Presentation and Clinical Course of Leptospirosis in a Referral Hospital in Far North Queensland, Tropical Australia
Abstract
1. Introduction
2. Methods
2.1. Statistical Analysis
2.2. Ethical Approval
3. Results
3.1. Disease Severity and Clinical Course
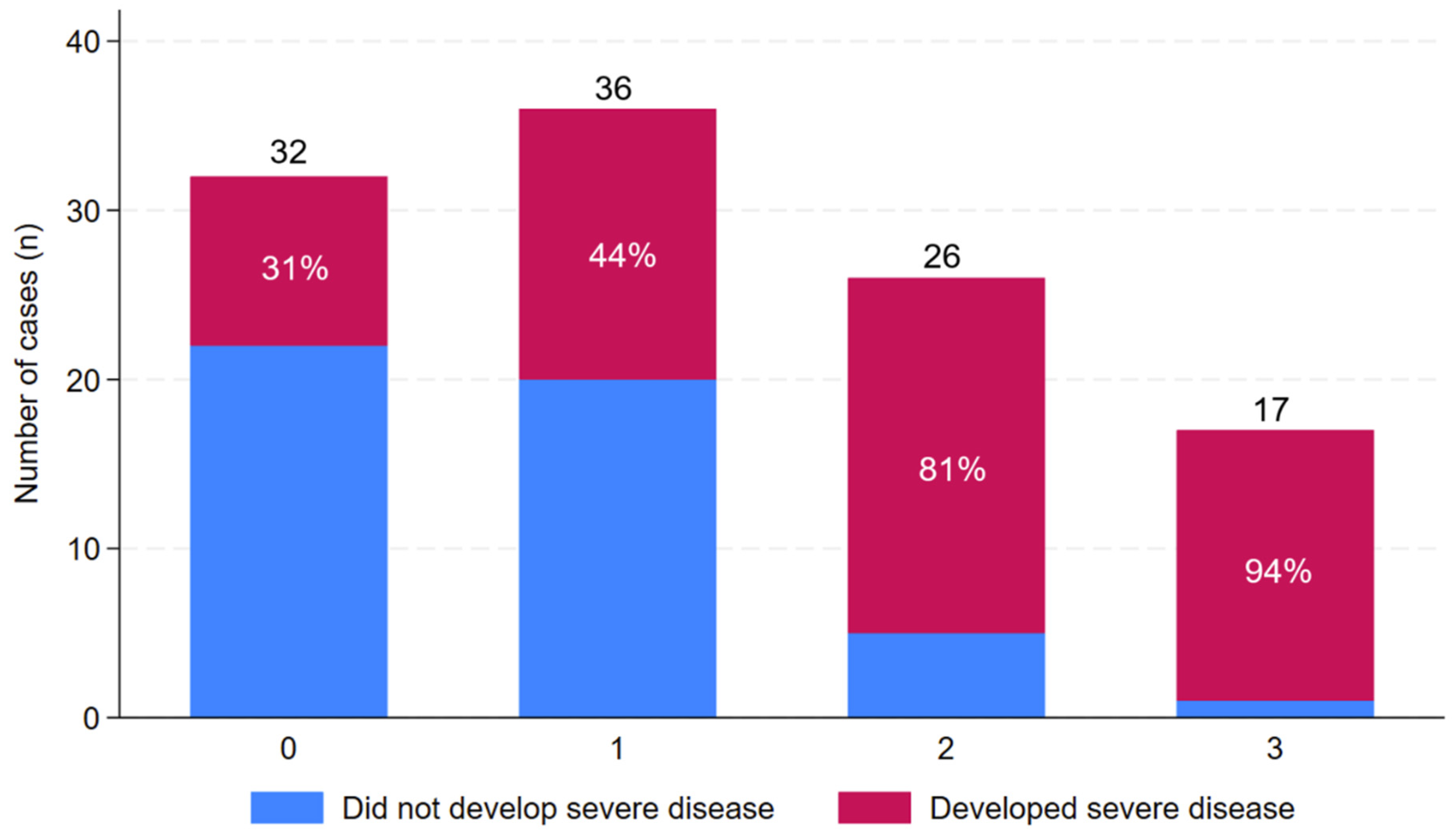
3.2. Correlation Between Age, Comorbidity and Subsequent Clinical Course
3.3. Diagnosis of Leptospirosis
3.4. Clinical Signs and Symptoms at Presentation and Correlation with Subsequent Clinical Course
3.5. Laboratory Values on Admission and Correlation with Subsequent Clinical Course
3.6. Chest Imaging Findings on Presentation and During Admission
3.7. Echocardiography
3.8. Antibiotic Therapy
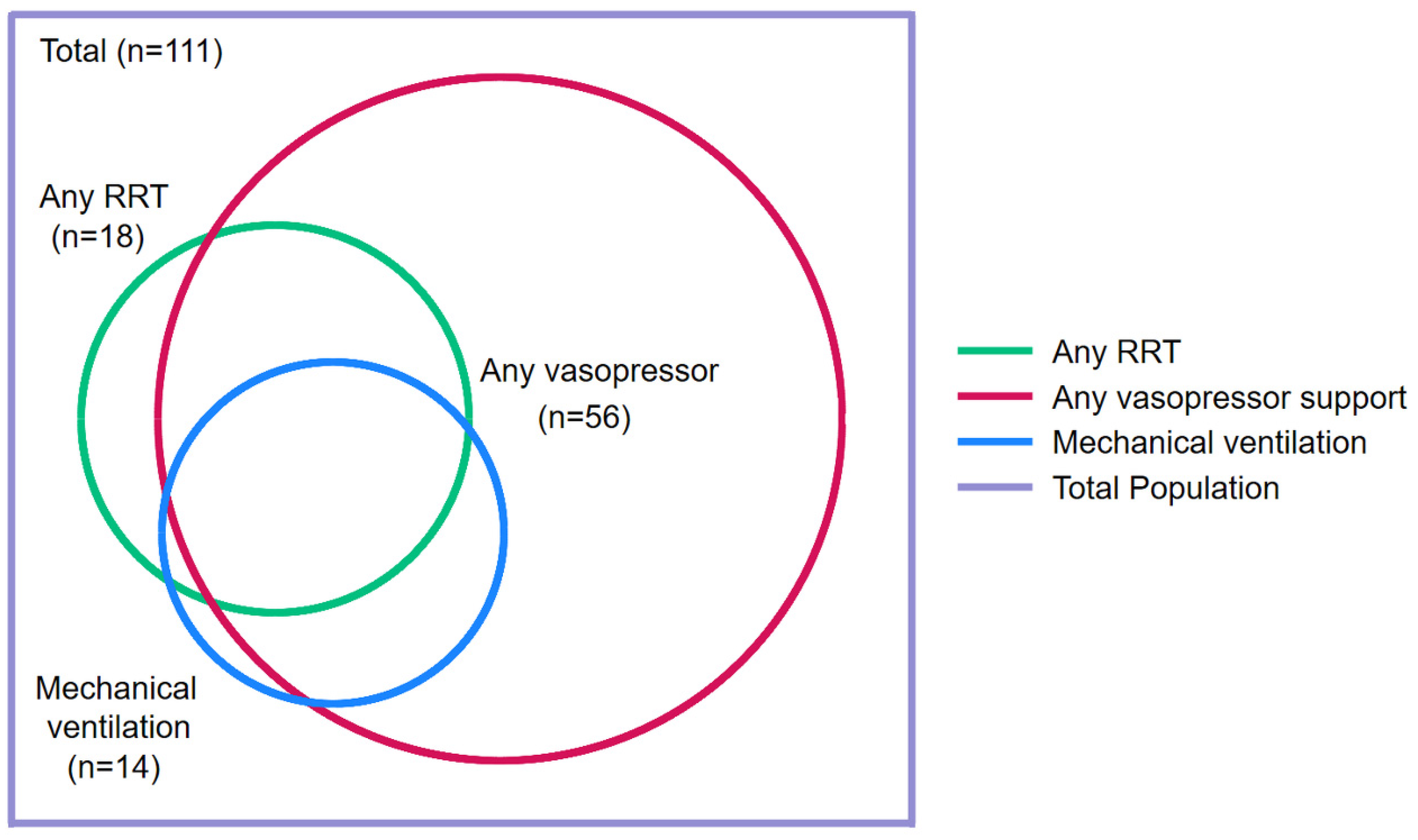
3.9. ICU Care
3.10. Other Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajapakse, S.; Fernando, N.; Dreyfus, A.; Smith, C.; Rodrigo, C. Leptospirosis. Nat. Rev. Dis. Primers 2025, 11, 32. [Google Scholar] [CrossRef]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Neglected Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Hagan, J.E.; Costa, F.; Calcagno, J.; Kane, M.; Martinez-Silveira, M.S.; Goris, M.G.; Stein, C.; Ko, A.I.; Abela-Ridder, B. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Neglected Trop. Dis. 2015, 9, e0004122. [Google Scholar] [CrossRef]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Obels, I.; Mughini-Gras, L.; Maas, M.; Brandwagt, D.; van den Berge, N.; Notermans, D.W.; Franz, E.; van Elzakker, E.; Pijnacker, R. Increased incidence of human leptospirosis and the effect of temperature and precipitation, the Netherlands, 2005 to 2023. Eurosurveillance 2025, 30, 2400611. [Google Scholar] [CrossRef]
- Dreesman, J.; Toikkanen, S.; Runge, M.; Hamschmidt, L.; Lusse, B.; Freise, J.F.; Ehlers, J.; Nockler, K.; Knorr, C.; Keller, B.; et al. Investigation and response to a large outbreak of leptospirosis in field workers in Lower Saxony, Germany. Zoonoses Public Health 2023, 70, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Pages, F.; Larrieu, S.; Simoes, J.; Lenabat, P.; Kurtkowiak, B.; Guernier, V.; Le Minter, G.; Lagadec, E.; Gomard, Y.; Michault, A.; et al. Investigation of a leptospirosis outbreak in triathlon participants, Reunion Island, 2013. Epidemiol. Infect. 