Abstract
Free-living amoebae (FLA) such as Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, Sappinia pedata, Vermamoeba vermiformis and Vahlkampfia spp. are causal agents of deadly and/or disabling infections in humans. Despite recent data showing an increase in infection cases worldwide, studies on the prevalence of these emerging pathogens in water sources are scarce. Moreover, climate change is believed to facilitate the expansion and persistence of these environmental pathogens, further emphasizing the need for comprehensive surveillance. Therefore, the current study investigates the variety and abundance of free-living amoebae in different water sources in the autonomous community of Castilla y León, Spain, during different seasons of the year. Vermamoeba vermiformis was the most prevalent species and was detected in rivers, swamps, irrigation waters, swimming pools and recreational fountains. Moreover, genera such as Acanthamoeba and Naegleria and Vahlkampfia were also identified. This study highlights the diversity of FLA in the region and their relationship with local water characteristics. Given that certain FLA species are opportunistic pathogens, these results emphasize the necessity of monitoring this area and water sources.
1. Introduction
Free-living amoebae (FLA) mostly exist in the environment such as water, soil, or dust without the requirement for a host [1,2]. Under favorable conditions, FLA adopt the form of trophozoites or the vegetative stage, in charge of feeding and multiplication. When external conditions are adverse, FLA turn to the cyst form or resistant stage, surrounding themselves with a highly robust wall to protect them against environmental hazards [3].
Among the many FLA genera found in nature, infections in humans and other animals are caused by Acanthamoeba spp., Naegleria fowleri, Sappinia pedata, Balamuthia mandrillaris, Vermamoeba vermiformis, and Vahlkampfia spp. [1,4,5]. Many of these amoebae cause brain infections, e.g., Acanthamoeba species, B. mandrillaris, and S. pedata, produce granulomatous amoebic encephalitis (GAE), whereas N. fowleri produces primary amoebic meningoencephalitis (PAM) [1,6,7]. However, Acanthamoeba spp., V. vermiformis and Vahlkampfia spp. are also causative agents of amoebic keratitis [8]. A recent study found that V. vermiformis is the cause of a painful ulcer next to the eye and has been found to be both an etiological agent and a pathogen reservoir [9]. Additionally, B. mandrillaris and Acanthamoeba species cause skin disorders in immunocompromised individuals [1].
FLA are increasingly considered as environmental vehicles of other microorganisms. In addition, it is important to mention that FLA primarily feed on other microorganisms, especially bacteria, through phagocytosis. In fact, several bacteria species have acquired resistance mechanisms to the FLA digestive enzymes (amoeba-resistant bacteria, ARB), using FLA as vehicles. Moreover, the cyst stage can facilitate the intracellular survival of bacteria, avoiding common water disinfection systems or agents, but they are not effective against FLA cysts [10,11]. As a result, FLA contribute to the recycling of nutrients in freshwater settings, which makes them important to the ecology of many ecosystems [12].
In Spain, research on FLA has grown due to their impact on public health. However, studies have been conducted mainly in the Canary Islands and have contributed significantly to the detection of these parasites in the environment [13,14,15,16]. On the other hand, researchers from the University of Zaragoza analyzed FLA in water and sludge from five wastewater treatment plants (WWTP) in the Ebro River basin [17] and other authors studied the presence of FLA during a year in water from four drinking water treatment plants (DWTP), seven wastewater treatment plants (WWTP) and six locations of influence (LI) on four river basins from Spain [18]. In general, these studies are essential to assess public health risks, especially in contexts of direct human exposure, to monitor environmental impacts and to design prevention and control measures. Additionally, there is no specific regulation in Spain on FLA in water, unlike other countries such as Mexico, Australia and France, which have limited the number of N. fowleri cells (100 cells/L), indicating a gap in the environmental control of these pathogens [19,20].
Therefore, due to the lack of knowledge about the environmental prevalence of FLA in Spain, particularly in a Continental Mediterranean climate, the aim of the present work was to increase the epidemiological knowledge of FLA through one year of sampling in different water sources located in the autonomous community of Castilla y León.
2. Materials and Methods
2.1. Location and Sampling
The sampling area was in the autonomous community of Castilla y León, covering the north-central part of the Iberian Peninsula in an elevated plateau (~800 m a.s.l.), surrounded by three mountain systems in the north, south and east. Castilla y León presents an area of 94,224 km2, and it is the largest region in Spain and one of the largest in Europe. The autonomous community is divided into 9 provinces: León, Zamora, Salamanca, Valladolid, Palencia, Burgos, Soria, Segovia, and Ávila (Figure 1). According to Köppen, Castilla y León presents a Mediterranean climate, characterized by long, cold winters (average January temperatures of 3 to 6 °C) and brief, warm summers (average temperatures of 19 to 22 °C). With an average annual rainfall of about 450 to 500 mm, which is especially visible in mountain ranges, there is very little rainfall throughout the summer. Moreover, distinct sub-climates can be identified because of the extensive area and orographic diversity of this region. A significant portion is in the subclimates of temperate with dry or temperate summer (Csb) or temperate with a dry season and temperate summer (Cfb), where the warmest month is typically below 22 °C but above 10 °C for at least five months. The sub-climate in several regions of the central plateau is categorized as either cold steppe (BSk), with average annual temperatures below 18 °C, or temperate with dry or hot summer (Csa), with summer temperatures above 22 °C. The climate at high elevations in mountainous regions is cold temperate, with dry summers (Dsb or Dsc) with average temperatures below 3 °C during the coldest months [21,22,23]. Furthermore, the geology of the region, dominated by sedimentary formations, plays a role in determining the pH and buffering capacity of surface waters.
Figure 1.
Geographical localization of the autonomous community of Castilla y León.
A total of 126 samples from 42 sampling point were collected from 4 provinces (Salamanca (SW1-28), Valladolid (VW1-6), Zamora (ZW1-6) and Burgos (BW1-2)) in the autonomous community of Castilla y León (41°45′16″ N 4°46′55″ O) during the years 2022 and 2023, in order to detect the presence of potentially pathogenic FLA in water samples. Water collection in sterile bottles (1.5 L) was performed three different seasons: from September to November of 2022, where the temperature was approximately 12 °C (t1, autumn); from January to February of 2023, where the temperature was approximately 5 °C (t2, winter); and from May to June of 2023, where the temperature was approximately 16 °C (t3, late spring). These water samples that were taken approximately every 2 months during the study period correspond to the recreational fountain (36/126), tap from private home (33/126), river (18/126), swamp (12/126), irrigation water (15/126), and swimming pool (12/126) (Table 1).

