Age-Related Impairment of Innate and Adaptive Immune Responses Exacerbates Herpes Simplex Viral Infection
Abstract
1. Introduction
2. Materials and Methods
3. Results
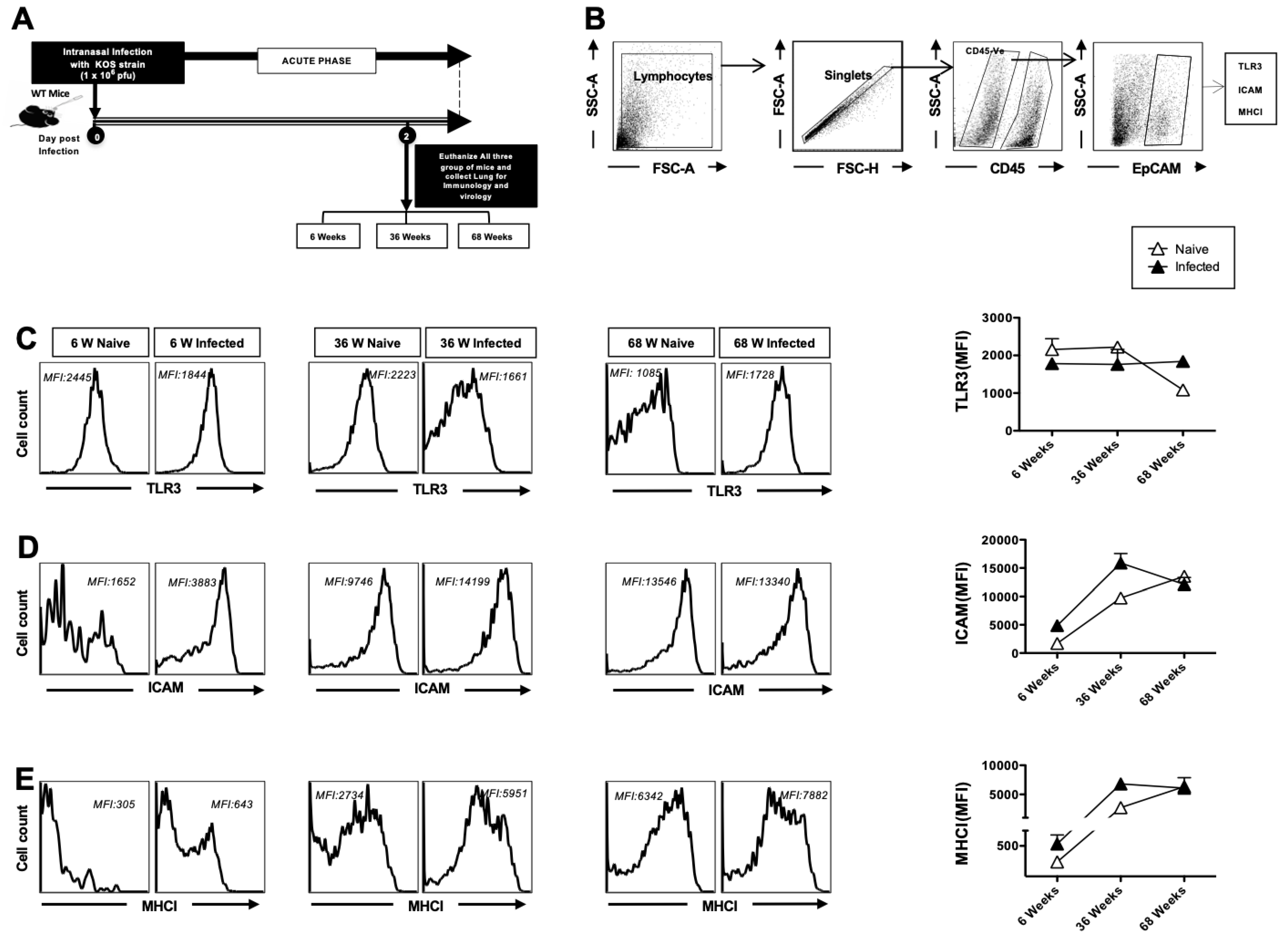
3.1. Decreased TLR-3 Expression and Increased Activation of Airway Epithelial Cells (AECs) in Aged Mice at Homeostasis
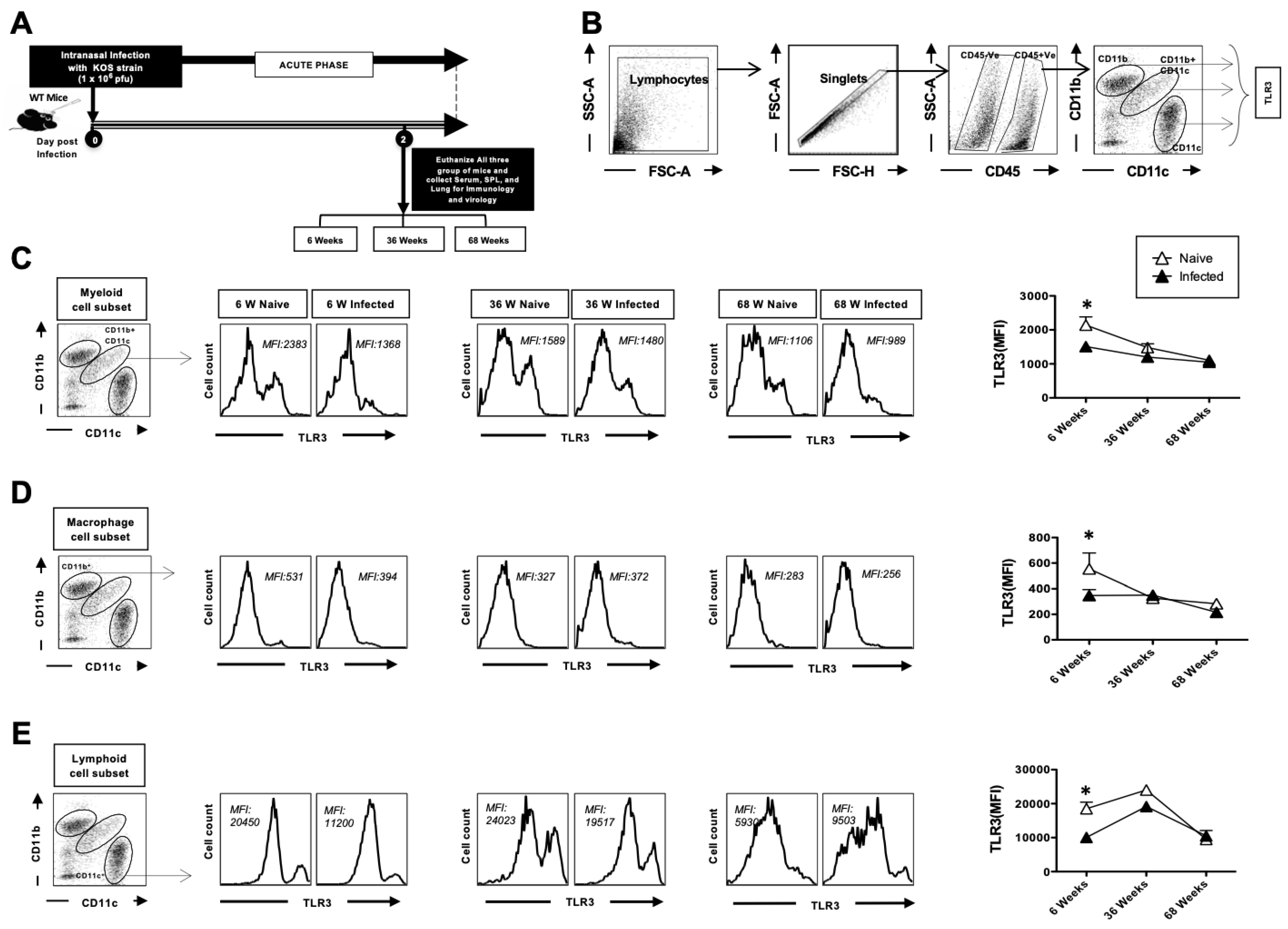
3.2. The Expression of TLR3 on Dendritic Cells and Macrophages in the Lungs Decreases with Age at Homeostasis
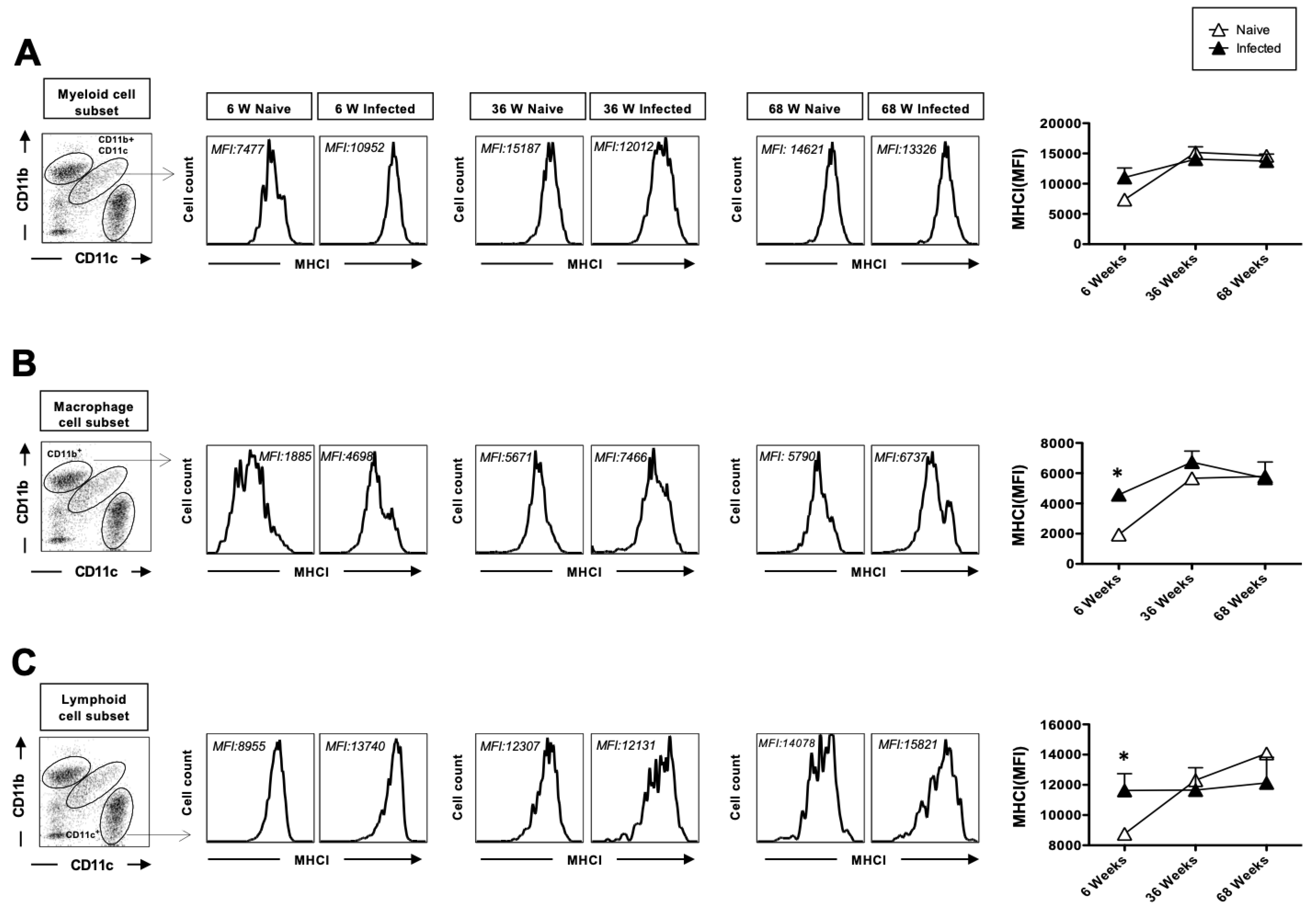
3.3. The Upregulation of MHC-I After Herpes Infection Is Impaired with Age
3.4. The Upregulation of MHC-II After Herpes Infection Is Impaired with Age and Exhibits Reduced Secretion of Anti-Inflammatory Cytokines in the Lungs After Acute HSV Infection
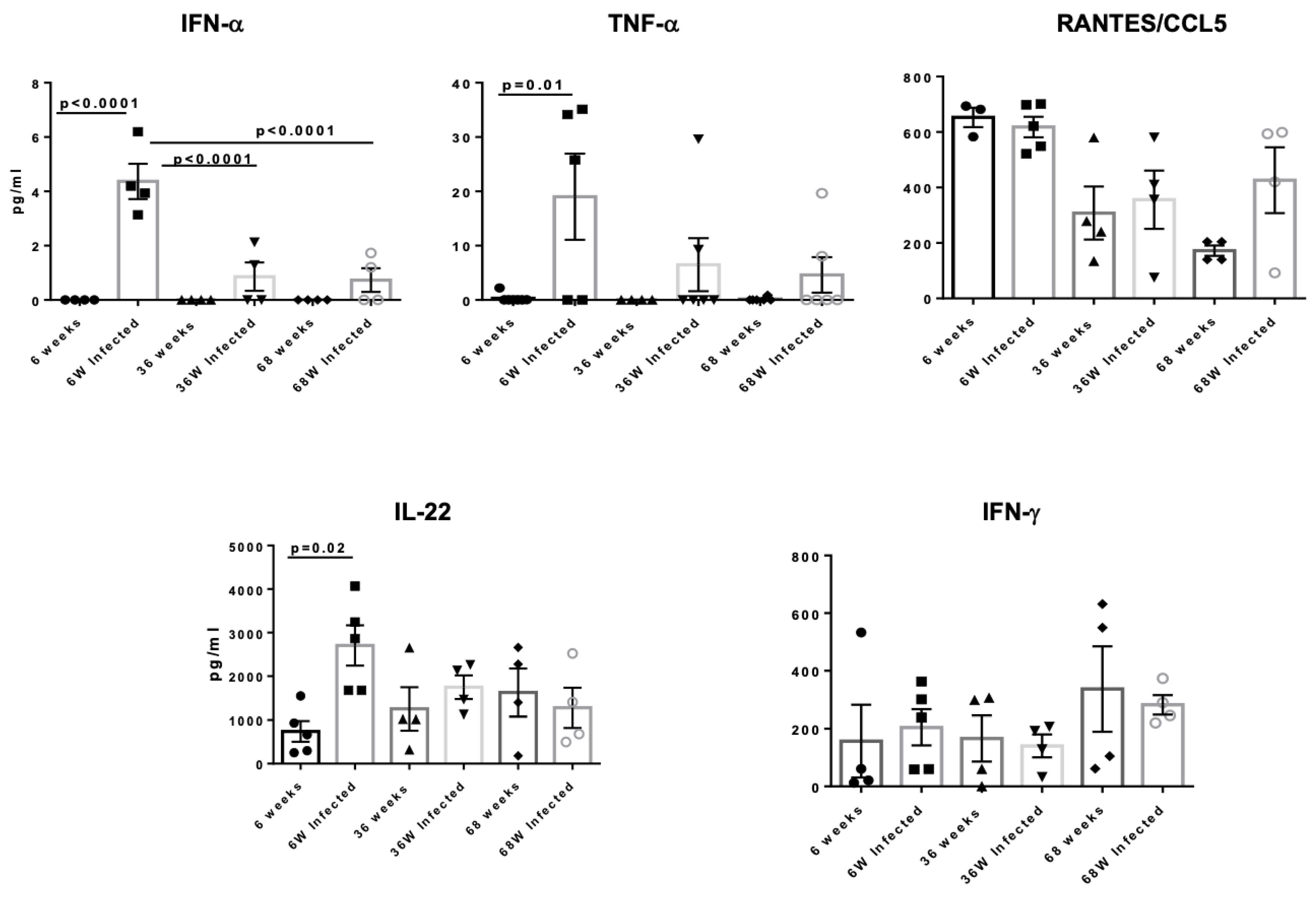
3.5. Aged Mice Display Reduced Secretion of Anti-Inflammatory Cytokines in the Lungs After Acute HSV Infection
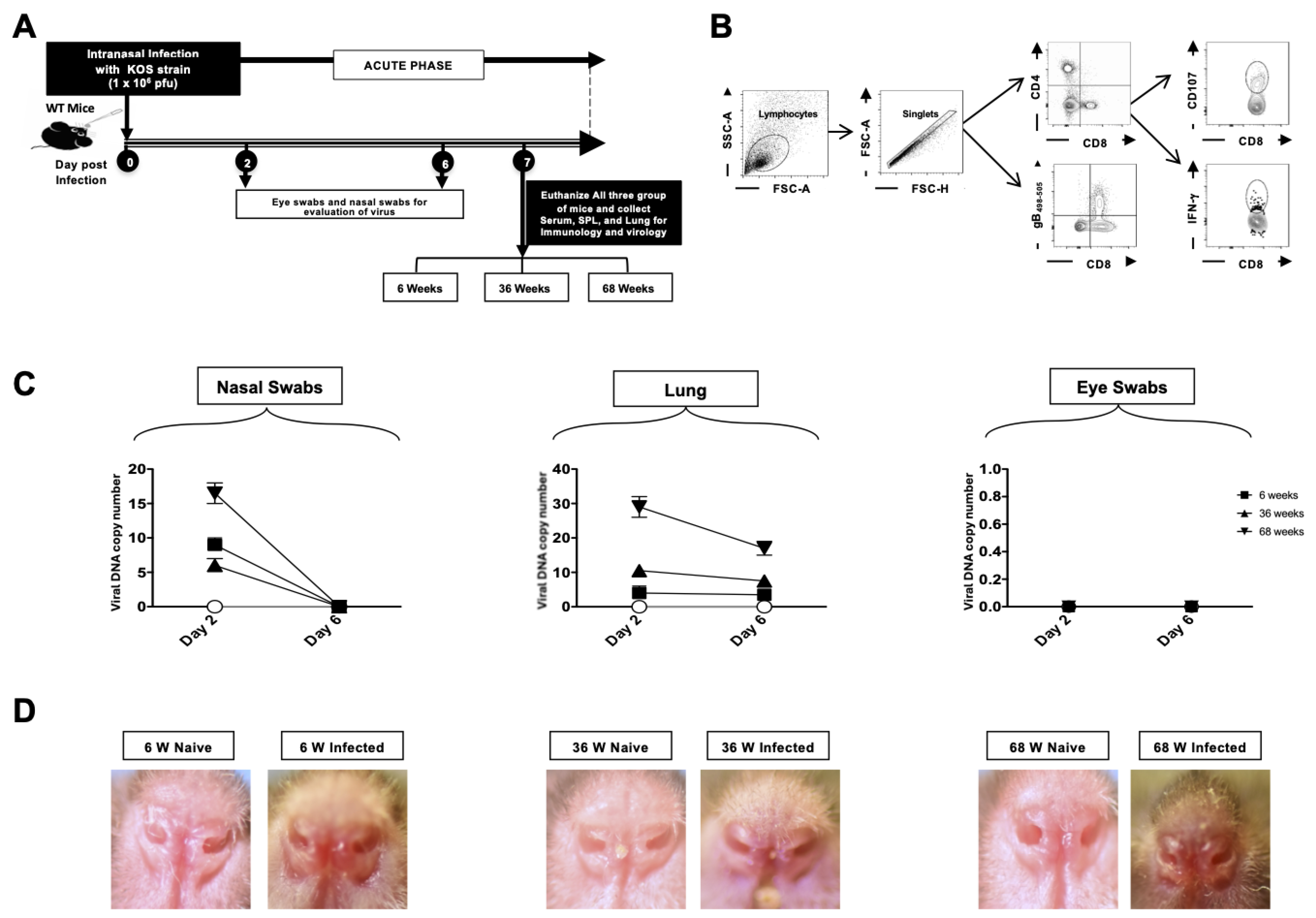
3.6. Viral Replication in the Lungs and Disease Severity Increase with Age
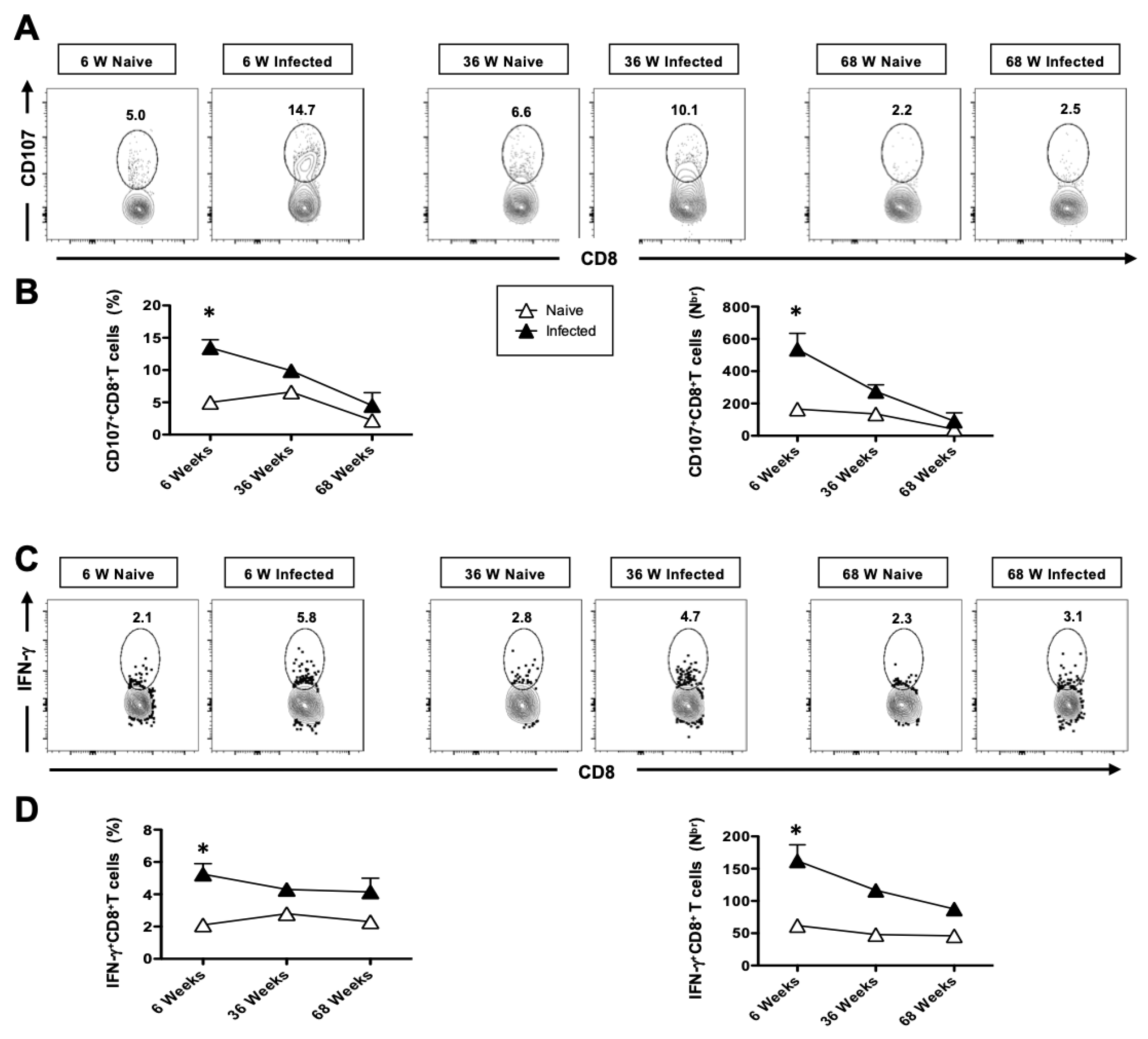
3.7. The Generation of Functional HSV-Specific CD8+ T Cells Is Impaired in Aged Mice Following Intranasal Infection with HSV-1
3.8. The Cytotoxic Functional Activity of CD8+ T Cells Is Reduced with Age in HSV-Infected Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitley, R.J.; Kimberlin, D.W.; Roizman, B. Herpes simplex viruses. Clin. Infect. Dis. 1998, 26, 541–553, quiz 554-5. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Spear, P.G. Infections with herpes simplex viruses (2). N. Engl. J. Med. 1986, 314, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.R.; Finland, M. Isolation of herpes virus from a case of atypical pneumonia and erythema multiforme exudativum with studies of four additional cases. Am. J. Med. Sci. 1949, 217, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Gaajetaan, G.R.; Bruggeman, C.A.; Stassen, F.R. The type I interferon response during viral infections: A “SWOT” analysis. Rev. Med. Virol. 2012, 22, 122–137. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Cook, D.N.; Pisetsky, D.S.; Schwartz, D.A. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 2004, 5, 975–979. [Google Scholar] [CrossRef]