2016, 144, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Baharom, M.; Ahmad, N.; Hod, R.; Ja’afar, M.H.; Arsad, F.S.; Tangang, F.; Ismail, R.; Mohamed, N.; Mohd Radi, M.F.; Osman, Y. Environmental and Occupational Factors Associated with Leptospirosis: A Systematic Review. Heliyon 2024, 10, e23473. [Google Scholar] [CrossRef]
- Reis, R.B.; Ribeiro, G.S.; Felzemburgh, R.D.; Santana, F.S.; Mohr, S.; Melendez, A.X.; Queiroz, A.; Santos, A.C.; Ravines, R.R.; Tassinari, W.S.; et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Neglected Trop. Dis. 2008, 2, e228. [Google Scholar] [CrossRef] [PubMed]
- Fairhead, L.J.; Smith, S.; Sim, B.Z.; Stewart, A.G.A.; Stewart, J.D.; Binotto, E.; Law, M.; Hanson, J. The seasonality of infections in tropical Far North Queensland, Australia: A 21-year retrospective evaluation of the seasonal patterns of six endemic pathogens. PLoS Glob. Public Health 2022, 2, e0000506. [Google Scholar] [CrossRef]
- Casanovas-Massana, A.; Pedra, G.G.; Wunder, E.A., Jr.; Diggle, P.J.; Begon, M.; Ko, A.I. Quantification of Leptospira interrogans Survival in Soil and Water Microcosms. Appl. Environ. Microbiol. 2018, 84, e00507-18. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Liu, Y.H.; Carter, A.; Kennedy, B.J.; Dermedgoglou, A.; Poulgrain, S.S.; Paavola, M.P.; Minto, T.L.; Luc, M.; Hanson, J. Severe leptospirosis in tropical Australia: Optimising intensive care unit management to reduce mortality. PLoS Neglected Trop. Dis. 2019, 13, e0007929. [Google Scholar] [CrossRef]
- Chawla, V.; Trivedi, T.H.; Yeolekar, M.E. Epidemic of leptospirosis: An ICU experience. J. Assoc. Physicians India 2004, 52, 619–622. [Google Scholar] [PubMed]
- Smith, S.; Hanson, J. Improving the mortality of severe leptospirosis. Intensive Care Med. 2020, 46, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Miailhe, A.F.; Mercier, E.; Maamar, A.; Lacherade, J.C.; Le Thuaut, A.; Gaultier, A.; Asfar, P.; Argaud, L.; Ausseur, A.; Ben Salah, A.; et al. Severe leptospirosis in non-tropical areas: A nationwide, multicentre, retrospective study in French ICUs. Intensive Care Med. 2019, 45, 1763–1773. [Google Scholar] [CrossRef]