Table 1.
Location of environmental water samples analyzed for FLA detection in the autonomous community of Castilla y León.
2.2. Free-Living Amoeba Isolation
A total of 1.5 L of each water sample was filtered by a vacuum filtration system using a 0.45 μm pore size filter (Pall, Madrid, Spain). After that, the membrane filters were inverted onto 2% Non-Nutrient Agar (NNA) plates with a layer of heat-killed E. coli. The plates were cultured at room temperature and checked under an inverted microscope every day for amoebic growth for a maximum of 15 days. Once FLA was found on a plate, it was cloned by diluting it with fresh NNA until, if possible, a monoxenic culture was obtained. Before the use of molecular methods, isolated FLA were categorized according to Page’s criteria at the genus level based on their morphology [24].
2.3. DNA Extraction
For molecular characterization, DNA from positive samples was extracted from 1 to 2 milliliters of amoebic culture suspension. The monoxenic amoeba culture was washed with 4 mL of Page’s Amoeba Solution (PAS) and scraped with a glass staff to create the amoeba suspension. As previously described [25] the suspension was centrifuged and was then put straight into the Maxwell® 16 tissue DNA purification kit sample cartridge (Promega, Madrid, Spain) in accordance with the manufacturer’s instructions. Extracted DNA yield and purity were quantified using the NanoDrop Lite Spectrophotometer (Fisher Scientific, Madrid, Spain).
2.4. PCR and Molecular Characterizations
Prior to being used as a template for PCR amplification of the 18S rRNA gene using various primer sets, extracted DNAs were kept at −20 °C. PCR was employed both as a confirmatory technique following preliminary morphological identification and as a molecular method for precise genus or species-level identification. The use of multiple primer sets was justified by the need to target different genera or families of free-living amoebae according to their morphological characteristics, allowing specific and efficient amplification: universal FLA-f 5′-CGCGGTAATTCCAGCTCCAATAGC-3′/FLA-r 5′-CAGGTTAAGGTCTCGTTCGTTAAC-3′ [26] and JDP-1f 5′-GGCCCAGATCGTTTACCGTGAA-3′ and JDP-2r 5′-TCTCACAAGCTGCTAGGGAGTCA-3′ for amoeba presenting morphology corresponding to Acanthamoeba spp. [27], Hv1227f 5′-TTACGAGGTCAGGACACTGT-3′ [28] and VermRV 5′-TGCCTCAAACTTCCATTCGC-3′ [29] for V. vermiformis and for the family Vahlkampfiidae we used these primers: VAHL1 5′-GTCTTCGTAGGTGAACCTGC-3′ and VAHL2 5′-CCGCTTACTGATATGCTTAA-3′ [30]. For all performed PCRs, amplification reactions were performed in a 50 μL mixture containing 80 ng DNA for FLA, 40 ng DNA for Acanthamoeba spp. and V. vermiformis and 60 ng DNA for VAHL, and the conditions for the PCRs shown in the table were established (Table 2). DNA is visualized with 2% agarose gel stained with Gel Red®. The positive PCR products obtained were sequenced by Macrogen Spain service. A homology search was performed with BLAST software within the GenBank database from the National Library of Medicine (NCBI).

Table 2.
PCR conditions for the different primers.
2.5. Phylogenetic Analysis
To establish the genetic correlation among the isolates, a sequence alignment was performed using the MAFFT software version 7 with the accurate L-INS-i method [31] and trimAl for the removal of poorly aligned sites when necessary [32]. All evolutionary analyses were performed on RAxML v.8.2.10 using the Maximum Composite Likelihood method, which was utilized to compute the evolutionary distances, expressed in base substitutions per site [33]. RAxML used the GTRGAMMA substitution model for nucleotide alignments. To assess the robustness of the clusters, a bootstrap analysis was performed with 500 replicates, indicating the percentage of support next to each node. The tree was rooted with an external group.
3. Results
From the total of 126 samples for 42 sampling points, 69 water samples were positive for the presence of FLA on NNA plates (69/126; 54.76%). After analysis of the 18S rRNA gene (the DF3 region in the case of Acanthamoeba) 59 water samples (59/69; 85.51%) were positive in the PCR.
Vermamoeba vermiformis was the most abundantly isolated species in this study (35/69; 50.72%). Acanthamoeba spp. was the second most frequently found (16/69; 23.19%), with the T4 genotype being the most frequently detected (10/16; 62.5%) followed by the T2 (4/16; 25%), T3 (1/16; 6.25%) and T16 genotypes (1/16; 6.25%). Free-living amoebae of the Vahlkampfiidae family, including Vahlkampfia avara and Naegleria pagei, for example, were isolated to a lesser degree (Table S1).
The obtained nucleotide sequences are deposited in GenBank with the following accession numbers: PP853450-PP853471, PP872540-PP872541, PQ806969-PQ806983 and PV249448-PV249451. All of them presented ˃95% of homology with the available DNA sequences in this database.
3.1. Regional Variation
The results obtained show that, according to provinces: of the 126 total samples collected, 84 were from the province of Salamanca, 18 from the provinces of Zamora and Valladolid, and 6 from Burgos.
Salamanca. Out of 84 samples tested, a prevalence of 55.95% (47/84) was obtained. V. vermiformis was the most predominant with 30.95% (26/84) (Figure 2). The genus Acanthamoeba was present in 14.29% of samples (12/84) and free-living amoebae of the family Vahlkampfiidae in 4.76% (4/84) (Figure 3). Other free-living amoebae were also identified in 8.33% of samples (7/84). Other FLA are those amoebae that are difficult to identify by the morphological approach. Salamanca showed higher diversity than the other provinces assessed. This large variety of FLA may be influenced by, among others, being the province with the largest number of samples.
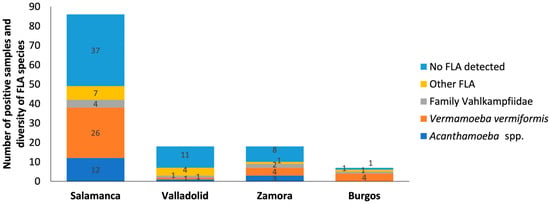
Figure 2.