- Hraiech, S.; Azoulay, E.; Papazian, L. Herpesviruses in Critically Ill Patients with ARDS. Encyclopedia of Respiratory Medicine. Encycl. Respir. Med. 2022, 373–385. [Google Scholar] [CrossRef]
- Duckworth, A.; Longhurst, H.J.; Paxton, J.K.; Scotton, C.J. The Role of Herpes Viruses in Pulmonary Fibrosis. Front. Med. 2021, 8, 704222. [Google Scholar] [CrossRef]
- De Vos, N.; Van Hoovels, L.; Vankeerberghen, A.; Van Vaerenbergh, K.; Boel, A.; Demeyer, I.; Creemers, L.; De Beenhouwer, H. Monitoring of herpes simplex virus in the lower respiratory tract of critically ill patients using real-time PCR: A prospective study. Clin. Microbiol. Infect. 2009, 15, 358–363. [Google Scholar] [CrossRef]
- Flurkey, K.; Currer, J.; Harrison, D. The Mouse in Aging Research. Mouse Biomed. Res. 2007, 3, 637–672. [Google Scholar] [CrossRef]
- Zhang, X.; Chentoufi, A.A.; Dasgupta, G.; Nesburn, A.B.; Wu, M.; Zhu, X.; Carpenter, D.; Wechsler, S.L.; You, S.; BenMohamed, L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009, 2, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dervillez, X.; Chentoufi, A.A.; Badakhshan, T.; Bettahi, I.; Benmohamed, L. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: Importance of MyD88. J. Immunol. 2012, 189, 4496–4509. [Google Scholar] [CrossRef]
- Steinert, E.M.; Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Manlove, L.S.; Igyarto, B.Z.; Southern, P.J.; Masopust, D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 2015, 161, 737–749. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Masopust, D. Cutting edge: Resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J. Immunol. 2014, 192, 2961–2964. [Google Scholar] [CrossRef]
- Holtzman, M.J.; Byers, D.E.; Alexander-Brett, J.; Wang, X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 2014, 14, 686–698. [Google Scholar] [CrossRef]
