A Pain in the Butt: The Association Between Endo-Parasite Diversity and Horn Growth in Rocky Mountain Bighorn Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Animals
2.2. Fecal Parasite Screening
2.3. Horn Growth Data
2.4. Data Analysis
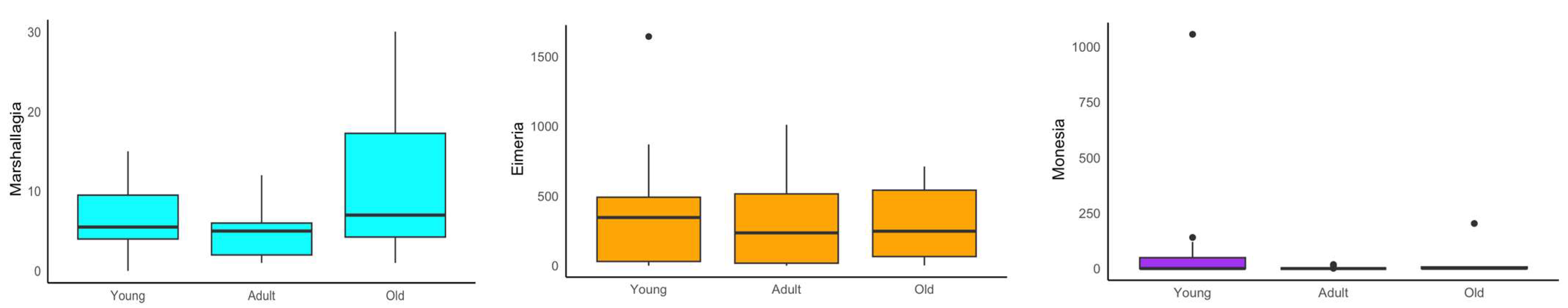
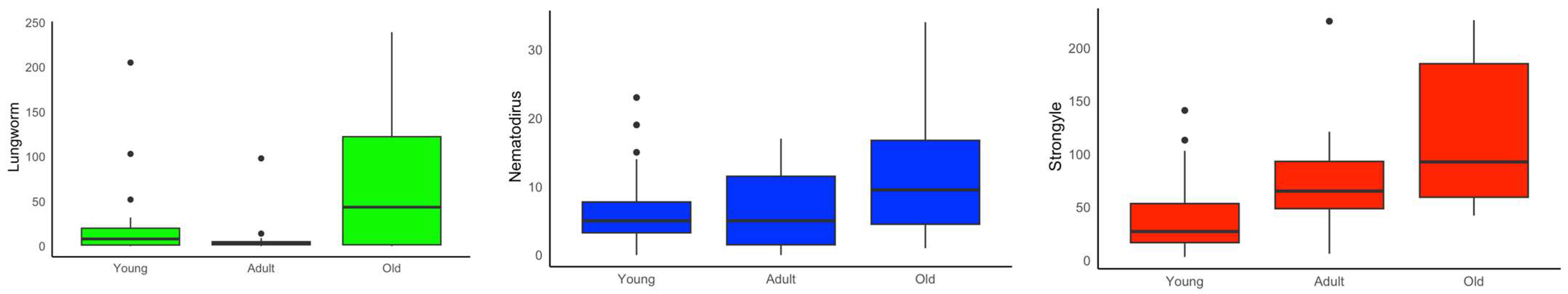
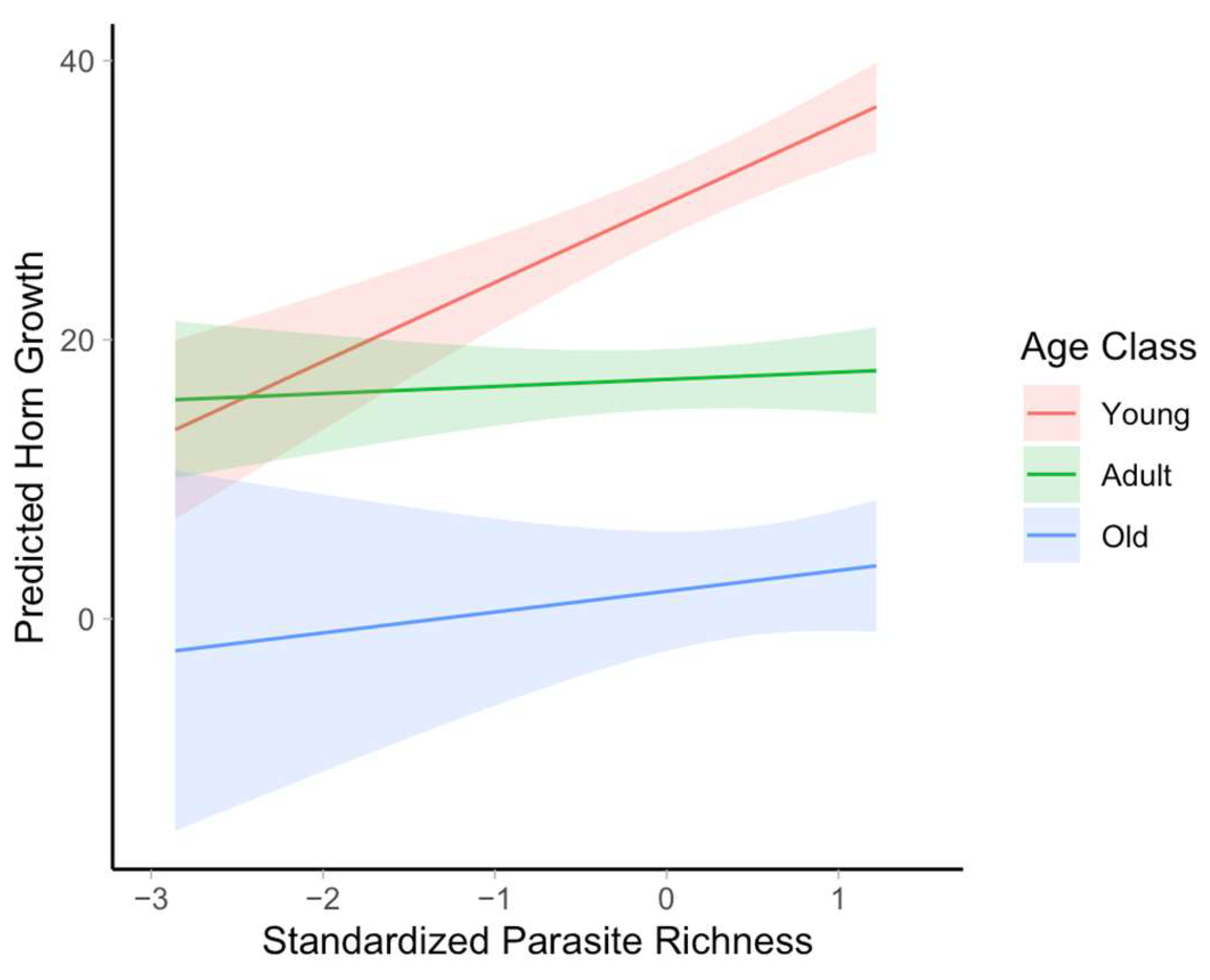
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; John Murray: London, UK, 1871; Volume 1. [Google Scholar]
- Zahavi, A. Mate Selection—A Selection for a Handicap. J. Theor. Biol. 1975, 53, 205–214. [Google Scholar] [CrossRef]
- Zahavi, A. The Cost of Honesty. J. Theor. Biol. 1977, 67, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.D.; Zuk, M. Heritable True Fitness and Bright Birds: A Role for Parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Folstad, I.; Karter, A.J. Parasites, Bright Males, and the Immunocompetence Handicap. Am. Nat. 1992, 139, 603–622. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Jolles, A.E. Horns Honestly Advertise Parasite Infection in Male and Female African Buffalo. Anim. Behav. 2008, 75, 2013–2021. [Google Scholar] [CrossRef]
- Thompson, C.W.; Hillgarth, N.; Leu, M.; McClure, H.E. High Parasite Load in House Finches (Carpodacus mexicanus) Is Correlated with Reduced Expression of a Sexually Selected Trait. Am. Nat. 1997, 149, 270–294. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Jolles, A.E. From Host Immunity to Pathogen Invasion: The Effects of Helminth Coinfection on the Dynamics of Microparasites. Integr. Comp. Biol. 2011, 51, 540–551. [Google Scholar] [CrossRef]
- Klein, S.L. The Effects of Hormones on Sex Differences in Infection: From Genes to Behavior. Neurosci. Biobehav. Rev. 2000, 24, 627–638. [Google Scholar] [CrossRef]