- Fonseka, C.L.; Dahanayake, N.J.; Mihiran, D.J.D.; Wijesinghe, K.M.; Liyanage, L.N.; Wickramasuriya, H.S.; Wijayaratne, G.B.; Sanjaya, K.; Bodinayake, C.K. Pulmonary haemorrhage as a frequent cause of death among patients with severe complicated Leptospirosis in Southern Sri Lanka. PLoS Neglected Trop. Dis. 2023, 17, e0011352. [Google Scholar] [CrossRef]
- Pongpan, S.; Thanatrakolsri, P.; Vittaporn, S.; Khamnuan, P.; Daraswang, P. Prognostic Factors for Leptospirosis Infection Severity. Trop. Med. Infect. Dis. 2023, 8, 112. [Google Scholar] [CrossRef]
- Lau, C.; Smythe, L.; Weinstein, P. Leptospirosis: An emerging disease in travellers. Travel Med. Infect. Dis. 2010, 8, 33–39. [Google Scholar] [CrossRef]
- National Notifiable Disease Surveillance System. Available online: https://nindss.health.gov.au/pbi-dashboard/ (accessed on 4 June 2025).
- Far North Queensland Regional Organisation of Councils. Agriculture. Available online: https://economy.id.com.au/fnqroc/value-of-agriculture (accessed on 26 June 2025).
- Smith, S.; Kennedy, B.J.; Dermedgoglou, A.; Poulgrain, S.S.; Paavola, M.P.; Minto, T.L.; Luc, M.; Liu, Y.H.; Hanson, J. A simple score to predict severe leptospirosis. PLoS Neglected Trop. Dis. 2019, 13, e0007205. [Google Scholar] [CrossRef]
- Salaveria, K.; Smith, S.; Liu, Y.H.; Bagshaw, R.; Ott, M.; Stewart, A.; Law, M.; Carter, A.; Hanson, J. The Applicability of Commonly Used Severity of Illness Scores to Tropical Infections in Australia. Am. J. Trop. Med. Hyg. 2021, 106, 257–267. [Google Scholar] [CrossRef]
- Bird, K.; Bohanna, I.; McDonald, M.; Wapau, H.; Blanco, L.; Cullen, J.; McLucas, J.; Forbes, S.; Vievers, A.; Wason, A.; et al. A good life for people living with disability: The story from Far North Queensland. Disabil. Rehabil. 2024, 46, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Leptospirosis—Laboratory Case Definition. Available online: https://www.health.gov.au/sites/default/files/2025-01/leptospirosis-laboratory-case-definition.pdf (accessed on 4 June 2025).
