Genetic Diversity of Enterocytozoon bieneusi in Diarrheic Shelter Dogs in Romania: First Molecular and Phylogenetic Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Molecular Analyses
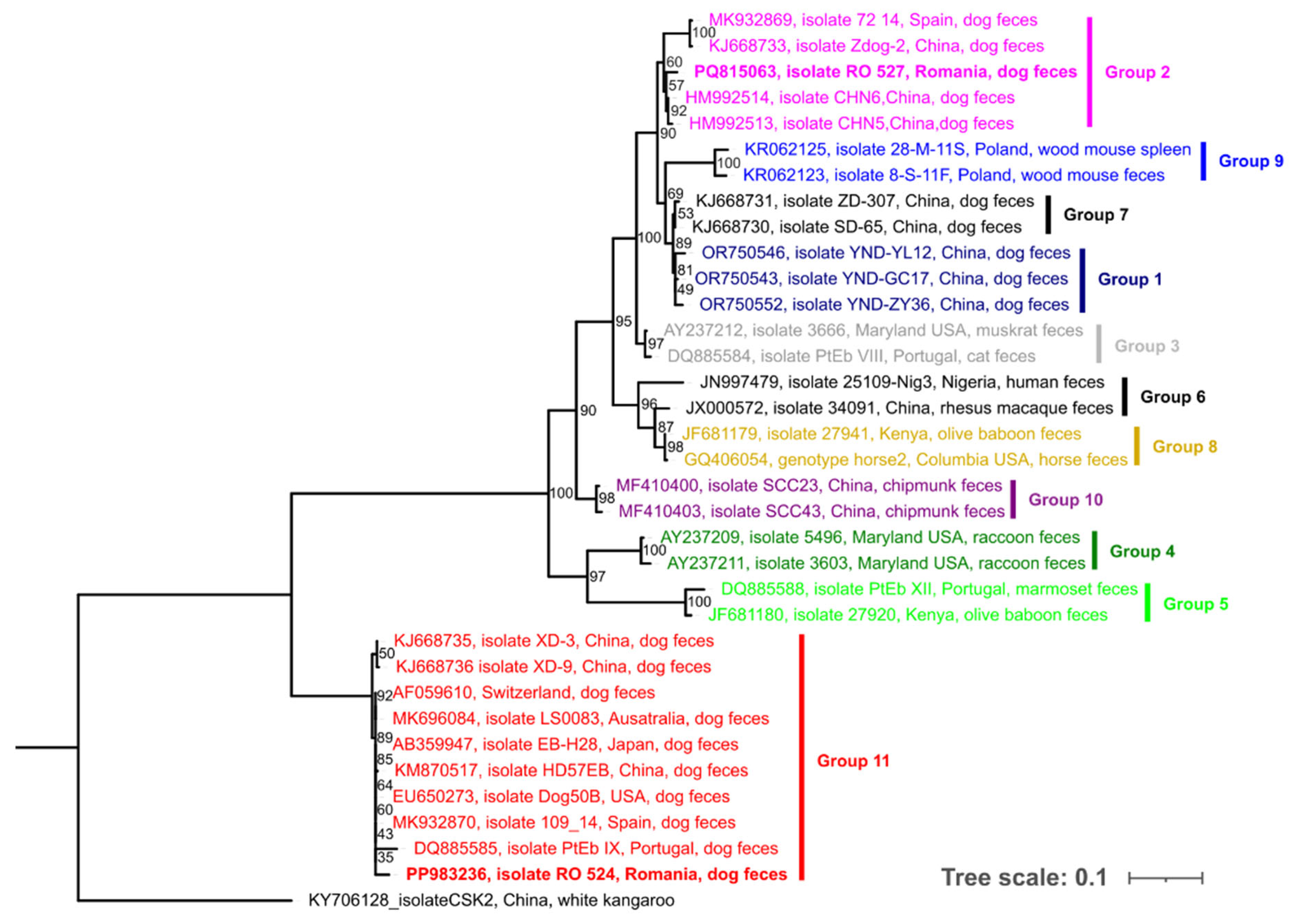
2.3. E. Bieneusi Genotyping and Phylogenetic Analysis
2.4. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, B.; Weiss, L.M. Microsporidia: Obligate intracellular pathogens within the fungal kingdom. Microbiol. Spectr. 2017, 5, 10. [Google Scholar] [CrossRef]
- Ruan, Y.; Xu, X.; He, Q.; Li, L.; Guo, J.; Bao, J.; Pan, G.; Li, T.; Zhou, Z. The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasit. Vectors 2021, 14, 186. [Google Scholar] [CrossRef]
- Bojko, J.; Reinke, A.W.; Stentiford, G.D.; Williams, B.; Rogers, M.S.; Bass, D. Microsporidia: A new taxonomic, evolutionary, and ecological synthesis. Trends Parasitol. 2022, 38, 642–659. [Google Scholar] [CrossRef]
- Santin, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Zhang, Y.; Gasser, R.B.A. Perspective on the Molecular Identification, Classification, and Epidemiology of Enterocytozoon bieneusi of Animals. Exp. Suppl. 2022, 114, 389–415. [Google Scholar]
- Li, W.; Feng, Y.; Santin, M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends. Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.; Becnel, J.; Weiss, L.; Keeling, P.; Didier, E.; Bjornson, S.; Freeman, M.; Brown, M.; Roesel, K.; Sokolova, Y. Microsporidia–emergent pathogens in the global food chain. Trends Parasitol. 2016, 32, 336–348. [Google Scholar] [CrossRef]
- Matos, O.; Lobo, M.L.; Xiao, L. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 2012, 2012, 981424. [Google Scholar] [CrossRef]
- Mathis, A.; Breitenmoser, A.C.; Deplazes, P. Detection of new Enterocytozoon genotypes in faecal samples of farm dogs and a cat. Parasite 1999, 6, 189–193. [Google Scholar] [CrossRef]
- Jian, J.; Zi, Y.; Wang, Y.; Yang, Y.; Su, Y.; Yao, L.; Li, B.; Peng, X.; Cao, J.; Shen, Y.; et al. Occurrence and genetic characterization of Enterocytozoon bieneusi in pet dogs in Yunnan Province, China. Parasite 2024, 31, 27. [Google Scholar] [CrossRef] [PubMed]
- Santín, M.; Fayer, R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: A consensus. J. Eukaryot. Microbiol. 2009, 56, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Dărăbuș, G.; Lupu, M.A.; Mederle, N.; Dărăbuș, R.G.; Imre, K.; Mederle, O.; Imre, M.; Paduraru, A.A.; Morariu, S.; Olariu, T.R. Epidemiology of Cryptosporidium Infection in Romania: A Review. Microorganisms 2023, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Mircean, V.; Györke, A.; Cozma, V. Prevalence and risk factors of Giardia duodenalis in dogs from Romania. Vet. Parasitol. 2012, 184, 325–329. [Google Scholar] [CrossRef]