- Lim, H.K.; Seppanen, M.; Hautala, T.; Ciancanelli, M.J.; Itan, Y.; Lafaille, F.G.; Dell, W.; Lorenzo, L.; Byun, M.; Pauwels, E.; et al. TLR3 deficiency in herpes simplex encephalitis: High allelic heterogeneity and recurrence risk. Neurology 2014, 83, 1888–1897. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Ivanov, S.; Renneson, J.; Fontaine, J.; Barthelemy, A.; Paget, C.; Fernandez, E.M.; Blanc, F.; De Trez, C.; Van Maele, L.; Dumoutier, L.; et al. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 2013, 87, 6911–6924. [Google Scholar] [CrossRef]
- Pociask, D.; Yan, X.; Kolls, J. IL-22 reduces the pulmonary injury and lethality of influenza infection (CCR4P.201). J. Immunol. 2015, 194 (Suppl. S1), 118-1. [Google Scholar] [CrossRef]
- Yoshikawa, T.T. Important infections in elderly persons. West. J. Med. 1981, 135, 441–445. [Google Scholar] [PubMed]
- Curran, D.; Doherty, T.M.; Lecrenier, N.; Breuer, T. Healthy ageing: Herpes zoster infection and the role of zoster vaccination. NPJ Vaccines 2023, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, M.; Imhof, A.; Klarer, A. Herpes simplex type 1 pneumonitis and acute respiratory distress syndrome in a patient with chronic lymphatic leukemia: A case report. J. Med. Case Rep. 2017, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Lopatko Lindman, K.; Weidung, B.; Olsson, J.; Josefsson, M.; Kok, E.; Johansson, A.; Eriksson, S.; Hallmans, G.; Elgh, F.; Lövheim, H. A genetic signature including apolipoprotein Eε4 potentiates the risk of herpes simplex-associated Alzheimer’s disease. Alzheimers Dement. 2019, 5, 697–704. [Google Scholar] [CrossRef]
- Linard, M.; Letenneur, L.; Garrigue, I.; Doize, A.; Dartigues, J.F.; Helmer, C. Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimers Dement. 2020, 16, 200–208. [Google Scholar] [CrossRef]
- Prakash, S.; Agrawal, S.; Vahed, H.; Ngyuen, M.; Benmohamad, L.; Gupta, S.; Agrawal, A. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol. 2014, 7, 1386–1394. [Google Scholar] [CrossRef]
- Gomez, M.I.; Prince, A. Airway epithelial cell signaling in response to bacterial pathogens. Pediatr. Pulmonol. 2008, 43, 11–19. [Google Scholar] [CrossRef]