- Taylor, A.J.; Paris, D.H.; Newton, P.N. A Systematic Review of the Mortality from Untreated Leptospirosis. PLoS Neglected Trop. Dis. 2015, 9, e0003866. [Google Scholar] [CrossRef] [PubMed]
- Guzman Perez, M.; Blanch Sancho, J.J.; Segura Luque, J.C.; Mateos Rodriguez, F.; Martinez Alfaro, E.; Solis Garcia Del Pozo, J. Current Evidence on the Antimicrobial Treatment and Chemoprophylaxis of Human Leptospirosis: A Meta-Analysis. Pathogens 2021, 10, 1125. [Google Scholar] [CrossRef]
- Ji, Z.; Jian, M.; Su, X.; Pan, Y.; Duan, Y.; Ma, W.; Zhong, L.; Yang, J.; Song, J.; Wu, X.; et al. Efficacy and safety of antibiotics for treatment of leptospirosis: A systematic review and network meta-analysis. Syst. Rev. 2024, 13, 108. [Google Scholar] [CrossRef]
- Win, T.Z.; Han, S.M.; Edwards, T.; Maung, H.T.; Brett-Major, D.M.; Smith, C.; Lee, N. Antibiotics for treatment of leptospirosis. Cochrane Database Syst. Rev. 2024, 3, CD014960. [Google Scholar] [CrossRef]
- Trott, D.J.; Abraham, S.; Adler, B. Antimicrobial Resistance in Leptospira, Brucella, and Other Rarely Investigated Veterinary and Zoonotic Pathogens. Microbiol. Spectr. 2018, 6, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, G.; Lefevre, P.; Chouvin, C.; D’Ortenzio, E. Jarisch-Herxheimer Reaction Among Patients with Leptospirosis: Incidence and Risk Factors. Am. J. Trop. Med. Hyg. 2017, 96, 791–794. [Google Scholar] [CrossRef]
- Bagshaw, R.J.; Stewart, A.G.A.; Smith, S.; Carter, A.W.; Hanson, J. The Characteristics and Clinical Course of Patients with Scrub Typhus and Queensland Tick Typhus Infection Requiring Intensive Care Unit Admission: A 23-year Case Series from Queensland, Tropical Australia. Am. J. Trop. Med. Hyg. 2020, 103, 2472–2477. [Google Scholar] [CrossRef]
- Price, C.; Smith, S.; Stewart, J.; Hanson, J. Acute Q Fever Patients Requiring Intensive Care Unit Support in Tropical Australia, 2015–2023. Emerg. Infect. Dis. 2025, 31, 332–335. [Google Scholar] [CrossRef]
- Susilawati, T.N.; McBride, W.J. Undiagnosed undifferentiated fever in Far North Queensland, Australia: A retrospective study. Int. J. Infect. Dis. 2014, 27, 59–64. [Google Scholar] [CrossRef]
- Dijkstra, F.; Riphagen-Dalhuisen, J.; Wijers, N.; Hak, E.; Van der Sande, M.A.; Morroy, G.; Schneeberger, P.M.; Schimmer, B.; Notermans, D.W.; Van der Hoek, W. Antibiotic therapy for acute Q fever in The Netherlands in 2007 and 2008 and its relation to hospitalization. Epidemiol. Infect. 2011, 139, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Gavey, R.; Stewart, A.G.A.; Bagshaw, R.; Smith, S.; Vincent, S.; Hanson, J. Respiratory manifestations of rickettsial disease in tropical Australia; Clinical course and implications for patient management. Acta Trop. 2025, 266, 107631. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- National Sepsis Program. Available online: https://www.safetyandquality.gov.au/our-work/national-sepsis-program (accessed on 21 March 2025).
- Leptospirosis. Available online: https://www.tg.org.au (accessed on 11 May 2025).
- Franklin, R.C.; King, J.C.; Aitken, P.J.; Elcock, M.S.; Lawton, L.; Robertson, A.; Mazur, S.M.; Edwards, K.; Leggat, P.A. Aeromedical retrievals in Queensland: A five-year review. Emerg. Med. Australas. 2021, 33, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Rygård, S.L.; Butler, E.; Granholm, A.; Møller, M.H.; Cohen, J.; Finfer, S.; Perner, A.; Myburgh, J.; Venkatesh, B.; Delaney, A. Low-dose corticosteroids for adult patients with septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2018, 44, 1003–1016. [Google Scholar] [CrossRef]
- Rodrigo, C.; Lakshitha de Silva, N.; Goonaratne, R.; Samarasekara, K.; Wijesinghe, I.; Parththipan, B.; Rajapakse, S. High dose corticosteroids in severe leptospirosis: A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 743–750. [Google Scholar] [CrossRef]
- Shenoy, V.V.; Nagar, V.S.; Chowdhury, A.A.; Bhalgat, P.S.; Juvale, N.I. Pulmonary leptospirosis: An excellent response to bolus methylprednisolone. Postgrad. Med. J. 2006, 82, 602–606. [Google Scholar] [CrossRef]
