CD4/CD8 Ratio Increase in Female Living with HIV Switching to Cabotegravir-Rilpivirine: A Real-Life 24 Weeks Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WLWH | Women living with HIV |
| PLWH | People living with HIV |
| SHiNe-SHiC | Sardinian HIV Network and Sicilian HIV Cohort |
| ART | Antiretroviral therapy |
| CVF | Confirmed virologic failure |
| LA | Long-acting |
| ISR | Injection site reaction |
| TND | Target not detected |
| DTG | Dolutegravir |
| CAB/RPV | Cabotegravir/rilpivirine |
| TAF | Tenofovir alafenimide |
| BIC | Bictegravir |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| BL | Baseline |
References
- UNAIDS. UNAIDS Data 2022. Available online: https://www.unaids.org/en/resources/documents/2023/2022_unaids_data (accessed on 20 May 2025).
- WHO. Global HIV Programme. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 17 May 2025).
- Mody, A.; Sohn, A.H.; Iwuji, C.; Tan, R.K.J.; Venter, F.; Geng, E.H. HIV Epidemiology, Prevention, Treatment, and Implementation Strategies for Public Health. Lancet 2024, 403, 471–492. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Fact Sheet 2024—Latest Global and Regional HIV Statistics on the Status of the AIDS Epidemic; UNAIDS: Geneva, Switzerland, 2024. [Google Scholar]
- Raffe, S.; Sabin, C.; Gilleece, Y. Comorbidities in Women Living with HIV: A Systematic Review. HIV Med. 2022, 23, 331–361. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, M.R.; Sherr, L.; Sonnenberg-Schwan, U.; Walmsley, S.L.; Johnson, M.; d’Arminio Monforte, A. Caring for Women Living with HIV: Gaps in the Evidence. J. Int. AIDS Soc. 2013, 16. [Google Scholar] [CrossRef]
- UNAIDS. HIV and Adolescent Girls and Young Women 2023 Global Aids Update Factsheet; UNAIDS: Geneva, Switzerland, 2023; ISBN 9789231004810. [Google Scholar]
- Liu, K.A.; DiPietro Mager, N.A. Women’s Involvement in Clinical Trials: Historical Perspective and Future Implications. Pharm. Pract. 2016, 14, 708. [Google Scholar] [CrossRef]