- Thrusfield, M.; Christley, R. Veterinary Epidemiology, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Buckholt, M.A.; Lee, J.H.; Tzipori, S. Prevalence of Enterocytozoon bieneusi in swine: An 18-month survey at a slaughterhouse inMassachusetts. Appl. Environ. Microbiol. 2002, 68, 2595–2599. [Google Scholar] [CrossRef]
- Simmons, M.P.; Ochoterena, H. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 2000, 49, 369–381. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Wang, H.; Lin, X.; Sun, Y.; Qi, N.; Lv, M.; Xiao, W.; Chen, Y.; Xiang, R.; Sun, M.; Zhang, L. Occurrence, risk factors and genotypes of Enterocytozoon bieneusi in dogs and cats in Guangzhou, southern China: High genotype diversity and zoonotic concern. BMC Vet. Res. 2020, 16, 201. [Google Scholar] [CrossRef]
- Karim, M.R.; Dong, H.; Yu, F.; Jian, F.; Zhang, L.; Wang, R.; Zhang, S.; Rume, F.I.; Ning, C.; Xiao, L. Genetic diversity in Enterocytozoon bieneusi isolates from dogs and cats in China: Host specificity and public health implications. J. Clin. Microbiol. 2014, 52, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhou, Z.; Deng, L.; Liu, H.; Zhong, Z.; Ma, X.; Zhang, K.; Wang, Y.; Fu, H.; Peng, G. Prevalence and new genotypes of Enterocytozoon bieneusi in sheltered dogs and cats in Sichuan province, southwestern China. Parasite 2021, 28, 31. [Google Scholar] [CrossRef]
- Santín, M.; Cortés Vecino, J.A.; Fayer, R. Enterocytozoon bieneusi genotypes in dogs in Bogota, Colombia. Am. J. Trop. Med. Hyg. 2008, 79, 215–217. [Google Scholar] [CrossRef]
- Delrobaei, M.; Jamshidi, S.; Shayan, P.; Ebrahimzade, E.; Ashrafi, T.I.; Rezaeian, M.; Rezaeian, M.; Mirjalali, H. Molecular Detection and Genotyping of Intestinal Microsporidia from Stray Dogs in Iran. Iran. J. Parasitol. 2019, 14, 159–166. [Google Scholar]
- Liu, H.; Xu, J.; Shen, Y.; Cao, J.; Yin, J. Genotyping and Zoonotic Potential of Enterocytozoon bieneusi in Stray Dogs Sheltered from Shanghai, China. Animals 2021, 11, 3571. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Kimata, I.; Iseki, M. Molecular evidence of Enterocytozoon bieneusi in Japan. J. Vet. Med. Sci. 2009, 71, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Phrompraphai, T.; Itoh, N.; Iijima, Y.; Ito, Y.; Kimura, Y. Molecular detection and genotyping of Enterocytozoon bieneusi in family pet dogs obtained from different routes in Japan. Parasitol. Int. 2019, 70, 86–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Koehler, A.V.; Wang, T.; Robertson, G.J.; Bradbury, R.S.; Gasser, R.B. Enterocytozoon bieneusi genotypes in people with gastrointestinal disorders in Queensland and Western Australia. Infect. Genet. Evol. 2018, 65, 293–299. [Google Scholar] [CrossRef]
- da Silva Fiuza, V.R.; Lopes, C.W.; de Oliveira, F.C.; Fayer, R.; Santín, M. New findings of Enterocytozoon bieneusi in beef and dairy cattle in Brazil. Vet Parasitol. 2016, 216, 46–51. [Google Scholar] [CrossRef]