- Busse, P.J.; Mathur, S.K. Age-related changes in immune function: Effect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699, quiz 700-691. [Google Scholar] [CrossRef]
- Busse, P.J.; Zhang, T.F.; Srivastava, K.; Schofield, B.; Li, X.M. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin. Exp. Allergy 2007, 37, 1392–1403. [Google Scholar] [CrossRef]
- Ho, J.C.; Chan, K.N.; Hu, W.H.; Lam, W.K.; Zheng, L.; Tipoe, G.L.; Sun, J.; Leung, R.; Tsang, K.W. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am. J. Respir. Crit. Care Med. 2001, 163, 983–988. [Google Scholar] [CrossRef]
- Svartengren, M.; Falk, R.; Philipson, K. Long-term clearance from small airways decreases with age. Eur. Respir. J. 2005, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the Ageing Immune System. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Liu, Z.M.; Yang, M.H.; Yu, K.; Lian, Z.X.; Deng, S.L. Toll-like receptor (TLRs) agonists and antagonists for COVID-19 treatments. Front. Pharmacol. 2022, 13, 989664. [Google Scholar] [CrossRef]
- Ngo, C.; Garrec, C.; Tomasello, E.; Dalod, M. The role of plasmacytoid dendritic cells (pDCs) in immunity during viral infections and beyond. Cell Mol. Immunol. 2024, 21, 1008–1035. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 2010, 327, 291–295. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 2009, 21, 185–193. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Rosenstiel, P.; Derer, S.; Till, A.; Hasler, R.; Eberstein, H.; Bewig, B.; Nikolaus, S.; Nebel, A.; Schreiber, S. Systematic expression profiling of innate immune genes defines a complex pattern of immunosenescence in peripheral and intestinal leukocytes. Genes. Immun. 2008, 9, 103–114. [Google Scholar] [CrossRef]
- Murciano, C.; Yanez, A.; O’Connor, J.E.; Gozalbo, D.; Gil, M.L. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol. Med. Microbiol. 2008, 53, 214–221. [Google Scholar] [CrossRef]