- UNAIDS. Women and Girls and HIV. Available online: https://www.unaids.org/sites/default/files/media_asset/women_girls_hiv_en.pdf (accessed on 17 May 2025).
- Ceccarelli, M.; Venanzi Rullo, E.; Marino, M.A.; d’Aleo, F.; Pellicanò, G.F.; D’Andrea, F.; Marino, A.; Cacopardo, B.; Celesia, B.M.; La Rocca, G.; et al. Non-AIDS Defining Cancers: A Comprehensive Update on Diagnosis and Management. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3849–3875. [Google Scholar] [CrossRef]
- D’andrea, F.; Ceccarelli, M.; Venanzi Rullo, E.; Facciolà, A.; D’aleo, F.; Cacopardo, B.; Iacobello, C.; Costa, A.; Altavilla, G.; Pellicanò, G.F.; et al. Cancer Screening in HIV-Infected Patients: Early Diagnosis in a High-Risk Population. World Cancer Res. J. 2018, 5, e1130. [Google Scholar] [CrossRef]
- Curno, M.J.; Rossi, S.; Hodges-Mameletzis, I.; Johnston, R.; Price, M.A.; Heidari, S. A Systematic Review of the Inclusion (or Exclusion) of Women in HIV Research. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 71, 181–188. [Google Scholar] [CrossRef]
- Colpani, A.; De Vito, A.; Marino, A.; Ceccarelli, M.; Celesia, B.M.; Conti, G.N.; Spampinato, S.; Moi, G.; Venanzi Rullo, E.; Pellicanò, G.F.; et al. Viro-Immunological Efficacy and Safety of Bictegravir/Emtricitabine/Tenofovir Alafenamide among Women Living with HIV: A 96-Week Post-Switch Analysis from the Real-Life SHiNe-SHiC Cohort. Biomedicines 2024, 12, 2311. [Google Scholar] [CrossRef]
- Spampinato, S.; Conti, G.; Marino, A.; Raimondo, V.; Celesia, B.; Pellicanò, G.; Puci, M.; Sotgiu, G.; Bruno, R.; Villari, N.; et al. Enhanced Metabolic Health and Immune Response with Bictegravir/Emtricitabine/TAF: Insights from a 96-week Retrospective Study. Biomed. Rep. 2024, 21, 179. [Google Scholar] [CrossRef]
- De Vito, A.; Caruana, G.; Clark, F.; Nunnari, G.; Pellicanò, G.F.; Angioni, G.; Freedman, A.; Babudieri, S.; Madeddu, G. Efficacy, Safety and Tolerability of Dolutegravir-Based Combination Antiretroviral Therapy in Clinical Practice in HIV-Infected Patients: Results from a Multicenter Study. Infect. Dis. Trop. Med. 2019, 5, e565. [Google Scholar] [CrossRef]