Distribution of free-living amoebae isolated in water samples for each province. The x-axis shows the provinces such as Salamanca, Valladolid, Zamora and Burgos. And the y-axis represents the number of positive samples and diversity of FLA species in the water samples. No FLA detected indicates the absence of free-living amoebae in the samples analyzed. Other FLA are amoebae not identified. Some of the samples contain more than one amoeba species.
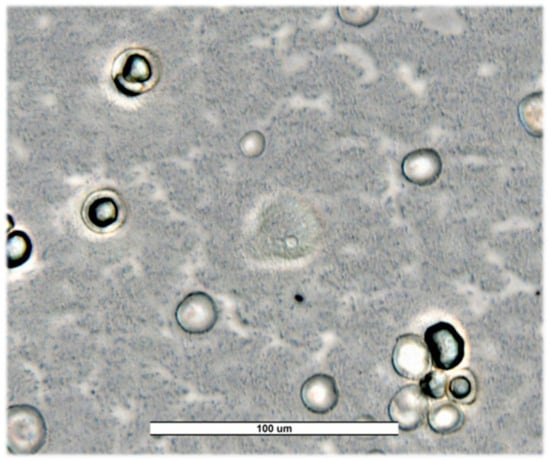
Figure 3.
Trophozoites and cysts of Naegleria americana from Salamanca’s river (SW23) for first sampling. Image was obtained with an ECHO Revolution hybrid microscope (40×). Scale bar represents 100 μM.
Valladolid. A prevalence of 38.89% (7/18) was obtained, with a predominance of other free-living amoebae (4/18; 22.22%), which could indicate the presence of less common species or the need for more advanced techniques for their identification (Figure 4). V. vermiformis, Acanthamoeba sp. T4 and Naegleria sp. were identified with a prevalence of 5.55% (1/18) for each of them.

Figure 4.
Trophozoites of Vannella spp. in a recreational fountain from Valladolid (VW5) for first sampling. Image was obtained with an ECHO Revolution hybrid microscope (40×). Scale bar represents 100 μM.
Zamora. A prevalence of 55.55% (10/18) was obtained. In this province, similar patterns to Salamanca were observed, V. vermiformis was the most commonly isolated species (4/18; 22.22%). Also, Acanthamoeba sp. T4 (3/18; 16.66%), V. avara (2/18; 11.11%) and other free-living amoebae (1/18; 5.55%) were found (Figure 5).
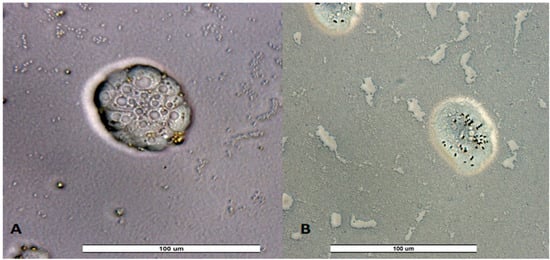
Figure 5.
Trophozoites of unidentified amoeba from a recreational fountain sample from Valladolid VW5 (A) and Zamora ZW4 (B) for third sampling. Image was obtained with an ECHO Revolution hybrid microscope (40×). Scale bar represents 100 μM.
Burgos. A prevalence of 83.33% (5/6) was obtained, with V. vermiformis species standing out (4/6; 66.66%). In addition, Vahlkampfia sp. (1/6; 16.66%) and other free-living amoebae (1/6; 16.66%) were identified. While there was hardly any diversity in this study area, the prevalence of V. vermiformis was high, suggesting favorable local conditions for the growth of this amoeba (Figure 6). Therefore, its presence in this study emphasizes its ability to survive in different environmental conditions, underlining its adaptability.
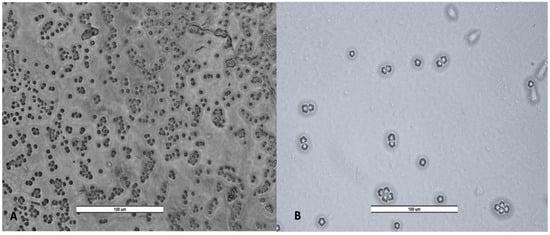
Figure 6.
Cyst of Vermamoeba vermiformis (A) and trophozoites and cyst of Acanthamoeba spp. (B). Image was obtained with a LEICA DM500 microscope (20×). Scale bar represents 100 μM.
3.2. Seasonal Variation
According to the season: Of the total of 126 samples distributed in 42 sampling points, 42 samples were taken for each of the stations (t1–t3), ensuring uniformity of the selected points and guaranteeing representativeness in each of the stations evaluated.
In the first sampling period (t1) a high prevalence and diversity of free-living amoebae was obtained in most provinces (24/42; 57.14%), with the species V. vermiformis (11/42) being the most abundant. However, the genus Acanthamoeba, species belonging to the family Vahlkampfiidae and other free-living amoebae were also found (Figure 7).
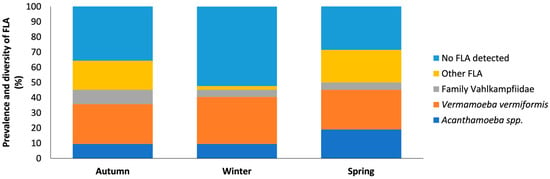
Figure 7.
Distribution of free-living amoebae isolated in water samples for each season. The x-axis shows the season (t1–t3). And the y-axis represents the prevalence and diversity of FLA in the water samples. No FLA detected indicates the absence of free-living amoebae in the samples analyzed. Other FLA are amoebae not identified.
The second sampling period (t2) showed a decrease in the prevalence and diversity of free-living amoebae (20/42; 47.61%). However, a higher abundance of V. vermiformis (13/42) than of the genus Acanthamoeba was observed. On the other hand, from the family Vahlkampfiidae only one species of the genus Vahlkampfia was identified: Vahlkampfia avara.
In the third sampling period (t3), prevalence and diversity were higher than in the rest of the samplings (25/42; 59.52%). It should be noted that V. vermiformis was the most frequently identified species as seen in the previous samplings (11/42). However, a variety of free-living amoebae were isolated, including the genus Acanthamoeba, the genus Vahlkampfia and Naegleria, and other FLA.