- Stout-Delgado, H.W.; Yang, X.; Walker, W.E.; Tesar, B.M.; Goldstein, D.R. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 2008, 181, 6747–6756. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Stockinger, S.; Torow, N.; Smoczek, A.; Lindner, C.; McInerney, G.; Backhed, F.; Baumann, U.; Pabst, O.; Bleich, A.; et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog. 2012, 8, e1002670. [Google Scholar] [CrossRef] [PubMed]
- Sironi, M.; Peri, A.M.; Cagliani, R.; Forni, D.; Riva, S.; Biasin, M.; Clerici, M.; Gori, A. TLR3 Mutations in Adult Patients With Herpes Simplex Virus and Varicella-Zoster Virus Encephalitis. J. Infect. Dis. 2017, 215, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998, 16, 111–135. [Google Scholar] [CrossRef]
- Agrawal, A.; Tay, J.; Ton, S.; Agrawal, S.; Gupta, S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J. Immunol. 2009, 182, 1138–1145. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Reitsema, R.D.; Kumawat, A.K.; Hesselink, B.C.; van Baarle, D.; van Sleen, Y. Effects of ageing and frailty on circulating monocyte and dendritic cell subsets. NPJ Aging 2024, 10, 17. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Dendritic cells in human aging. Exp. Gerontol. 2007, 42, 421–426. [Google Scholar] [CrossRef]
- Effros, R.B. Replicative senescence of CD8 T cells: Potential effects on cancer immune surveillance and immunotherapy. Cancer Immunol. Immunother. 2004, 53, 925–933. [Google Scholar] [CrossRef]
- Gupta, S.; Bi, R.; Su, K.; Yel, L.; Chiplunkar, S.; Gollapudi, S. Characterization of naive, memory and effector CD8+ T cells: Effect of age. Exp. Gerontol. 2004, 39, 545–550. [Google Scholar] [CrossRef]
- Haynes, L.; Maue, A.C. Effects of aging on T cell function. Curr. Opin. Immunol. 2009, 21, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.E.; Goodbourn, S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008, 89 Pt. 1, 1–47. [Google Scholar] [CrossRef]
- Diebold, S.S.; Montoya, M.; Unger, H.; Alexopoulou, L.; Roy, P.; Haswell, L.E.; Al-Shamkhani, A.; Flavell, R.; Borrow, P.; Reis e Sousa, C. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 2003, 424, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef]