- Paternò Raddusa, M.S.; Marino, A.; Celesia, B.M.; Spampinato, S.; Giarratana, C.; Venanzi Rullo, E.; Cacopardo, B.; Nunnari, G. Atherosclerosis and Cardiovascular Complications in People Living with HIV: A Focused Review. Infect. Dis. Rep. 2024, 16, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Swindells, S.; Andrade-Villanueva, J.-F.; Richmond, G.J.; Rizzardini, G.; Baumgarten, A.; Masiá, M.; Latiff, G.; Pokrovsky, V.; Bredeek, F.; Smith, G.; et al. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression. N. Engl. J. Med. 2020, 382, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Swindells, S.; Lutz, T.; Van Zyl, L.; Porteiro, N.; Stoll, M.; Mitha, E.; Shon, A.; Benn, P.; Huang, J.O.; Harrington, C.M.; et al. Week 96 Extension Results of a Phase 3 Study Evaluating Long-Acting Cabotegravir with Rilpivirine for HIV-1 Treatment. AIDS 2022, 36, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.T.; Richmond, G.; Rizzardini, G.; Thalme, A.; Girard, P.M.; Wong, A.; Porteiro, N.; Swindells, S.; Reynes, J.; Noe, S.; et al. Long-Acting Cabotegravir and Rilpivirine Dosed Every 2 Months in Adults With Human Immunodeficiency Virus 1 Type 1 Infection: 152-Week Results From ATLAS-2M, a Randomized, Open-Label, Phase 3b, Noninferiority Study. Clin. Infect. Dis. 2023, 76, 1646–1654. [Google Scholar] [CrossRef]
- Overton, E.T.; Richmond, G.; Rizzardini, G.; Jaeger, H.; Orrell, C.; Nagimova, F.; Bredeek, F.; García Deltoro, M.; Swindells, S.; Andrade-Villanueva, J.F.; et al. Long-Acting Cabotegravir and Rilpivirine Dosed Every 2 Months in Adults with HIV-1 Infection (ATLAS-2M), 48-Week Results: A Randomised, Multicentre, Open-Label, Phase 3b, Non-Inferiority Study. Lancet 2020, 396, 1994–2005. [Google Scholar] [CrossRef]