Thus, in the first and third sampling period, a higher prevalence and diversity of free-living amoebae was observed in all provinces, except Zamora. In this province, unlike the general pattern, a higher prevalence was recorded in the second sampling (t2), suggesting a variation in environmental conditions or local factors that favored the increase in these amoebae in that period. In conclusion, our data shows a clear seasonal variation in FLA in the samples from the same sites taken in different seasons.
3.3. Influence of Type of Water Source
According to type of water: The different types of water analyzed (rivers, recreational fountain, irrigation water, swimming pools, swamps and drinking water) showed diverse patterns of prevalence and richness/diversity of FLA.
Natural waters, as well as rivers and swamps, showed a remarkable prevalence of FLA; e.g., all river samples were positive for FLA culture (18/18; 100%), while from swamps only eight (8/12; 66.67%) exhibited a higher prevalence of V. vermiformis, Acanthamoeba spp., and less frequently, amoebae of the family Vahlkampfiidae (Figure 8).
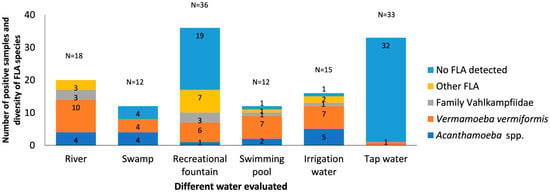
Figure 8.
Distribution of free-living amoebae isolated in water samples for type of water. The x-axis shows the different types of water samples collected in different provinces such as Salamanca, Valladolid, Zamora, and Burgos. And the y-axis represents the number of positive samples and diversity of FLA species in the water samples. No FLA detected indicates the absence of free-living amoebae in the samples analyzed. Other FLA are amoebae not identified. Some of the samples contain more than one amoeba species.
Recreational fountains and swimming pools classified as recreational waters show results that differ from each other. Recreational fountains showed a 47.22% prevalence of FLA (17/36; 47.22%), with V. vermiformis being the most prevalent, possibly due to their greater exposure to the environment. In contrast, swimming pools showed a high prevalence of FLA (11/12; 91.66%) but lower diversity, but V. vermiformis was consistently present, suggesting its adaptability to chlorinated environments and potential failures in disinfection systems.
The irrigation water presents a prevalence of 93.33% (14/15). It was an important reservoir of V. vermiformis and Acanthamoeba spp., especially in agricultural areas. This finding highlights the importance of monitoring these sources, given their direct contact with crops and their role in public health.
The detection of FLA in only one drinking water sample (1/33; 3.03%) supports the high efficacy of the treatment systems in the region.
The phylogenetic relationship of the FLA strains isolated in water samples is presented in Figure 9, Figure 10 and Figure 11, respectively.
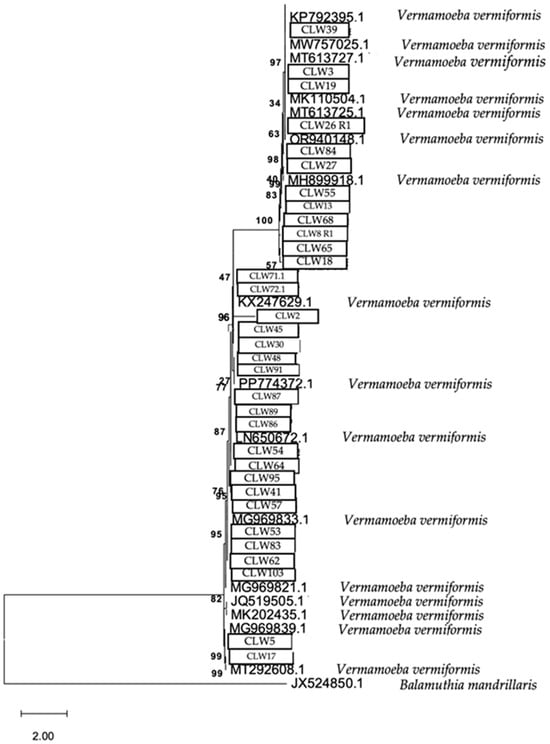
Figure 9.
Phylogenetic connections among the species Vermamoeba vermiformis obtained from water samples in this study. The isolates obtained in this study are marked within boxes. Other species sequences were obtained from GenBank and their accession numbers are shown. The tree is computed using the Maximum Likelihood method and measured in the number of substitutions per site. The tree is rooted with Balamuthia mandrillaris as the outgroup. The percentage of replicate trees, in which the associated taxa are clustered together in the bootstrap test, is shown next to the branches (in bold). Scale bar = 2.00 substitutions/site.
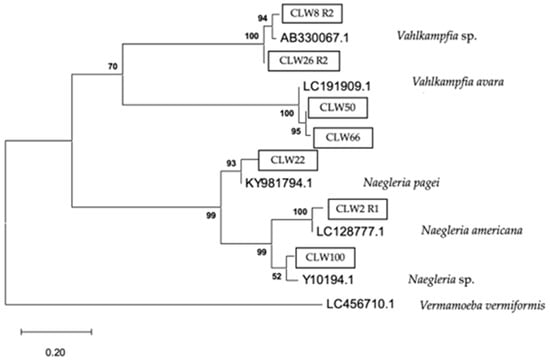
Figure 10.
Phylogenetic connections among the family Vahlkampfiidae obtained from water samples in this study. The isolates obtained in this study are marked within boxes. Other species sequences were obtained from GenBank and their accession numbers are shown. The tree is computed using the Maximum Likelihood method and measured in the number of substitutions per site. The tree is rooted with Vermamoeba vermiformis as the outgroup. The percentage of replicate trees, in which the associated taxa are clustered together in the bootstrap test, is shown next to the branches (in bold). Scale bar = 0.20 substitutions/site.
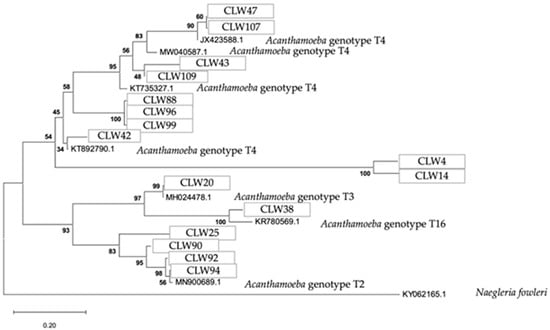
Figure 11.