- Psarras, A.; Alase, A.; Antanaviciute, A.; Carr, I.M.; Md Yusof, M.Y.; Wittmann, M.; Emery, P.; Tsokos, G.C.; Vital, E.M. Functionally impaired plasmacytoid dendritic cells and non-haematopoietic sources of type I interferon characterize human autoimmunity. Nat. Commun. 2020, 11, 6149. [Google Scholar] [CrossRef]
- Katashiba, Y.; Miyamoto, R.; Hyo, A.; Shimamoto, K.; Murakami, N.; Ogata, M.; Amakawa, R.; Inaba, M.; Nomura, S.; Fukuhara, S.; et al. Interferon-alpha and interleukin-12 are induced, respectively, by double-stranded DNA and single-stranded RNA in human myeloid dendritic cells. Immunology 2011, 132, 165–173. [Google Scholar] [CrossRef]
- Shodell, M.; Siegal, F.P. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand. J. Immunol. 2002, 56, 518–521. [Google Scholar] [CrossRef]
- Canaday, D.H.; Amponsah, N.A.; Jones, L.; Tisch, D.J.; Hornick, T.R.; Ramachandra, L. Influenza-induced production of interferon-alpha is defective in geriatric individuals. J. Clin. Immunol. 2010, 30, 373–383. [Google Scholar] [CrossRef]
- Qian, F.; Wang, X.; Zhang, L.; Lin, A.; Zhao, H.; Fikrig, E.; Montgomery, R.R. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 2011, 203, 1415–1424. [Google Scholar] [CrossRef]
- Feng, E.; Balint, E.; Poznanski, S.M.; Ashkar, A.A.; Loeb, M. Aging and Interferons: Impacts on Inflammation and Viral Disease Outcomes. Cells 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Pociask, D.A.; Scheller, E.V.; Mandalapu, S.; McHugh, K.J.; Enelow, R.I.; Fattman, C.L.; Kolls, J.K.; Alcorn, J.F. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 2013, 182, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- McAleer, J.P.; Kolls, J.K. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol. Rev. 2014, 260, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Yi, P.; Liang, Y.; Yuan, D.M.K.; Jie, Z.; Kwota, Z.; Chen, Y.; Cong, Y.; Fan, X.; Sun, J. A tightly regulated IL-22 response maintains immune functions and homeostasis in systemic viral infection. Sci. Rep. 2017, 7, 3857. [Google Scholar] [CrossRef]
- Sonnenberg, G.F.; Nair, M.G.; Kirn, T.J.; Zaph, C.; Fouser, L.A.; Artis, D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 2010, 207, 1293–1305. [Google Scholar] [CrossRef]