- Pitre, T.; Drover, K.; Chaudhuri, D.; Zeraaktkar, D.; Menon, K.; Gershengorn, H.B.; Jayaprakash, N.; Spencer-Segal, J.L.; Pastores, S.M.; Nei, A.M.; et al. Corticosteroids in Sepsis and Septic Shock: A Systematic Review, Pairwise, and Dose-Response Meta-Analysis. Crit. Care Explor. 2024, 6, e1000. [Google Scholar] [CrossRef]
- Fonseka, C.L.; Lekamwasam, S. Role of Plasmapheresis and Extracorporeal Membrane Oxygenation in the Treatment of Leptospirosis Complicated with Pulmonary Hemorrhages. J. Trop. Med. 2018, 2018, 4520185. [Google Scholar] [CrossRef]
- Klement-Frutos, E.; Tarantola, A.; Gourinat, A.C.; Floury, L.; Goarant, C. Age-specific epidemiology of human leptospirosis in New Caledonia, 2006–2016. PLoS ONE 2020, 15, e0242886. [Google Scholar] [CrossRef]
- Spichler, A.; Athanazio, D.A.; Vilaca, P.; Seguro, A.; Vinetz, J.; Leake, J.A. Comparative analysis of severe pediatric and adult leptospirosis in Sao Paulo, Brazil. Am. J. Trop. Med. Hyg. 2012, 86, 306–308. [Google Scholar] [CrossRef]
- Stewart, A.G.A.; Smith, S.; Binotto, E.; Hanson, J. Clinical Features of Rickettsial Infection in Children in Tropical Australia-A Report of 15 Cases. J. Trop. Pediatr. 2020, 66, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.A.; Costa, E.; Costa, Y.A.; Sacramento, E.; de Oliveira Junior, A.R.; Lopes, M.B.; Lopes, G.B. Comparative study of the in-hospital case-fatality rate of leptospirosis between pediatric and adult patients of different age groups. Rev. Inst. Med. Trop. Sao Paulo 2004, 46, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Evangelista, K.V.; Coburn, J. Leptospira as an emerging pathogen: A review of its biology, pathogenesis and host immune responses. Future Microbiol. 2010, 5, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Herrmann-Storck, C.; Saint-Louis, M.; Foucand, T.; Lamaury, I.; Deloumeaux, J.; Baranton, G.; Simonetti, M.; Sertour, N.; Nicolas, M.; Salin, J.; et al. Severe leptospirosis in hospitalized patients, Guadeloupe. Emerg. Infect. Dis. 2010, 16, 331–334. [Google Scholar] [CrossRef]
- Katz, A.R.; Ansdell, V.E.; Effler, P.V.; Middleton, C.R.; Sasaki, D.M. Assessment of the clinical presentation and treatment of 353 cases of laboratory-confirmed leptospirosis in Hawaii, 1974–1998. Clin. Infect. Dis. 2001, 33, 1834–1841. [Google Scholar] [CrossRef]
- Stewart, A.G.A.; Smith, S.; Binotto, E.; McBride, W.J.H.; Hanson, J. The epidemiology and clinical features of rickettsial diseases in North Queensland, Australia: Implications for patient identification and management. PLoS Neglected Trop. Dis. 2019, 13, e0007583. [Google Scholar] [CrossRef]
- Mar Minn, M.; Aung, N.M.; Kyaw, Z.; Zaw, T.T.; Chann, P.N.; Khine, H.E.; McLoughlin, S.; Kelleher, A.D.; Tun, N.L.; Oo, T.Z.C.; et al. The comparative ability of commonly used disease severity scores to predict death or a requirement for ICU care in patients hospitalised with possible sepsis in Yangon, Myanmar. Int. J. Infect. Dis. 2021, 104, 543–550. [Google Scholar] [CrossRef]
- Hanson, J.; Lee, S.J.; Mohanty, S.; Faiz, M.A.; Anstey, N.M.; Price, R.N.; Charunwatthana, P.; Yunus, E.B.; Mishra, S.K.; Tjitra, E.; et al. Rapid clinical assessment to facilitate the triage of adults with falciparum malaria, a retrospective analysis. PLoS ONE 2014, 9, e87020. [Google Scholar] [CrossRef]
- Niriella, M.A.; Liyanage, I.K.; Udeshika, A.; Liyanapathirana, K.V.A.; De Silva, A.P.; de Silva, H.J. Identification of dengue patients with high risk of severe disease, using early clinical and laboratory features, in a resource-limited setting. Arch. Virol. 2020, 165, 2029–2035. [Google Scholar] [CrossRef]
- Galdino, G.S.; de Sandes-Freitas, T.V.; de Andrade, L.G.M.; Adamian, C.M.C.; Meneses, G.C.; da Silva Junior, G.B.; de Francesco Daher, E. Development and validation of a simple machine learning tool to predict mortality in leptospirosis. Sci. Rep. 2023, 13, 4506. [Google Scholar] [CrossRef] [PubMed]