- Folstad, I.; Nilssen, A.C.; Halvorsen, O.; Andersen, J. Why Do Male Reindeer (Rangifer T. Tarandus) Have Higher Abundance of Second and Third Instar Larvae of Hypoderma Tarandi Than Females? Oikos 1989, 55, 87–92. [Google Scholar] [CrossRef]
- Smith, B.L. Antler Size and Winter Mortality of Elk: Effects of Environment, Birth Year, and Parasites. J. Mammal. 1998, 79, 1038. [Google Scholar] [CrossRef][Green Version]
- Salvador, A.; Veiga, J.P.; Martin, J.; Lopez, P.; Abelenda, M.; Puertac, M. The Cost of Producing a Sexual Signal: Testosterone Increases the Susceptibility of Male Lizards to Ectoparasitic Infestation. Behav. Ecol. 1996, 7, 145–150. [Google Scholar] [CrossRef]
- Coltman, D.W.; O’Donoghue, P.; Jorgenson, J.T.; Hogg, J.T.; Strobeck, C.; Festa-Bianchet, M. Undesirable Evolutionary Consequences of Trophy Hunting. Nature 2003, 426, 655–658. [Google Scholar] [CrossRef]
- Schindler, S.; Ruckstuhl, K.E.; Neuhaus, P. Male Mating Behaviour Affects Growth of Secondary Sexual Traits: A Mechanism for Rapid Phenotypic Change. Anim. Behav. 2020, 169, 129–138. [Google Scholar] [CrossRef]
- Douhard, M.; Pigeon, G.; Festa-Bianchet, M.; Coltman, D.W.; Guillemette, S.; Pelletier, F. Environmental and Evolutionary Effects on Horn Growth of Male Bighorn Sheep. Oikos 2017, 126, 1031–1041. [Google Scholar] [CrossRef]
- Hoefs, M.; Bunch, T.D. Lumpy jaw in wild sheep and its evolutionary implications. J. Wildl. Dis. 2001, 37, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Hogg, J.T.; Manlove, K.R.; LaSharr, T.N.; Shannon, J.M.; McWhirter, D.E.; Miyasaki, H.; Monteith, K.L.; Cross, P.C. Disease and Secondary Sexual Traits: Effects of Pneumonia on Horn Size of Bighorn Sheep. J. Wildl. Manag. 2022, 86, e22154. [Google Scholar] [CrossRef]