- Jiang, J.; Fisher, E.M.; Murasko, D.M. CD8 T cell responses to influenza virus infection in aged mice. Ageing Res. Rev. 2011, 10, 422–427. [Google Scholar] [CrossRef]
- Cadar, A.N.; Martin, D.E.; Bartley, J.M. Targeting the hallmarks of aging to improve influenza vaccine responses in older adults. Immun. Ageing 2023, 20, 23. [Google Scholar] [CrossRef]
- Effros, R.B.; Walford, R.L. The immune response of aged mice to influenza: Diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983, 81, 298–305. [Google Scholar] [CrossRef]
- Parks, O.B.; Eddens, T.; Sojati, J.; Lan, J.; Zhang, Y.; Oury, T.D.; Ramsey, M.; Erickson, J.J.; Byersdorfer, C.A.; Williams, J.V. Terminally exhausted CD8(+) T cells contribute to age-dependent severity of respiratory virus infection. Immun. Ageing 2023, 20, 40. [Google Scholar] [CrossRef]
- Larsson, M.; Messmer, D.; Somersan, S.; Fonteneau, J.F.; Donahoe, S.M.; Lee, M.; Dunbar, P.R.; Cerundolo, V.; Julkunen, I.; Nixon, D.F.; et al. Requirement of mature dendritic cells for efficient activation of influenza A-specific memory CD8+ T cells. J. Immunol. 2000, 165, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.B.; Weiss, K.A.; Pewe, L.L.; Harty, J.T.; Varga, S.M. Aged mice exhibit a severely diminished CD8 T cell response following respiratory syncytial virus infection. J. Virol. 2013, 87, 12694–12700. [Google Scholar] [CrossRef]
- de Miguel-Díez, J.; Núñez Villota, J.; Santos Pérez, S.; Manito Lorite, N.; Alcázar Navarrete, B.; Delgado Jiménez, J.F.; Soler-Cataluña, J.J.; Pascual Figal, D.; Sobradillo Ecenarro, P.; Gómez Doblas, J.J. Multidisciplinary Management of Patients With Chronic Obstructive Pulmonary Disease and Cardiovascular Disease. Arch. Bronconeumol. 2024, 60, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Kim, I.H.; Kisseleva, T.; Brenner, D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015, 31, 184–191. [Google Scholar] [CrossRef]