- Jaeger, H.; Overton, E.T.; Richmond, G.; Rizzardini, G.; Andrade-Villanueva, J.F.; Mngqibisa, R.; Hermida, A.O.; Thalme, A.; Belonosova, E.; Ajana, F.; et al. Long-Acting Cabotegravir and Rilpivirine Dosed Every 2 Months in Adults with HIV-1 Infection (ATLAS-2M), 96-Week Results: A Randomised, Multicentre, Open-Label, Phase 3b, Non-Inferiority Study. Lancet HIV 2021, 8, e679–e689. [Google Scholar] [CrossRef]
- Orkin, C.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Overton, E.T.; Girard, P.-M.; Oka, S.; Walmsley, S.; Bettacchi, C.; Brinson, C.; et al. Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection. N. Engl. J. Med. 2020, 382, 1124–1135. [Google Scholar] [CrossRef]
- Orkin, C.; Oka, S.; Philibert, P.; Brinson, C.; Bassa, A.; Gusev, D.; Degen, O.; García, J.G.; Morell, E.B.; Tan, D.H.S.; et al. Long-Acting Cabotegravir plus Rilpivirine for Treatment in Adults with HIV-1 Infection: 96-Week Results of the Randomised, Open-Label, Phase 3 FLAIR Study. Lancet HIV 2021, 8, e185–e196. [Google Scholar] [CrossRef]
- Orkin, C.; Bernal Morell, E.; Tan, D.H.S.; Katner, H.; Stellbrink, H.-J.; Belonosova, E.; DeMoor, R.; Griffith, S.; Thiagarajah, S.; Van Solingen-Ristea, R.; et al. Initiation of Long-Acting Cabotegravir plus Rilpivirine as Direct-to-Injection or with an Oral Lead-in in Adults with HIV-1 Infection: Week 124 Results of the Open-Label Phase 3 FLAIR Study. Lancet HIV 2021, 8, e668–e678. [Google Scholar] [CrossRef]
- Iannone, V.; Rossotti, R.; Bana, N.B.; Cavazza, G.; D’Amico, F.; Lombardi, F.; Salvo, P.F.; Baldin, G.; Di Giambenedetto, S.; Bernacchia, D.; et al. Unconventional Use of Injectable Long-Acting Cabotegravir and Rilpivirine against HIV-1 in PWH in Clinical Need: 52 Weeks Real-World Data. BMC Infect. Dis. 2025, 25, 105. [Google Scholar] [CrossRef]