- Li, W.; Diao, R.; Yang, J.; Xiao, L.; Lu, Y.; Li, Y.; Song, M. High diversity of human pathogenic Enterocytozoon bieneusi genotypes in swine in Northeast China. Parasitol Res. 2014, 113, 1147–1153. [Google Scholar] [CrossRef]
- Santín, M.; Calero-Bernal, R.; Carmena, D.; Mateo, M.; Balseiro, A.; Barral, M.; Lima-Barbero, J.F.; Habela, M.Á. Molecular characterization of Enterocytozoon bieneusi in wild carnivores in Spain. J. Eukaryot. Microbiol. 2018, 65, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sak, B.; Brady, D.; Pelikánová, M.; Květoňová, D.; Rost, M.; Kostka, M.; Pelikánová, M.; Tolarová, V.; Hůzová, Z.; Kváč, M. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J. Clin. Microbiol. 2011, 49, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
| Individual Animal and Epidemiological Data | Occurrence [%(n/Total)] | 95% Confidence Interval | E. bieneusi GenoTypes (No.) | p-Value |
|---|---|---|---|---|
| Age | 0.026 * | |||
| Juvenile | 23.1 (6/26) | 11.0–42.1 | PtEb IX (6), | |
| Adult | 5.8 (5/86) | 25.1–12.9 | BEB4, PtEb IX (4) | |
| Gender | 0.940 | |||
| Female | 8.5 (4/47) | 3.4–19.9 | PtEb IX (4), | |
| Male | 10.8 (7/65) | 5.3–20.6 | PtEb IX (6), BEB4 | |
| Breed | 0.850 | |||
| Pure | 10.5 (2/19) | 2.9–3.1 | PtEb IX, BEB4 | |
| Crossbreed | 9.7 (9/93) | 5.2–17.4 | PtEb IX (9) | |
| Season | 0.707 | |||
| Spring | 12.1 (4/33) | 4.8–27.3 | PtEb IX (3), BEB4 | |
| Summer | 5.9 (1/17) | 1.1–27.0 | PtEb IX | |
| Autumn | 5.0 (1/20) | 0.9–23.6 | PtEb IX | |
| Winter | 11.9 (5/42) | 5.2–25.0 | PtEb IX (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imre, M.; Dărăbuș, G.; Morariu, S.; Szabó, K.; Ilie, M.-S.; Florea, T.; Pocinoc, A.; Awwad, R.; Imre, K. Genetic Diversity of Enterocytozoon bieneusi in Diarrheic Shelter Dogs in Romania: First Molecular and Phylogenetic Evidence. Pathogens 2025, 14, 641. https://doi.org/10.3390/pathogens14070641
Imre M, Dărăbuș G, Morariu S, Szabó K, Ilie M-S, Florea T, Pocinoc A, Awwad R, Imre K. Genetic Diversity of Enterocytozoon bieneusi in Diarrheic Shelter Dogs in Romania: First Molecular and Phylogenetic Evidence. Pathogens. 2025; 14(7):641. https://doi.org/10.3390/pathogens14070641
Chicago/Turabian StyleImre, Mirela, Gheorghe Dărăbuș, Sorin Morariu, Krisztián Szabó, Marius-Stelian Ilie, Tiana Florea, Alexandra Pocinoc, Reem Awwad, and Kálmán Imre. 2025. "Genetic Diversity of Enterocytozoon bieneusi in Diarrheic Shelter Dogs in Romania: First Molecular and Phylogenetic Evidence" Pathogens 14, no. 7: 641. https://doi.org/10.3390/pathogens14070641
APA StyleImre, M., Dărăbuș, G., Morariu, S., Szabó, K., Ilie, M.-S., Florea, T., Pocinoc, A., Awwad, R., & Imre, K. (2025). Genetic Diversity of Enterocytozoon bieneusi in Diarrheic Shelter Dogs in Romania: First Molecular and Phylogenetic Evidence. Pathogens, 14(7), 641. https://doi.org/10.3390/pathogens14070641