Phylogenetic connections among the genus Acanthamoeba obtained from water samples in this study. The isolates obtained in this study are marked within boxes. Other species sequences were obtained from GenBank and their accession numbers are shown. The tree is computed using the Maximum Likelihood method and measured in the number of substitutions per site. The tree is rooted with Naegleria fowleri as the outgroup. The percentage of replicate trees, in which the associated taxa are clustered together in the bootstrap test, is shown next to the branches (in bold). Scale bar = 0.20 substitutions/site.
The analytical procedure of the tree of the species V. vermiformis encompassed 1.972 aligned positions in the final dataset (Figure 9).
The analytical procedure of the tree of the family Vahlkampfiidae encompassed 818 aligned positions in the final dataset (Figure 10).
The analytical procedure of the tree of the genus Acanthamoeba encompassed 2.558 aligned positions in the final dataset (Figure 11).
4. Discussion
This manuscript shows the prevalence of the potentially pathogenic free-living amoebae in water from human related environments from Castilla y León, the largest region of the Iberian Peninsula and one of the largest territories of the European Union. FLA are distributed worldwide; i.e., they are cosmopolitan in nature [34]. Considering that these FLA represent a risk to both the environment and human health, different authors have been reporting their presence in a multitude of environments. However, there are currently few environmental studies on FLA epidemiology in Spain, and most of them have focused on the Canary Islands, especially on the island of Tenerife, which has been the most sampled island over time, reporting V. vermiformis species, the genera Acanthamoeba and Naegleria [35,36,37,38]. Previously, Lorenzo-Morales et al. (2005) focused on demonstrating the presence of Acanthamoeba in waters of Tenerife Island [36]. However, a study was conducted in later years that expanded the search for free-living amoebae on the same island, finding a greater diversity of FLA, including Naegleria fultoni, Cercozoa spp. and Thecamoeba spp., which are rare [16]. Compared to our current study, it is worth highlighting that the prevalence of the species V. vermiformis in the water samples was high, in line with what was observed on the island of Tenerife, Canary Islands. V. vermiformis is a ubiquitous and thermotolerant amoeba that is among the most common free-living amoebae. It has demonstrated pathogenic potential and has also been associated with harmful bacteria [39,40]. Previous studies have also reported the identification of this species in artificial recreational aquatic environments [41,42]. Contrasting these studies, a greater diversity of FLA was found in Castilla y León, as can be seen in Section 3; for example, Vahlkampfia avara was isolated, having been noted as a causative agent of keratitis [43]. Although Acanthamoeba spp. are the primary cause of amoebic keratitis, there have been various reported cases of coinfections of Acanthamoeba and Vahlkampfia genera and/or Vahlkampfia-related corneal injury [44,45,46]. This could be due to the completely different climate than that of the Canary Islands. In addition, the significant influence of the type of sample, province and season is evaluated.
Likewise, a brief investigation has recently been conducted over the course of a year in drinking water treatment plants (DWTPs), wastewater treatment plants (WWTPs), and other environmental waters, including reservoirs and rivers in the central region of Spain, which is located in the continental Mediterranean climate zone [47]. In comparison with this study, a fundamental difference lies in the type of sampled sources: while the study by Magnet and colleagues focused on treated waters within the urban water supply and sanitation systems, the present study focused on natural, recreational, and irrigation waters, that is, open ecosystems with greater direct contact with the environment. Therefore, while Magnet et al. concentrated on assessing health risks associated with treated water distribution systems, the present work provides valuable information on community-level risks related to the use of water for recreation and agriculture, highlighting the need to extend surveillance measures beyond urban and potable systems, into rural and semi-urban environments.
On the other hand, both studies agree in identifying the genus Acanthamoeba, particularly the T4 genotype, as one of the most abundant groups, reflecting its global prevalence and recognized pathogenic potential. Acanthamoeba is the genus best known to produce pathology in humans and it is also the one most commonly found in the environment [4]. This is relevant because of its association with serious human pathologies such as cerebral infections like granulomatous amoebic encephalitis (GAE), Acanthamoeba keratitis (AK), and less frequently, skin lesions [48,49].
Moreover, Magnet et al. observed seasonal variation, with a higher presence of FLA in spring and summer, which is consistent with the pattern observed in Castilla y León (maximum prevalence in spring and autumn). This seasonal behavior is related to optimal temperature conditions and nutrient availability that favor the trophic phase of amoebae.
Thus, the present work is the first to investigate these potential protozoa in the central north of the Iberian Peninsula (Autonomous Community of Castilla y León), providing new data that enrich the understanding of the biodiversity of these amoebae in aquatic environments in Spain. Moreover, our results contribute to the growing body of evidence supporting the need for continuous surveillance and standardized protocols for the detection of FLA, which are essential for the prevention of public health risks.
In this context, it is crucial to implement preventive measures aimed at reducing human exposure to these potentially pathogenic amoebae. Among the most important recommendations are the proper maintenance and disinfection of drinking water systems and swimming pools, strict control of water treatment conditions including the effective use of disinfectants such as chlorine and environmental monitoring to detect the presence of FLA at an early stage. Additionally, the public should be informed about the risks associated with contact with untreated or poorly maintained water, especially among vulnerable groups such as immunocompromised individuals.
Finally, considering that some FLA species can resist conventional treatments and proliferate in hospital and domestic environments, the implementation of specific management and control protocols in these settings is also a priority. The integration of molecular techniques alongside culture-based methods in environmental surveillance programs may enhance the detection and characterization of these amoebae, thus facilitating more informed decision-making in public health [41,50,51].
As mentioned, the results obtained in this study show great heterogeneity in the prevalence and diversity of FLA according on each province of Castilla y León, the sampling period, and the type of water. The lack of previous studies in the autonomous community of Castilla y León limits the comparison of historical data. However, the wide distribution of FLA evidences the need for more detailed investigations in other regions with similar climatic and geographical characteristics. Thus, the analysis by province revealed remarkable variations in the diversity and prevalence of FLA. On the other hand, the different types of water analyzed (rivers, recreational fountains, irrigation water, swimming pools, swamps and drinking water) showed diverse patterns of prevalence and richness of FLA, considering that natural waters, as well as rivers and swamps, showed a remarkable diversity of FLA, with a higher incidence of V. vermiformis, Acanthamoeba spp., and less frequently, amoebae of the family Vahlkampfiidae. High exposure to pollutants and environmental variations favors the proliferation of these species, especially during autumn and spring when climatic conditions are more moderate. In contrast, FLA were detected in just one drinking water sample, suggesting that the vast majority were negative and highlighting the efficiency of local treatment systems. However, past studies have indicated that certain FLA can survive the control of drinking water treatment plants, so continued monitoring is recommended [47,51].