- Marotto, P.C.; Ko, A.I.; Murta-Nascimento, C.; Seguro, A.C.; Prado, R.R.; Barbosa, M.C.; Cleto, S.A.; Eluf-Neto, J. Early identification of leptospirosis-associated pulmonary hemorrhage syndrome by use of a validated prediction model. J. Infect. 2010, 60, 218–223. [Google Scholar] [CrossRef]
- Sreevalsan, T.V.; Chandra, R. Relevance of Polymerase Chain Reaction in Early Diagnosis of Leptospirosis. Indian J. Crit. Care Med. 2024, 28, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Win, T.Z.; Perinpanathan, T.; Mukadi, P.; Smith, C.; Edwards, T.; Han, S.M.; Maung, H.T.; Brett-Major, D.M.; Lee, N. Antibiotic prophylaxis for leptospirosis. Cochrane Database Syst. Rev. 2024, 3, CD014959. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, J.; Kuffner, T.; Tappero, J.; Whitworth, W.; Mize, A.; Kaiser, R.; McNicholl, J. HLA-DQ6 and ingestion of contaminated water: Possible gene-environment interaction in an outbreak of Leptospirosis. Genes Immun. 2004, 5, 197–202. [Google Scholar] [CrossRef]
- Agampodi, S.B.; Matthias, M.A.; Moreno, A.C.; Vinetz, J.M. Utility of quantitative polymerase chain reaction in leptospirosis diagnosis: Association of level of leptospiremia and clinical manifestations in Sri Lanka. Clin. Infect. Dis. 2012, 54, 1249–1255. [Google Scholar] [CrossRef]
- Hochedez, P.; Theodose, R.; Olive, C.; Bourhy, P.; Hurtrel, G.; Vignier, N.; Mehdaoui, H.; Valentino, R.; Martinez, R.; Delord, J.M.; et al. Factors Associated with Severe Leptospirosis, Martinique, 2010–2013. Emerg. Infect. Dis. 2015, 21, 2221–2224. [Google Scholar] [CrossRef]
- Munoz-Zanzi, C.; Dreyfus, A.; Limothai, U.; Foley, W.; Srisawat, N.; Picardeau, M.; Haake, D.A. Leptospirosis-Improving Healthcare Outcomes for a Neglected Tropical Disease. Open Forum Infect. Dis. 2025, 12, ofaf035. [Google Scholar] [CrossRef]
- Tshokey, T.; Ko, A.I.; Currie, B.J.; Munoz-Zanzi, C.; Goarant, C.; Paris, D.H.; Dance, D.A.B.; Limmathurotsakul, D.; Birnie, E.; Bertherat, E.; et al. Leptospirosis, melioidosis, and rickettsioses in the vicious circle of neglect. PLoS Neglected Trop. Dis. 2025, 19, e0012796. [Google Scholar] [CrossRef]


| Variable | All n = 111 | No Severe Disease a n = 48 | Severe Disease a n = 63 | p |
|---|---|---|---|---|
| Age (years) | 38 (24–55) | 32 (19–48) | 41 (26–63) | 0.03 |
| Child (age < 16 years) | 6 (5) | 3 (6) | 3 (5) | 1.00 |
| Male sex | 94 (85) | 38 (79) | 56 (89) | 0.19 |
| Remote residence b | 17 (15) | 6 (12) | 11 (18) | 0.60 |
| Rural or remote residence c | 89 (80) | 36 (75) | 53 (84) | 0.23 |
| Wet season presentation d | 67 (60) | 31 (63) | 36 (58) | 0.44 |
| Days of symptoms before presentation | 4 (2–5) | 4 (2–5) | 5 (3–6) | 0.07 |
| Any comorbidity e | 13 (12) | 3 (6) | 10 (16) | 0.15 |
| Diabetes mellitus e | 2 (2) | 1 (2) | 1 (2) | 1.0 |
| Cardiac failure e | 3 (3) | 0 | 3 (5) | 0.26 |
| Ischaemic heart disease e | 2 (2) | 0 | 2 (3) | 0.51 |
| Chronic kidney disease e | 0 | 0 | 0 | - |
| Lung disease e | 5 (5) | 1 (2) | 4 (6) | 0.39 |
| Liver disease e | 5 (5) | 1 (2) | 4 (6) | 0.39 |
| Malignancy e | 2 (2) | 0 | 2 (3) | 0.51 |
| Autoimmune disease e | 0 | 0 | 0 | - |
| Immunosuppressed e | 0 | 0 | 0 | - |
| Hazardous Alcohol use e | 29 (26) | 12 (24) | 17 (27) | 0.81 |
| Smoker e | 41 (37) | 14 (29) | 27 (43) | 0.14 |
| Serovar Zanoni | 21/59 (37) | 7/30 (23) | 14/29 (48) | 0.06 |
| Serovar Australis | 12/59 (20) | 6/30 (20) | 6/29 (21) | 0.72 |
| Variable | Number with Data | All n = 111 | No Severe Disease a n = 48 | Severe Disease a n = 63 | p |
|---|---|---|---|---|---|
| Subjective symptoms | |||||
| Headache | 111 | 80 (72) | 40 (83) | 40 (63) | 0.02 |
| Fevers | 111 | 106 (96) | 45 (94) | 61 (97) | 0.65 |
| Rigors | 111 | 40 (36) | 18 (38) | 22 (35) | 0.78 |
| Confusion | 111 | 8 (7) | 3 (6) | 5 (8) | 1.00 |
| Fatigue | 111 | 43 (39) | 14 (29) | 29 (47) | 0.07 |
| Abdominal pain | 111 | 42 (38) | 20 (41) | 22 (35) | 0.47 |
| Myalgia | 111 | 83 (75) | 34 (71) | 49 (79) | 0.40 |
| Arthralgia | 111 | 48 (43) | 17 (35) | 31 (49) | 0.15 |