- Jackaman, C.; Tomay, F.; Duong, L.; Abdol Razak, N.B.; Pixley, F.J.; Metharom, P.; Nelson, D.J. Aging and cancer: The role of macrophages and neutrophils. Ageing Res. Rev. 2017, 36, 105–116. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Torrance, B.L.; Haynes, L. Cellular senescence is a key mediator of lung aging and susceptibility to infection. Front. Immunol. 2022, 13, 1006710. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Zhou, L.; Ruscetti, M. Senescent macrophages: A new “old” player in lung cancer development. Cancer Cell 2023, 41, 1201–1203. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, S.; Wright, R.E., 3rd; Rattan, R.; Kumar, A. Aging, But Not Sex and Genetic Diversity, Impacts the Pathobiology of Bacterial Endophthalmitis. Invest. Ophthalmol. Vis. Sci. 2020, 61, 5. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, R.; Karan, S.; Lekbach, Y.; Quadiri, A.; Tohidian, A.; Maurya, C.; Ng, S.X.L.; Chow, R.; Garcia, A.; Agrawal, A.; et al. Age-Related Impairment of Innate and Adaptive Immune Responses Exacerbates Herpes Simplex Viral Infection. Pathogens 2025, 14, 624. https://doi.org/10.3390/pathogens14070624
Srivastava R, Karan S, Lekbach Y, Quadiri A, Tohidian A, Maurya C, Ng SXL, Chow R, Garcia A, Agrawal A, et al. Age-Related Impairment of Innate and Adaptive Immune Responses Exacerbates Herpes Simplex Viral Infection. Pathogens. 2025; 14(7):624. https://doi.org/10.3390/pathogens14070624
Chicago/Turabian StyleSrivastava, Ruchi, Sweta Karan, Yassir Lekbach, Afshana Quadiri, Ava Tohidian, Chhaya Maurya, Sarah Xue Le Ng, Reilly Chow, America Garcia, Anshu Agrawal, and et al. 2025. "Age-Related Impairment of Innate and Adaptive Immune Responses Exacerbates Herpes Simplex Viral Infection" Pathogens 14, no. 7: 624. https://doi.org/10.3390/pathogens14070624
APA StyleSrivastava, R., Karan, S., Lekbach, Y., Quadiri, A., Tohidian, A., Maurya, C., Ng, S. X. L., Chow, R., Garcia, A., Agrawal, A., Vahed, H., Chentoufi, A. A., & BenMohamed, L. (2025). Age-Related Impairment of Innate and Adaptive Immune Responses Exacerbates Herpes Simplex Viral Infection. Pathogens, 14(7), 624. https://doi.org/10.3390/pathogens14070624