- Iannone, V.; Lombardi, F.; Ciccullo, A.; Lamanna, F.; Salvo, P.F.; Sanfilippo, A.; Baldin, G.; Borghetti, A.; Torti, C.; Di Giambenedetto, S. Real World Data from an Italian Outpatient Clinical Setting and from Home Care Assistance of Treatment-Experienced PWH Switching to CAB + RPV Regimen: A Prospective Observational Study. AIDS Behav. 2025, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Rosas Cancio-Suárez, M.; Moreno, A.; Del Campo Terrón, S.; Vivancos, M.J.; García-Ruiz De Morales, A.G.; Martínez-Sanz, J.; Ron, R.; Sánchez-Izquierdo, S.; Vélez-Díaz-Pallarés, M.; Moreno, S.; et al. Real-World Efficacy and Tolerability of CAB+RPV LA in Women: Addressing the Gender Gap in HIV Treatment Research. J. Antimicrob. Chemother. 2025, 80, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.H.S.; Antinori, A.; Eu, B.; Galindo Puerto, M.J.; Kinder, C.; Sweet, D.; Van Dam, C.N.; Sutton, K.; Sutherland-Phillips, D.; Berni, A.; et al. Weight and Metabolic Changes With Long-Acting Cabotegravir and Rilpivirine or Bictegravir/Emtricitabine/Tenofovir Alafenamide. JAIDS J. Acquir. Immune Defic. Syndr. 2025, 98, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.D.; Gistand, N.; Grochowski, J.; Mayorga-Munoz, F.; Imbert, E.; Szumowski, J.D.; Oskarsson, J.; Shiels, M.; Dilworth, S.; Appa, A.; et al. Viral Suppression Rates at 48 Weeks in People With HIV Starting Long-Acting Cabotegravir/Rilpivirine With Initial Viremia. Clin. Infect. Dis. 2025, 80, 864–870. [Google Scholar] [CrossRef]
- Muccini, C.; Gianotti, N.; Lolatto, R.; Nozza, S.; Diotallevi, S.; Castagna, A. CD4+/CD8+ Improvement after Switch from a Second-Generation Integrase Inhibitor Regimen to Long-Acting Cabotegravir and Rilpivirine. AIDS 2024, 38, 1890–1892. [Google Scholar] [CrossRef]
- Elliot, E.R.; Polli, J.W.; Patel, P.; Garside, L.; Grove, R.; Barnett, V.; Roberts, J.; Byrapuneni, S.; Crauwels, H.; Ford, S.L.; et al. Efficacy, Safety, and Pharmacokinetics by Body Mass Index Category in Phase 3/3b Long-Acting Cabotegravir Plus Rilpivirine Trials. J. Infect. Dis. 2024, 230, e34–e42. [Google Scholar] [CrossRef]