| Diarrhoea | 111 | 41 (37) | 14 (29) | 27 (43) | 0.14 |
| Nausea/vomiting | 111 | 74 (67) | 35 (73) | 39 (62) | 0.14 |
| Chest pain | 111 | 9 (8) | 4 (8) | 5 (8) | 1.00 |
| Dyspnoea | 111 | 16 (14) | 2 (4) | 14 (22) | 0.01 |
| Cough | 111 | 33 (30) | 11 (23) | 22 (35) | 0.17 |
| URTI symptoms | 111 | 15 (14) | 6 (12) | 9 (15) | 1.00 |
| Haemoptysis | 111 | 12 (11) | 0 | 12 (19) | 0.001 |
| Abnormal bleeding or bruising | 111 | 11 (10) | 3 (6) | 8 (13) | 0.34 |
| Objective examination findings | |||||
| Hepatomegaly | 111 | 11 (10) | 4 (8) | 7 (11) | 0.75 |
| Splenomegaly | 111 | 0 | - | - | - |
| Lymphadenopathy | 111 | 6 (5) | 1 (2) | 5 (8) | 0.23 |
| Conjunctival suffusion | 111 | 23 (21) | 11 (23) | 12 (19) | 0.62 |
| Skin rash | 111 | 19 (17) | 11 (23) | 8 (13) | 0.21 |
| Abnormal chest auscultation | 111 | 44 (40) | 13 (27) | 31 (49) | 0.02 |
| Vital signs at presentation | |||||
| Oliguria b | 111 | 42 (38) | 9 (19) | 33 (52) | <0.001 |
| Fever > 38.0 °C | 111 | 21 (38) | 7 (15) | 14 (22) | 0.34 |
| Supplemental oxygen administered | 111 | 23 (21) | 2 (4) | 21 (33) | <0.001 |
| Respiratory rate ≥ 22 breaths/minute | 111 | 44 (40) | 14 (29) | 30 (48) | 0.049 |
| Heart rate ≥ 100 beats/min | 111 | 54 (49) | 17 (35) | 37 (59) | 0.02 |
| Systolic blood pressure <100 mmHg | 111 | 56 (50) | 12 (25) | 44 (70) | <0.001 |
| Disease severity score | |||||
| SPiRO score c | 111 | 1 (0–2) | 1 (0–1) | 2 (1–3) | <0.001 |
| Variable | Reference Range a | Number with Data | All n = 111 | No Severe Disease b n = 48 | Severe Disease b n = 63 | p |
|---|---|---|---|---|---|---|
| Haemoglobin initial | 115–160 g/dL | 111 | 134 (111–147) | 139 (129–150) | 131 (120–145) | 0.02 |
| Haemoglobin lowest | 115–160 g/dL | 111 | 112 (98–123) | 122 (110–129) | 103 (86–119) | <0.0001 |
| White cell initial | 4.0–11.0 × 109/L | 111 | 9.3 (6.8–11.9) | 8.2 (6.3–11.0) | 9.8 (7.2–12.8) | 0.046 |
| White cell highest | 4.0–11.0 × 109/L | 111 | 12.8 (9.5–18.3) | 10.6 (8.1–13.3) | 15.9 (11.9–22.1) | <0.0001 |
| White cell lowest | 4.0–11.0 × 109/L | 111 | 5.7 (4.2–7.8) | 5.5 (4.2–6.9) | 6.1 (4.2–8.4) | 0.32 |
| Platelet initial | 140–400 × 109/L | 111 | 119 (72–165) | 146 (113–197) | 84 (47–141) | <0.0001 |
| Platelet count lowest | 140–400 × 109/L | 111 | 84 (33–121) | 115 (90–147) | 54 (24–90) | <0.0001 |
| Neutrophil initial | 2.0–8.0 × 109/L | 111 | 8.2 (5.5–10.6) | 6.7 (4.6–9.3) | 8.5 (6.3–11.7) | 0.02 |
| Neutrophil highest | 2.0–8.0 × 109/L | 111 | 10.7 (8.1–14.2) | 9.0 (6.1–11.8) | 12.8 (10.0–19.9) | <0.0001 |
| Neutrophil lowest | 2.0–8.0 × 109/L | 111 | 3.5 (2.7–5.6) | 3.4 (2.5–4.6) | 4.0 (2.8–6.1) | 0.10 |
| Lymphocyte initial | 1.0–4.0 × 109/L | 111 | 0.5 (0.3–0.7) | 0.6 (0.4–0.8) | 0.4 (0.3–0.6) | 0.005 |
| Lymphocyte lowest | 1.0–4.0 × 109/L | 111 | 0.3 (0.2–0.5) | 0.4 (0.3–0.6) | 0.3 (0.2–0.4) | 0.0001 |
| INR initial | 0.9–1.2 | 85 | 1.1 (1.1–1.3) | 1.1 (1.1–1.1) | 1.1 (1.1–1.3) | 0.02 |
| INR highest | 0.9–1.2 | 85 | 1.2 (1.1–1.3) | 1.1 (1.1–1.1) | 1.2 (1.1–1.4) | 0.0002 |
| APTT initial | 25–38 s | 111 | 31 (29–34) | 31 (29–33) | 31 (28–34) | 0.52 |
| APTT highest | 25–38 s | 111 | 32 (30–36) | 31 (29–33) | 33 (30–39) | 0.004 |
| Variable | Reference Range a | Number with Data | All n = 111 | No Severe Disease b n = 48 | Severe Disease b n = 63 | p |
|---|---|---|---|---|---|---|
| Initial serum sodium | 135–145 mmol/L | 111 | 133 (129–135) | 134 (130–137) | 132 (128–135) | 0.01 |
| Lowest serum sodium | 135–145 mmol/L | 111 | 132 (129–135) | 133 (130–136) | 131 (126–134) | 0.001 |
| Initial serum potassium | 3.5–5.2 mmol/L | 111 | 3.7 (3.4–4.0) | 3.7 (3.4–4.0) | 3.6 (3.4–4.0) | 0.55 |
| Lowest serum potassium | 3.5–5.2 mmol/L | 111 | 4.4 (4.0–4.9) | 4.2 (3.8–4.7) | 4.6 (4.2–4.9) | 0.0003 |
| eGFR initial | >90 mL/min/1.73 m2 | 99 | 64 (25–90) | 77 (27–90) | 54 (25–78) | 0.12 |
| eGFR lowest | >90 mL/min/1.73 m2 | 99 | 38 (14–78) | 76 (18–90) | 25 (14–54) | 0.002 |
| Initial serum creatinine | 45–90 µmol/L | 111 | 113 (88–205) | 100 (81–197) | 124 (93–232) | 0.04 |
