawa, I.; Kuroki, T.; Yamai, S.; Sugiyama, H.; Yagita, K.; Endo, T. Prevalence of Cryptosporidium parvum infections in weaned piglets and fattening porkers in Kanagawa Prefecture, Japan. Jpn. J. Infect. Dis. 2001, 54, 23–26. [Google Scholar] [CrossRef]
- Wang, N.; Wang, K.; Liu, Y.; Zhang, X.; Zhao, J.; Zhang, S.; Zhang, L. Molecular characterization of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in laboratory rodents in China. Parasite 2022, 29, 46. [Google Scholar] [CrossRef]
- Corsi, G.I.; Tichkule, S.; Sannella, A.R.; Vatta, P.; Asnicar, F.; Segata, N.; Jex, A.R.; van Oosterhout, C.; Cacciò, S.M. Recent genetic exchanges and admixture shape the genome and population structure of the zoonotic pathogen Cryptosporidium parvum. Mol. Ecol. 2023, 32, 2633–2645. [Google Scholar] [CrossRef]
- Agyabeng-Dadzie, F.; Xiao, R.; Kissinger, J.C. Cryptosporidium genomics—Current understanding, advances, and applications. Curr. Trop. Med. Rep. 2024, 11, 92–103. [Google Scholar] [CrossRef]
- Feng, Y.; Ryan, U.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef]
- Keidel, J.; Daugschies, A. Integration of halofuginone lactate treatment and disinfection with p-chloro-m-cresol to control natural cryptosporidiosis in calves. Vet. Parasitol. 2013, 196, 321–326. [Google Scholar] [CrossRef]
- Burnet, J.B.; Penny, C.; Ogorzaly, L.; Cauchie, H.M. Spatial and temporal distribution of Cryptosporidium and Giardia in a drinking water resource: Implications for monitoring and risk assessment. Sci. Total Environ. 2014, 472, 1023–1035. [Google Scholar] [CrossRef]
- Zahedi, A.; Paparini, A.; Jian, F.; Robertson, I.; Ryan, U. Public health significance of zoonotic Cryptosporidium species in wildlife: Critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 2015, 5, 88–109. [Google Scholar] [CrossRef]
| Production Type | Number of Farms (%) | Number of Samples per Production Stage | ||||
|---|---|---|---|---|---|---|
| Lactation-P1 | Nursery-P2 | Fattening-P3 | Wean-to-Finish-WTF | Total (%) | ||
| 3-stages | 4 | 10 | 23 | 12 | NA | 32 (44.4%) |
| 2-stages | 6 | 15 | NA | NA | 23 | 40 (55.6%) |
| Total | 10 | 25 | 12 | 12 | 23 | 72 (100%) |
| Farm | Production Type | N° Collected Samples | Positive Samples (%) | Number of Samples per Production Stage | |||
|---|---|---|---|---|---|---|---|
| Lactation-P1 | Nursery-P2 | Fattening-P3 | Wean-to-Finish-WTF | ||||
| A | 3-stages | 8 | 6 (75%) a | - | 3 | 3 | NA |
| B | 3-stages | 7 | 1 (14.3%) b | 1 | - | - | NA |
| D | 2-stages | 5 | 2 (40.0%) b | - | NA | NA | 2 |
| F | 2-stages | 6 | 2 (33.3%) b | - | NA | NA | 2 |
| J | 2-stages | 6 | 1 (16.7%) b | 1 | NA | NA | - |
| Total | 32 | 12 (37.5%) | 2 | 3 | 3 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garza-Moreno, L.; León, C.; Quílez, J. Occurrence and Molecular Characterization of Cryptosporidium spp. in Swine Farms in Northeastern Spain. Pathogens 2025, 14, 665. https://doi.org/10.3390/pathogens14070665
Garza-Moreno L, León C, Quílez J. Occurrence and Molecular Characterization of Cryptosporidium spp. in Swine Farms in Northeastern Spain. Pathogens. 2025; 14(7):665. https://doi.org/10.3390/pathogens14070665
Chicago/Turabian StyleGarza-Moreno, Laura, Celia León, and Joaquín Quílez. 2025. "Occurrence and Molecular Characterization of Cryptosporidium spp. in Swine Farms in Northeastern Spain" Pathogens 14, no. 7: 665. https://doi.org/10.3390/pathogens14070665
APA StyleGarza-Moreno, L., León, C., & Quílez, J. (2025). Occurrence and Molecular Characterization of Cryptosporidium spp. in Swine Farms in Northeastern Spain. Pathogens, 14(7), 665. https://doi.org/10.3390/pathogens14070665