Considering that the sampling was performed in three different periods (t1-t3), it was possible to identify seasonal patterns in the prevalence of FLA. On the one hand, the autumn and spring sampling period were the seasons with the highest prevalence of FLA. During these samplings, climatic conditions (moderate temperatures and higher humidity) are ideal for the growth of amoebae [52] as is the case with some groups of parasites [53]. In contrast, the second sampling showed a lower overall detection of FLA, which is attributed to lower temperatures that hinder trophozoite activity and favor their cyst state. These data corroborate/agree with studies described by Magnet et al. and Vingataramin et al. which show a similar seasonal pattern with peaks in autumn and spring [47,54]. There is even a report from a subtropical climate zone, Tulsa (Oklahoma, USA), that reported seasonality in the prevalence of Acanthamoeba in ponds in the United States [55].
According to the Köppen classification, the autonomous community of Castilla y León has a continental Mediterranean climate with certain subclimates in specific provinces due to geographical factors such as altitude and hydrography. This is a key factor in the distribution of free-living amoebae. Given that in temperate summer climates (Csb) established in the provinces of Salamanca, Zamora, and Burgos, the diversity of free-living amoebae, including V. vermiformis, Acanthamoeba spp. and genera of the family Vahlkampfiidae, is abundant. Also, the presence of large rivers, e.g., the Tormes River in Salamanca or the Duero River in Zamora, generates more humid microclimates that favor the persistence of FLA. In contrast, in more extreme climates such as BSk, which mainly occurs in Valladolid, there is a low diversity of FLA with a predominance of species resistant to environmental stress conditions such as Acanthamoeba spp. since low winter temperatures and lower humidity reduce trophozoite activity and favor the formation of resistant cysts.
Overall, the results of this study show the influence of the climate of each province, according to the Köppen classification, and the type of water on the distribution and abundance of FLA in Castilla y León. The combination of temperate and more extreme climates within the region determines the diversity and adaptability of these amoebae, evidencing the need to implement continuous surveillance and control measures, especially in recreational and agricultural waters, to protect public health against emerging pathogens such as Acanthamoeba spp. and V. vermiformis.
It is essential to emphasize that the results obtained in this study do not depend exclusively on environmental or seasonal factors, but are deeply influenced by several methodological aspects, such as sampling design, isolation protocol, incubation temperature used during primary culture, and strain selection procedure. These factors can significantly condition the recovery of FLA, favoring especially those species that show a higher growth capacity under laboratory conditions, or that are present in the sample at higher concentrations. In particular, the incubation temperature is decisive for the activation of the trophic state of the amoebae, which directly influences the success of the culture and, therefore, the detection of the species present.
5. Conclusions
This study provides evidence of the environmental of FLA in Castilla y León, highlighting the importance of implementing epidemiological surveillance strategies in susceptible water sources. Given the geographical and seasonal variability of FLA, it is essential to develop specific control policies for each type of water source, with special attention to recreational and agricultural waters, to reduce the risks associated with these potentially pathogenic protozoa.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14070637/s1, Table S1: Additional details, results, including tables showing the presence of free-living amoebae in each of the water samples from Castilla y León for the three sampling periods.
Author Contributions
Conceptualization, R.M. and J.L.-M.; Methodology, P.P.-P. and I.R.-E.; Software, P.P.-P.; Validation, R.M. and J.L.-M.; Formal Analysis, P.P.-P.; Investigation, P.P.-P. and I.R.-E.; Resources, J.E.P., R.M. and J.L.-M.; Data Curation, P.P.-P.; Writing—Original Draft Preparation, P.P.-P.; Writing—Review and Editing, R.M. and J.L.-M.; Visualization, J.E.P., R.M. and J.L.-M.; Supervision, R.M. and J.L.-M.; Project Administration, J.E.P., R.M. and J.L.-M.; Funding Acquisition, J.E.P., R.M. and J.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the Consorcio Centro de Investigación Biomédica (CIBER) de Enfermedades Infecciosas (CIBERINFEC); Instituto de Salud Carlos III, 28006 Madrid, Spain (CB21/13/00100); Project 2022CLISA26 from Fundación Cajacanarias y Fundación Bancaria la Caixa. P.P-P. (TESIS2021010070) was funded by a grant from the Agencia Canaria de Investigación, Innovación y Sociedad de la Información de la Consejería de Economía, Conocimiento y Empleo, by Fondo Social Europeo (FSE) Programa Operativo Integrado de Canarias 2014-2020, Eje 3 Tema Prioritario 74 (85%); Cabildo Insular de Tenerife 2023–2028 (Proyecto CC20230222, CABILDO.23), and Ministerio de Sanidad, Spain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schuster, F.L.; Visvesvara, G.S. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef]