- Spampinato, S.; Spampinato, S.; Saia, A.G.; Russotto, Y.; Micali, C.; Marino, A.; Ceccarelli, M.; Venanzi Rullo, E.; Pellicanò, G.F.; Nunnari, G. Real-Life Efficacy and Satisfaction of Long-Acting ART Cabotegravir-Rilpivirine in HIV-Infected Individuals. Infect. Dis. Trop. Med. 2023, 9, e1207. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Age, median (IQR) | 50 (36–57) |
| Ethnicity (n,%) | |
| Caucasian | 50 (92.6%) |
| African | 3 (5.6%) |
| Latin American | 1 (1.8%) |
| Years since HIV onset (Median, IQR) | 17.5 (6.75–29) |
| CD4 Nadir (Median, IQR) | 258 (120–500) |
| Zenith VL (Median, IQR) | 64,440 (8851–244,000) |
| Transmission Route (n, %) | |
| Sexual | 49 (90.74%) |
| Injection drug user (IDU) | 5 (9.26%) |
| Smokers (n,%) | 19 (35.18%) |
| Alcohol use (n,%) | 14 (25.93%) |
| Comorbidities (n,%) | |
| Hypertension | 9 (16.36%) |
| Dyslipidemia | 15 (27.27%) |
| Cancer | 1 (1.81%) |
| Diabetes | 3 (5.45%) |
| Anxiety | 6 (10.91%) |
| Steatosis | 2 (3.63%) |
| Previous Regimens (n,%) | |
| DTG-based 2DR | 38 (70.37%) |
| DTG-based 3DR | 3 (5.55%) |
| TAF-based | 9 (16.67%) |
| Doravirine-based | 4 (7.41%) |
| Parameter | Value at BL | Value at 24 w |
|---|---|---|
| WBC/mm3, Median (IQR), | 6800 (5658–7775) | 6900 (5543–7650) |
| Haemoglobin, g/dL, Mean (±SD) | 13 (±1.2) | 13 (±1.1) |
| Total cholesterol, mg/dL, Mean (±SD) | 207 (±44) | 194 (±35) |
| HDL Cholesterol, mg/dL, Mean (±SD) | 55 (±16) | 59 (±13) |
| LDL Cholesterol, mg/dL, Mean (±SD) | 129 (±39) | 115 (±35) |
| Triglycerides, mg/dL, Mean (±SD) | 92 (±39) | 99 (±54) |
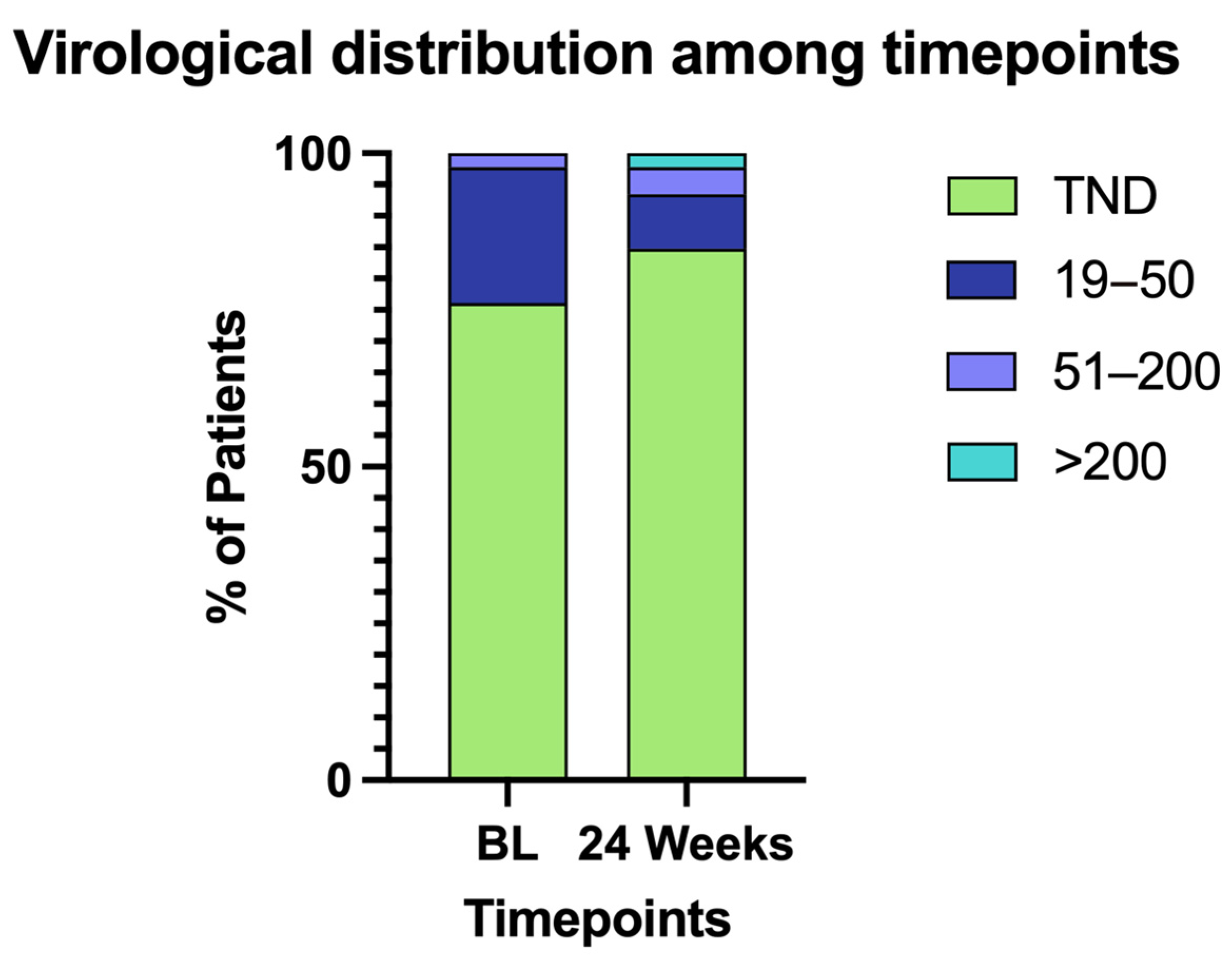
| TND | 19–50 | 51–200 | >200 | |
|---|---|---|---|---|
| BL | 35 | 10 | 1 | 0 |
| 24 Weeks | 39 | 4 | 2 | 1 |
| 24 Weeks | |||||
|---|---|---|---|---|---|
| TND | 19–50 | 51–200 | >200 | ||
| Baseline | TND | 30 | 2 | 2 | 1 |
| 19–50 | 8 | 2 | 0 | 0 | |
| 51–200 | 1 | 0 | 0 | 0 | |
| Parameter | Value at BL | Value at 24 w | p Value |
|---|---|---|---|
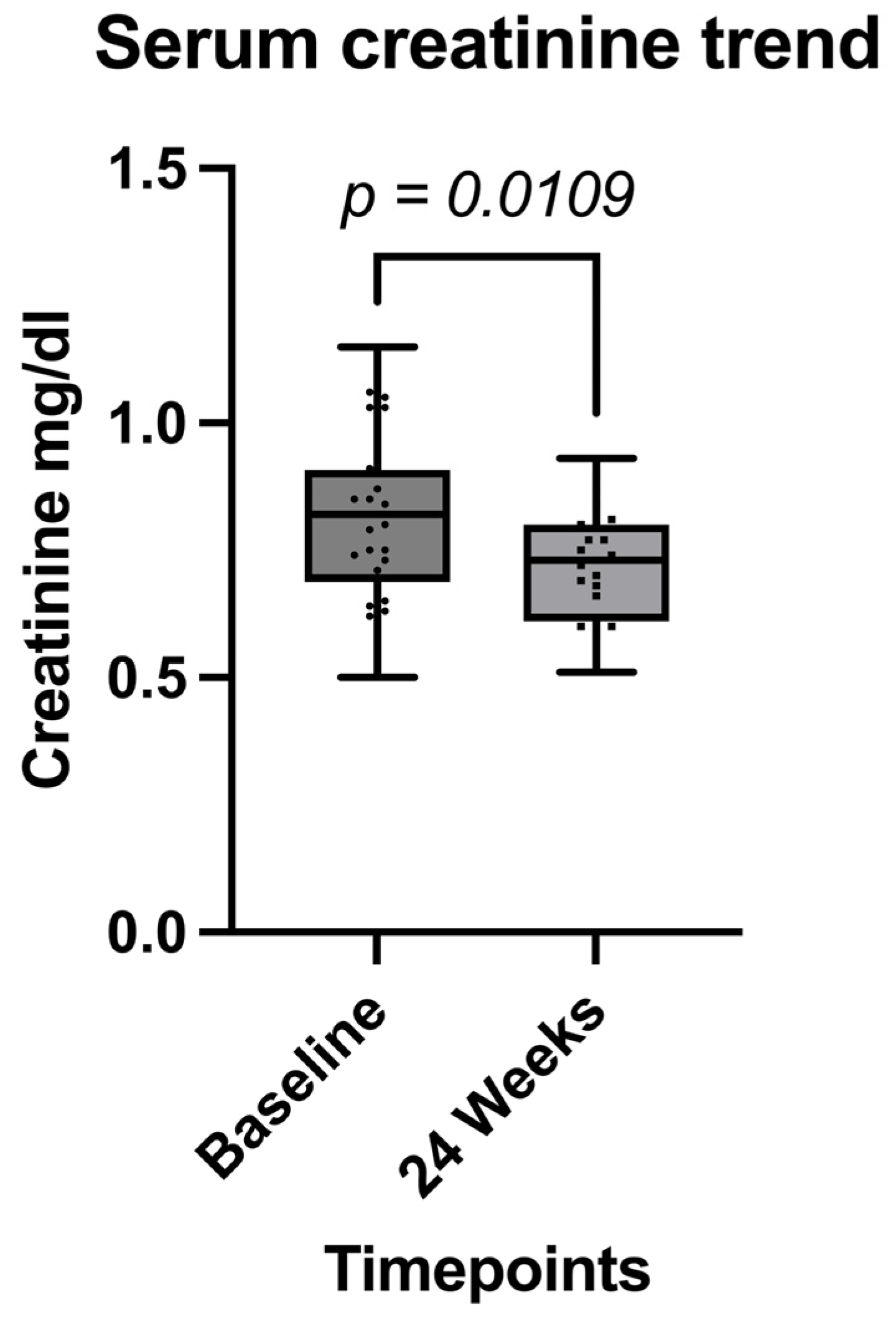
| Serum creatinine (mg/dL, mean, ±SD) | 0.82 (±0.16) | 0.72 (±0.12) | 0.0109 |