- Petney, T.N.; Andrews, R.H. Multiparasite Communities in Animals and Humans: Frequency, Structure and Pathogenic Significance. Int. J. Parasitol. 1998, 28, 377–393. [Google Scholar] [CrossRef]
- Rijal, S.; Neuhaus, P.; Thorley, J.; Caulkett, N.; Kutz, S.; Ruckstuhl, K.E. Patterns of Gastrointestinal Parasite Infections in Bighorn Sheep, Ovis canadensis, with Respect to Host Sex and Seasonality. Int. J. Parasitol. Parasites Wildl. 2024, 24, 100950. [Google Scholar] [CrossRef]
- Ruckstuhl, K.E. Foraging Behaviour and Sexual Segregation in Bighorn Sheep. Anim. Behav. 1998, 56, 99–106. [Google Scholar] [CrossRef]
- Becklund, W.W.; Senger, C.M. Parasites of Ovis canadensis Canadensis in Montana, with a Checklist of the Internal and External Parasites of the Rocky Mountain Bighorn Sheep in North America. J. Parasitol. 1967, 53, 157–165. [Google Scholar] [CrossRef]
- Bryan, R.P.; Kerr, J.D. The Relation between the Natural Worm Burden of Steers and the Faecal Egg Count Differentiated to Species. Vet. Parasitol. 1989, 30, 327–334. [Google Scholar] [CrossRef]
- Stear, M.J.; Bishop, S.C.; Doligalska, M.; Duncan, J.L.; Holmes, P.H.; Irvine, J.; McCRIRIE, L.; McKELLAR, Q.A.; Sinski, E.; Murray, M. Regulation of Egg Production, Worm Burden, Worm Length and Worm Fecundity by Host Responses in Sheep Infected with Ostertagia circumcincta. Parasite Immunol. 1995, 17, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.C.; Neuhaus, P.; Ruckstuhl, K.E. Hornography: Photogrammetry and 2D Measurement Can Be Used to Assess Bighorn Sheep (Ovis canadensis) Horn Morphology. Proc. Bienn. Symp. North. Wild Sheep Goat Counc. 2022, 23, 57–62. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.11.1-00001. 2025. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 19 June 2025).