- Król-Turmińska, K.; Olender, A. Human infections caused by free-living amoebae. Ann. Agric. Environ. Med. 2017, 24, 254–260. [Google Scholar] [CrossRef]
- Vaerewijck, M.J.M.; Baré, J.; Lambrecht, E.; Sabbe, K.; Houf, K. Interactions of Foodborne Pathogens with Free-living Protozoa: Potential Consequences for Food Safety. Compr. Rev. Food Sci. Food Saf. 2014, 13, 924–944. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef]
- Trabelsi, H.; Dendana, F.; Sellami, A.; Sellami, H.; Cheikhrouhou, F.; Neji, S.; Makni, F.; Ayadi, A. Pathogenic free-living amoebae: Epidemiology and clinical review. Pathol. Biol. 2012, 60, 399–405. [Google Scholar] [CrossRef]
- Hara, T.; Yagita, K.; Sugita, Y. Pathogenic free-living amoebic encephalitis in Japan. Neuropathology 2019, 39, 251–258. [Google Scholar] [CrossRef]
- Pana, A.; Vijayan, V.; Anilkumar, A.C. Amebic Meningoencephalitis. 2023. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Pinna, A.; Porcu, T.; Boscia, F.; Cano, A.; Erre, G.; Mattana, A. Free-Living Amoebae Keratitis. Cornea 2017, 36, 785–790. [Google Scholar] [CrossRef]
- Scheid, P.L.; Lâm, T.T.; Sinsch, U.; Balczun, C. Vermamoeba vermiformis as etiological agent of a painful ulcer close to the eye. Parasitol. Res. 2019, 118, 1999–2004. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Delafont, V.; Rodier, M.H.; Cateau, E.; Héchard, Y. Free-living amoebae and squatters in the wild: Ecological and molecular features. FEMS Microbiol. Rev. 2019, 43, 415–434. [Google Scholar] [CrossRef]
- Balczun, C.; Scheid, P.L. Free-Living Amoebae as Hosts for and Vectors of Intracellular Microorganisms with Public Health Significance. Viruses 2017, 9, 65. [Google Scholar] [CrossRef]
- Mella, C.; Medina, G.; Flores-Martin, S.; Toledo, Z.; Simaluiza, R.J.; Pérez-Pérez, G.; Fernández, H. Interaction between zoonotic bacteria and free-living amoebas: A new angle of an epidemiological polyhedron of public health importance? Arch. Med. Vet. 2016, 48, 1–10. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Todd, C.D.; Martín-Navarro, C.M.; López-Arencibia, A.; Cabello-Vilchez, A.M.; González, A.C.; Córdoba-Lanús, E.; Lindo, J.F.; Valladares, B.; Piñero, J.E.; et al. Isolation and characterization of Acanthamoeba strains from soil samples in Gran Canaria, Canary Islands, Spain. Parasitol. Res. 2014, 113, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Batlle, M.; Rizo-Liendo, A.; Viera-Santana, R.A.; Afonso-Morales, S.; López-Arencibia, A.; Sifaoui, I.; Chiboub, O.; Bethencourt-Estrella, C.J.; Nicolás-Hernández, D.S.; Rodríguez-Expósito, R.L.; et al. Isolation and Molecular Identification of Naegleria australiensis in Irrigation Water of Fuerteventura Island, Spain. Acta Parasitol. 2019, 64, 331–335. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Díaz, F.J.; Sifaoui, I.; Rodríguez-Expósito, R.; Rizo-Liendo, A.; Piñero, J.E.; Lorenzo-Morales, J. Free living amoebae isolation in irrigation waters and soils of an insular arid agroecosystem. Sci. Total Environ. 2021, 753, 141833. [Google Scholar] [CrossRef]
- Pérez-Pérez, P.; Reyes-Batlle, M.; Morchón, R.; Piñero, J.E.; Lorenzo-Morales, J. Isolation and molecular identification of pathogenic free-living amoebae from environmental samples in Tenerife, Canary Islands, Spain. ACS Est. Water. 2025, 5, 2861–2889. [Google Scholar] [CrossRef]
- Benito, M.; LaPlante, D.; Fernández, M.T.; Miguel, N.; Lasheras, A.M.; Gómez, J.; Ormad, M.P.; Rubio, E.; Goñi, M.P. Amebas de vida libre en aguas residuales y fangos: Su papel como reservorio natural de bacterias potencialmente patogénas. Rev. Esp. Saúde Ambient. 2018, 18, 69–77. Available online: https://ojs.diffundit.com/index.php/rsa/article/view/892 (accessed on 19 November 2024).
- Magnet, A.; Fenoy, S.; Galván, A.L.; Izquierdo, F.; Rueda, C.; Fernandez Vadillo, C.; Del Aguila, C. A year long study of the presence of free-living amoeba in Spain. Water Res. 2013, 47, 6966–6972. [Google Scholar] [CrossRef]
- Lares-Villa, F.; Hernández-Peña, C. Concentration of Naegleria fowleri in natural waters used for recreational purposes in Sonora, Mexico (November 2007–October 2008). Exp. Parasitol. 2010, 126, 33–36. [Google Scholar] [CrossRef]
- Cabanes, P.A.; Wallet, F.; Pringuez, E.; Pernin, P. Assessing the risk of primary amoebic meningoencephalitis from swimming in the presence of environmental Naegleria fowleri. Appl. Environ. Microbiol. 2001, 67, 2927–2931. [Google Scholar] [CrossRef]
- Agencia Estatal de Meteorología, Ministerio de Agricultura, Alimentación y Medio Ambiente. Iberian Climate Atlas. Air Temperature and Precipitation (1971–2000); Agencia Estatal de Meteorología: Madrid, Spain, 2021.
- Research Innovation Strategy for Smart Specialisation (RIS3) of Castilla y León 2014–2020. 2021. Available online: https://fuescyl.com/images/03innovacion_conocimiento/Comisionado/RIS3_Castilla_y_Leon_2014-2020_(eng).pdf (accessed on 13 December 2024).