| Highest serum creatinine | 45–90 µmol/L | 111 | 179 (102–382) | 107 (85–337) | 211 (142–433) | 0.0008 |
| Initial serum bicarbonate | 22–32 mmol/L | 111 | 23 (21–25) | 24 (22–26) | 23 (21–25) | 0.07 |
| Lowest serum bicarbonate | 22–32 mmol/L | 111 | 20 (17–22) | 22 (19–23) | 19 (16–21) | 0.0001 |
| Initial serum bilirubin | <20 µmol/L | 111 | 18 (12–28) | 16 (10–26) | 19 (13–29) | 0.19 |
| Highest serum bilirubin | <20 µmol/L | 111 | 26 (19–48) | 20 (12–45) | 29 (21–50) | 0.004 |
| Initial serum ALT | <34 IU/mL | 111 | 68 (27–115) | 68 (26–126) | 67 (27–110) | 0.91 |
| Highest serum ALT | <34 IU/mL | 111 | 121 (68–208) | 131 (69–189) | 120 (68–220) | 0.66 |
| Initial serum AST | <31 IU/mL | 111 | 63 (34–135) | 67 (32–113) | 59 (34–143) | 0.37 |
| Highest serum AST | <31 IU/mL | 111 | 131 (74–210) | 102 (67–166) | 153 (80–287) | 0.02 |
| Initial serum GGT | <38 IU/mL | 111 | 51 (22–120) | 52 (23–159) | 48 (22–103) | 0.66 |
| Highest serum GGT | <38 IU/mL | 111 | 135 (70–235) | 142 (69–297) | 135 (70–217) | 0.57 |
| Initial serum SAP | 30–110 IU/mL | 111 | 98 (67–171) | 113 (70–164) | 89 (64–174) | 0.49 |
| Highest serum SAP | 30–110 IU/mL | 111 | 146 (109–208) | 143 (114–221) | 152 (91–208) | 0.88 |
| Initial serum LDH | 120–250 IU/mL | 111 | 296 (236–359) | 295 (238–358) | 296 (234–386) | 0.66 |
| Highest serum LDH | 120–250 IU/mL | 111 | 400 (327–534) | 359 (280–401) | 480 (377–690) | <0.0001 |
| Initial serum CK | 34–145 IU/mL | 83 | 281 (104–1020) | 124 (73–438) | 486 (118–1160) | 0.01 |
| Highest serum CK | 34–145 IU/mL | 83 | 350 (114–1020) | 124 (73–438) | 621 (124–1225) | 0.004 |
| Initial serum CRP | <5 mg/L | 107 | 190 (138–287) | 156 (102–234) | 233 (165–323) | 0.002 |
| Highest serum CRP | <5 mg/L | 107 | 227 (159–323) | 187 (137–238) | 258 (195–353) | 0.0002 |
| Initial serum lactate | 0.5–2.2 mmol/L | 105 | 1.5 (1.1–2.3) | 1.3 (1.0–1.8) | 1.7 (1.2–2.4) | 0.02 |
| Highest serum lactate | 0.5–2.2 mmol/L | 105 | 2.0 (1.4–2.7) | 1.5 (1.2–2.3) | 2.2 (1.8–3.6) | 0.0001 |
| Elevated initial serum troponin c | - | 111 | 25/111 (23%) | 2/48 (4%) | 23/63 (37%) | <0.0001 |
| Elevated serum troponin during hospitalisation c | - | 111 | 29/111 (26%) | 2/48 (4%) | 27/63 (43%) | <0.0001 |
| Variable | Number with Data | All n = 111 | No Severe Disease a n = 48 | Severe Disease a n = 63 | p |
|---|---|---|---|---|---|
| Abnormal initial chest imaging | 109 | 37/109 (34) | 10/46 (22) | 27 (43) | 0.02 |
| Any abnormal chest imaging during hospitalisation | 109 | 63/109 (58) | 17/46 (37) | 46 (73) | <0.001 |
| Multilobar involvement | 109 | 49/109 (45) | 12/46 (26) | 38 (60) | <0.001 |
| Alveolar changes | 109 | 54/109 (49) | 10/46 (22) | 44 (70) | <0.001 |
| Interstitial changes | 109 | 25/109 (23) | 6/46 (13) | 19 (30) | 0.04 |
| Pleural effusion | 109 | 19/109 (17) | 8/46 (17) | 12 (19) | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratton, H.; Rosengren, P.; Kinneally, T.; Prideaux, L.; Smith, S.; Hanson, J. Presentation and Clinical Course of Leptospirosis in a Referral Hospital in Far North Queensland, Tropical Australia. Pathogens 2025, 14, 643. https://doi.org/10.3390/pathogens14070643
Stratton H, Rosengren P, Kinneally T, Prideaux L, Smith S, Hanson J. Presentation and Clinical Course of Leptospirosis in a Referral Hospital in Far North Queensland, Tropical Australia. Pathogens. 2025; 14(7):643. https://doi.org/10.3390/pathogens14070643
Chicago/Turabian StyleStratton, Hayley, Patrick Rosengren, Toni Kinneally, Laura Prideaux, Simon Smith, and Josh Hanson. 2025. "Presentation and Clinical Course of Leptospirosis in a Referral Hospital in Far North Queensland, Tropical Australia" Pathogens 14, no. 7: 643. https://doi.org/10.3390/pathogens14070643
APA StyleStratton, H., Rosengren, P., Kinneally, T., Prideaux, L., Smith, S., & Hanson, J. (2025). Presentation and Clinical Course of Leptospirosis in a Referral Hospital in Far North Queensland, Tropical Australia. Pathogens, 14(7), 643. https://doi.org/10.3390/pathogens14070643