- R Core Team. R: A Language and Environment for Statistical ## Computing; R Foundation of Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Defolie, C.; Merkling, T.; Fichtel, C. Patterns and Variation in the Mammal Parasite–Glucocorticoid Relationship. Biol. Rev. 2020, 95, 74–93. [Google Scholar] [CrossRef]
- Bellay, S.; Oda, F.H.; Almeida-Neto, M.; De Oliveira, E.F.; Takemoto, R.M.; Balbuena, J.A. Host Age Predicts Parasite Occurrence, Richness, and Nested Infracommunities in a Pilot Whale-Helminth Network. Parasitol. Res. 2020, 119, 2237–2244. [Google Scholar] [CrossRef]
- Lo, C.M.; Morand, S.; Galzin, R. Parasite Diversity\host Age and Size Relationship in Three Coral-Reef Fishes from French Polynesia. Int. J. Parasitol. 1998, 28, 1695–1708. [Google Scholar] [CrossRef]
- Gustafson, K.D.; Newman, R.A.; Pulis, E.E.; Cabarle, K.C. A Skeletochronological Assessment of Age–Parasitism Relationships in Wood Frogs (Lithobates sylvaticus). J. Herpetol. 2015, 49, 122–130. [Google Scholar] [CrossRef]
- Zuk, M.; Thornhill, R.; Ligon, J.D.; Johnson, K. Parasites and Mate Choice in Red Jungle Fowl. Am. Zool. 1990, 30, 235–244. [Google Scholar] [CrossRef]
- Coltman, D.W.; Festa-Bianchet, M.; Jorgenson, J.T.; Strobeck, C. Age-Dependent Sexual Selection in Bighorn Rams. Proc. R. Soc. Lond. B 2002, 269, 165–172. [Google Scholar] [CrossRef]
- Hogg, J.T. Mating in Bighorn Sheep: Multiple Creative Male Strategies. Science 1984, 225, 526–529. [Google Scholar] [CrossRef]
- Aleuy, O.A.; Ruckstuhl, K.; Hoberg, E.P.; Veitch, A.; Simmons, N.; Kutz, S.J. Diversity of Gastrointestinal Helminths in Dall’s Sheep and the Negative Association of the Abomasal Nematode, Marshallagia marshalli, with Fitness Indicators. PLoS ONE 2018, 13, e0192825. [Google Scholar] [CrossRef] [PubMed]
- Craig, B.H.; Jones, O.R.; Pilkington, J.G.; Pemberton, J.M. Re-Establishment of Nematode Infra-Community and Host Survivorship in Wild Soay Sheep Following Anthelmintic Treatment. Vet. Parasitol. 2009, 161, 47–52. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. Reproductive Success in Red Deer. Sci. Am. 1985, 252, 86–92. [Google Scholar] [CrossRef]
- Wilson, A.J.; Nussey, D.H. What Is Individual Quality? An Evolutionary Perspective. Trends Ecol. Evol. 2010, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, N.J.; Dochtermann, N.A. Quantifying Individual Variation in Behaviour: Mixed-effect Modelling Approaches. J. Anim. Ecol. 2013, 82, 39–54. [Google Scholar] [CrossRef] [PubMed]
| Effect | Sum of Squares | Mean Squares | df | F-Value | p-Value |
|---|---|---|---|---|---|