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen–Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Page, F.C. A New Key to Fresh Water and Soil Gymnamoebae; Freshwater Biological Association: Cumbria, UK, 1988; 122p. [Google Scholar]
- Reyes-Batlle, M.; Zamora-Herrera, J.; Vargas-Mesa, A.; Valerón-Tejera, M.A.; Wagner, C.; Martín-Navarro, C.M.; López-Arencibia, A.; Sifaoui, I.; Martínez-Carretero, E.; Valladares, B.; et al. Acanthamoeba genotypes T2, T4, and T11 in soil sources from El Hierro island, Canary Islands, Spain. Parasitol. Res. 2016, 115, 2953–2956. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, N.; Schild, M.; Panaiotov, S.; Kurdova-Mintcheva, R.; Gottstein, B.; Walochnik, J.; Aspöck, H.; Lucas, M.S.; Müller, N. The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol. Res. 2004, 92, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.M.; Booton, G.C.; Hay, J. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 2001, 39, 1903–1911. [Google Scholar] [CrossRef]
- Kuiper, M.W.; Valster, R.M.; Wullings, B.A.; Boonstra, H.; Smidt, H.; van der Kooij, D. Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time, P.C.R. Appl. Environ. Microbiol. 2006, 72, 5750–5756. [Google Scholar] [CrossRef]
- Córdoba-Lanús, E.; Reyes-Batlle, M.; Domínguez-de-Barros, A.; Pérez-Pérez, P.; Expósito-Rodríguez, R.L.; García-Ramos, A.; Sifaoui, I.; García-Pérez, O.; Aneiros-Giraldez, G.; Piñero, J.E.; et al. Multiplex qPCR assay to detect Acanthamoeba spp., Vermamoeba vermiformis, Naegleria fowleri and Balamuthia mandrillaris in different water sources. Am. J. Trop. Med. Hyg. 2024, 111, 785–790. [Google Scholar] [CrossRef]
- De Jonckheere, J.F.; Brown, S. The identification of vahlkampfiid amoebae by ITS sequencing. Protist 2005, 156, 89–96. [Google Scholar] [CrossRef]
- Kazutaka, K.; Standley, D.M. Standley, MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Rodríguez-Zaragoza, S. Ecology of free-living amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Niyyati, M.; Martín-Navarro, C.M.; López-Arencibia, A.; Valladares, B.; Martínez-Carretero, E.; Piñero, J.E.; Lorenzo-Morales, J. Unusual Vermamoeba Vermiformis Strain Isolated from Snow in Mount Teide, Tenerife, Canary Islands, Spain. Nov. Biomed. 2015, 3, 189–192. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Ortega-Rivas, A.; Foronda, P.; Martínez, E.; Valladares, B. Isolation and identification of pathogenic Acanthamoeba strains in Tenerife, Canary Islands, Spain from water sources. Parasitol. Res. 2005, 95, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Monteverde-Miranda, C.A.; Jiménez, C.; Tejedor, M.L.; Valladares, B.; Ortega-Rivas, A. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann. Agric. Environ. Med. 2005, 12, 233–236. [Google Scholar]
- Reyes-Batlle, M.; Wagner, C.; López-Arencibia, A.; Sifaoui, I.; Martínez-Carretero, E.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Isolation and molecular characterization of a Naegleria strain from a recreational water fountain in Tenerife, Canary Islands, Spain. Acta Parasitol. 2017, 62, 265–268. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Khan, N.A. The Increasing Importance of Vermamoeba vermiformis. J. Eukaryot. Microbiol. 2021, 68, e12857. [Google Scholar] [CrossRef]
- Delafont, V.; Rodier, M.H.; Maisonneuve, E.; Cateau, E. Vermamoeba vermiformis: A Free-Living Amoeba of Interest. Microbial. Ecol. 2018, 76, 991–1001. [Google Scholar] [CrossRef]
- Nazar, M.; Haghighi, A.; Taghipour, N.; Ortega-Rivas, A.; Tahvildar-Biderouni, F.; Mojarad, E.N.; Eftekhar, M. Molecular identification of Hartmannella vermiformis and Vannella persistens from man-made recreational water environments, Tehran, Iran. Parasitol. Res. 2012, 111, 835–839. [Google Scholar] [CrossRef]
- Armand, B.; Motazedian, M.H.; Asgari, Q. Isolation and identification of pathogenic free-living amoeba from surface and tap water of Shiraz City using morphological and molecular methods. Parasitol. Res. 2016, 115, 63–68. [Google Scholar] [CrossRef]
- Moran, S.; Mooney, R.; Henriquez, F.L. Diagnostic Considerations for Non-Acanthamoeba Amoebic Keratitis and Clinical Outcomes. Pathogens 2022, 11, 219. [Google Scholar] [CrossRef]
- Niyyati, M.; Lorenzo-Morales, J.; Rezaie, S.; Rahimi, F.; Martín-Navarro, C.M.; Mohebali, M.; Maghsood, A.M.; Farnia, S.; Valladares, B.; Rezaeian, M. First report of a mixedinfection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp. Parasitol. 2010, 126, 89–90. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Lorenzo-Morales, J.; Irigoyen, C.; Morcillo-Laiz, R.; López-Vélez, R.; Muñoz-Negrete, F.; Piñero, J.E.; Valladares, B. Co-isolation of Vahlkampfia and Acanthamoeba in acanthamoeba-like keratitis in a Spanish population. Cornea 2013, 32, 608–614. [Google Scholar] [CrossRef]
- Alexandrakis, G.; Miller, D.; Huang, A.J. Amebic keratitis due to Vahlkampfia infection following corneal trauma. Arch. Ophthalmol. 1998, 116, 950–951. [Google Scholar]
- Magnet, A.; Galván, A.L.; Fenoy, S.; Izquierdo, F.; Rueda, C.; Fernandez-Vadillo, C.; Pérez-Irezábal, J.; Bandyopadhyay, K.; Visvesvara, G.S.; da Silva, A.J.; et al. Molecular characterization of Acanthamoeba isolated in water treatment plants and comparison with clinical isolates. Parasitol. Res. 2012, 111, 383–392. [Google Scholar] [CrossRef]
- Booton, G.C.; Visvesvara, G.S.; Byers, T.J.; Kelly, D.J.; Fuerst, P.A. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 2005, 43, 1689–1693. [Google Scholar] [CrossRef]
- Ledee, D.R.; Iovieno, A.; Miller, D.; Mandal, N.; Diaz, M.; Fell, J.; Fini, M.E.; Alfonso, E.C. Molecular identification of T4 and T5 genotypes in isolates from acanthamoeba keratitis patients. J. Clin. Microbiol. 2009, 47, 1458–1462. [Google Scholar] [CrossRef]
- World Health Organization. Volume 2: Swimming pools and similar environments. In Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Corsaro, D.; Pages, G.S.; Catalan, V.; Loret, J.F.; Greub, G. Biodiversity of amoebae and amoeba-associated bacteria in water treatment plants. Int. J. Hyg. Environ. Health 2010, 213, 158–166. [Google Scholar] [CrossRef]
- Resumen anual del clima en España 2023. Available online: https://www.aemet.es/documentos/es/serviciosclimaticos/vigilancia_clima/resumenes_climat/anuales/res_anual_clim_2023.pdf (accessed on 16 December 2024).
- Bryant, A.S.; Hallem, E.A. Temperature-dependent behaviors of parasitic helminths. Neurosci. Lett. 2018, 687, 290–303. [Google Scholar] [CrossRef]
- Vingataramin, Y.; Quétel, I.; Pons, M.A.; Talarmin, A.; Marcelino, I. Spatiotemporal distribution of thermophilic free-living amoebae in recreational waters: A 5-year survey in Guadeloupe (French West Indies). Sci. Total Environ. 2024, 941, 173318. [Google Scholar] [CrossRef]
- John, D.T.; Howard, M.J. Seasonal distribution of pathogenic free-living amebae in Oklahoma waters. Parasitol. Res. 1995, 81, 193–201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).