| CD4/mm3 (Median, IQR) | 849 (641–1111) | 850 (658–1138) | 0.1914 |
| CD8/mm3 (Median, IQR) | 736 (534–928) | 722 (528–863) | 0.4217 |
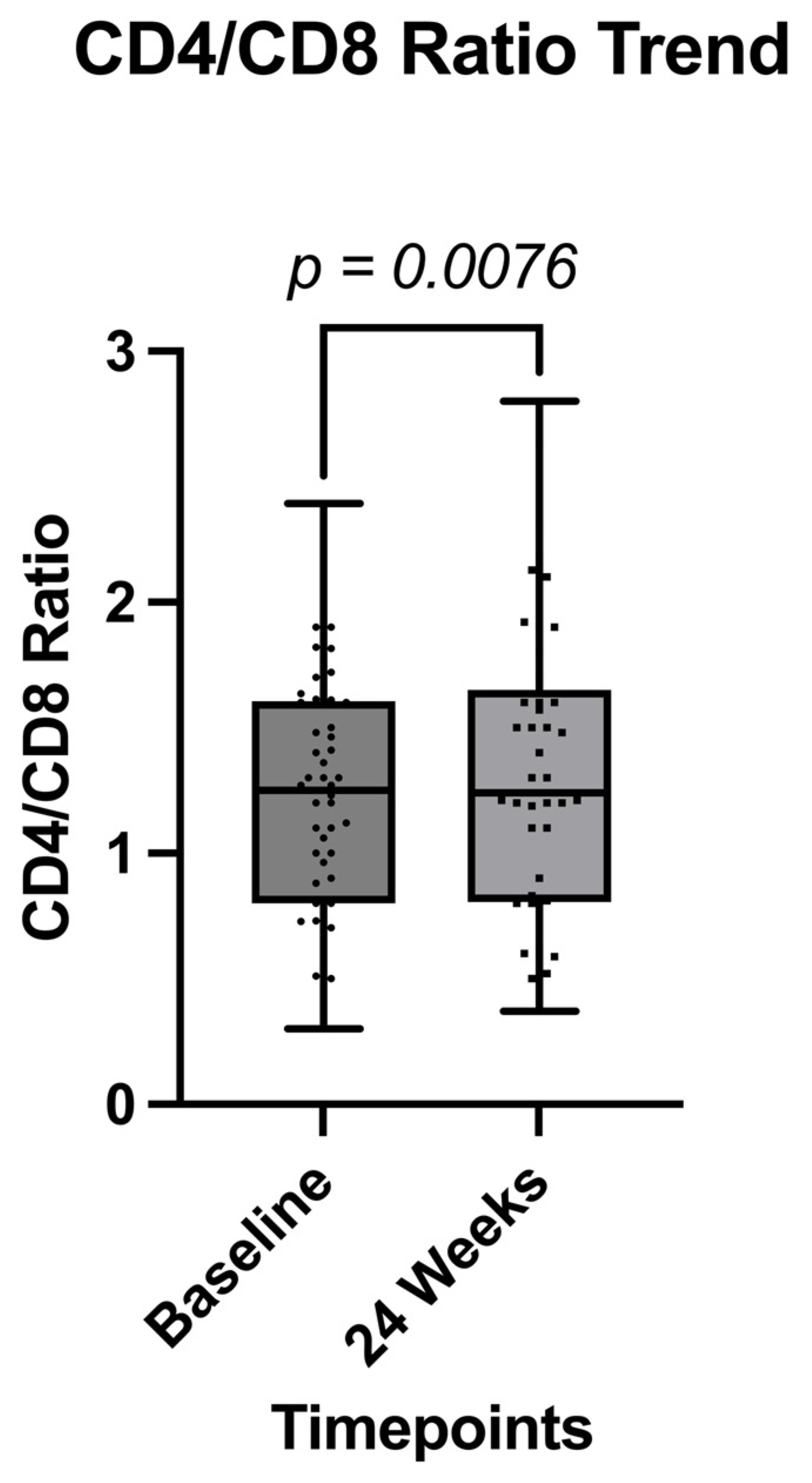
| CD4/CD8 Ratio (Mean, ±SD) | 1.2 (±0.51) | 1.3 (±0.59) | 0.0076 |
| Virological suppression, absolute count (VL < 50) | 45 (97.8%) | 43 (95.5%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spampinato, S.; Venanzi Rullo, E.; Conti, G.N.; De Vito, A.; Marino, A.; Cirelli, T.; Coco, V.; Mirabile, A.; Fontana del Vecchio, R.; Franco, A.; et al. CD4/CD8 Ratio Increase in Female Living with HIV Switching to Cabotegravir-Rilpivirine: A Real-Life 24 Weeks Evaluation. Pathogens 2025, 14, 633. https://doi.org/10.3390/pathogens14070633
Spampinato S, Venanzi Rullo E, Conti GN, De Vito A, Marino A, Cirelli T, Coco V, Mirabile A, Fontana del Vecchio R, Franco A, et al. CD4/CD8 Ratio Increase in Female Living with HIV Switching to Cabotegravir-Rilpivirine: A Real-Life 24 Weeks Evaluation. Pathogens. 2025; 14(7):633. https://doi.org/10.3390/pathogens14070633
Chicago/Turabian StyleSpampinato, Serena, Emmanuele Venanzi Rullo, Giuseppe Nicolò Conti, Andrea De Vito, Andrea Marino, Teresa Cirelli, Viviana Coco, Alessia Mirabile, Rossella Fontana del Vecchio, Antonina Franco, and et al. 2025. "CD4/CD8 Ratio Increase in Female Living with HIV Switching to Cabotegravir-Rilpivirine: A Real-Life 24 Weeks Evaluation" Pathogens 14, no. 7: 633. https://doi.org/10.3390/pathogens14070633
APA StyleSpampinato, S., Venanzi Rullo, E., Conti, G. N., De Vito, A., Marino, A., Cirelli, T., Coco, V., Mirabile, A., Fontana del Vecchio, R., Franco, A., Montineri, A., Frasca, C., Gullotta, C., Paternò Raddusa, M. S., Russotto, Y., Fugooah, A., Pulvirenti, S., Sofia, S., Pantò, G., ... Nunnari, G. (2025). CD4/CD8 Ratio Increase in Female Living with HIV Switching to Cabotegravir-Rilpivirine: A Real-Life 24 Weeks Evaluation. Pathogens, 14(7), 633. https://doi.org/10.3390/pathogens14070633