| Age class | 1682.85 | 841.43 | 2 | 83.72 | <0.001 |
| Strongyle egg counts | Data2.84 | 2.84 | 1 | 0.28 | 0.593 |
| Nematodirus egg counts | 4.72 | 4.72 | 1 | 0.47 | 0.498 |
| Moniezia egg counts | 12.40 | 12.40 | 1 | 1.23 | 0.275 |
| Eimeria oocyst counts | 0.17 | 0.17 | 1 | 0.02 | 0.898 |
| Parasite Species Richness | 91.34 | 91.34 | 1 | 9.09 | 0.005 |
| Year | 65.11 | 65.11 | 1 | 6.48 | 0.018 |
| Age class × Parasite Species Richness | 192.17 | 96.08 | 2 | 9.56 | <0.001 |
| Predictor | Estimate | SE | t Test | df | p-Value | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|
| (Intercept) | 20.047 | 1.294 | 15.497 | 36.01 | <0.001 | 17.42 | 22.67 |
| Age class—Old | −15.185 | 2.317 | −6.554 | 17.83 | <0.001 | −20.06 | −10.31 |
| Age class—Young | 12.612 | 1.330 | 9.480 | 37.92 | <0.001 | 9.92 | 15.30 |
| Strongyle | −0.392 | 0.737 | −0.531 | 37.47 | 0.598 | −1.89 | 1.10 |
| Nematodirus | 0.450 | 0.657 | 0.686 | 31.89 | 0.498 | −0.89 | 1.79 |
| Moniezia | −3.092 | 2.784 | −1.111 | 31.53 | 0.275 | −8.77 | 2.58 |
| Eimeria | −0.095 | 0.731 | −0.130 | 36.68 | 0.897 | −1.58 | 1.39 |
| Parasite species richness | 0.511 | 0.928 | 0.550 | 32.83 | 0.586 | −1.38 | 2.40 |
| Year—2017 | −3.722 | 1.462 | −2.545 | 24.14 | 0.018 | −6.74 | −0.71 |
| Age class—Old × Parasite species richness | 0.981 | 2.051 | 0.478 | 30.06 | 0.636 | −3.21 | 5.17 |
| Age class—Young × Parasite species richness | 5.159 | 1.210 | 4.265 | 37.15 | <0.0001 | 2.71 | 7.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, T.C.; Rijal, S.; Alves, J.; Neuhaus, P.; Kutz, S.; Ruckstuhl, K.E. A Pain in the Butt: The Association Between Endo-Parasite Diversity and Horn Growth in Rocky Mountain Bighorn Sheep. Pathogens 2025, 14, 623. https://doi.org/10.3390/pathogens14070623
Henry TC, Rijal S, Alves J, Neuhaus P, Kutz S, Ruckstuhl KE. A Pain in the Butt: The Association Between Endo-Parasite Diversity and Horn Growth in Rocky Mountain Bighorn Sheep. Pathogens. 2025; 14(7):623. https://doi.org/10.3390/pathogens14070623
Chicago/Turabian StyleHenry, Tanisha C., Samridhi Rijal, Joana Alves, Peter Neuhaus, Susan Kutz, and Kathreen E. Ruckstuhl. 2025. "A Pain in the Butt: The Association Between Endo-Parasite Diversity and Horn Growth in Rocky Mountain Bighorn Sheep" Pathogens 14, no. 7: 623. https://doi.org/10.3390/pathogens14070623
APA StyleHenry, T. C., Rijal, S., Alves, J., Neuhaus, P., Kutz, S., & Ruckstuhl, K. E. (2025). A Pain in the Butt: The Association Between Endo-Parasite Diversity and Horn Growth in Rocky Mountain Bighorn Sheep. Pathogens, 14(7), 623. https://doi.org/10.3390/pathogens14070623







