Genetic Identification of Brazilian Mammalian Hosts of Trypanosoma cruzi: Improving Blood Meal Source Discrimination in Vector-Borne Transmission
Abstract
1. Introduction
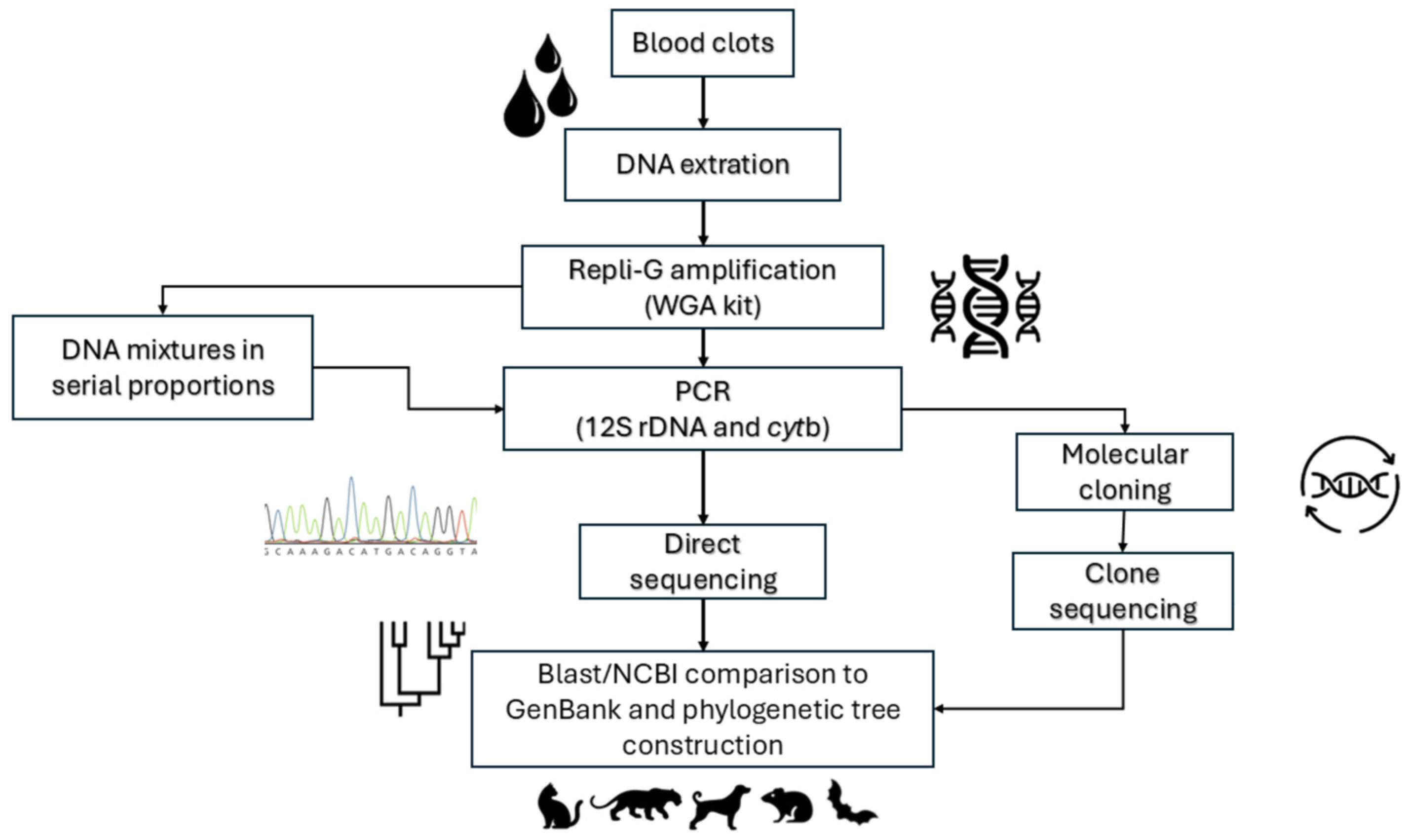
2. Materials and Methods
2.1. Blood Clot Samples to Evaluate the Specificity and Sensitivity of the BMS Molecular Approach
2.2. DNA Extraction from Blood Clots
2.3. Molecular Identification of Mammalian Species and T. cruzi Infection
3. Results
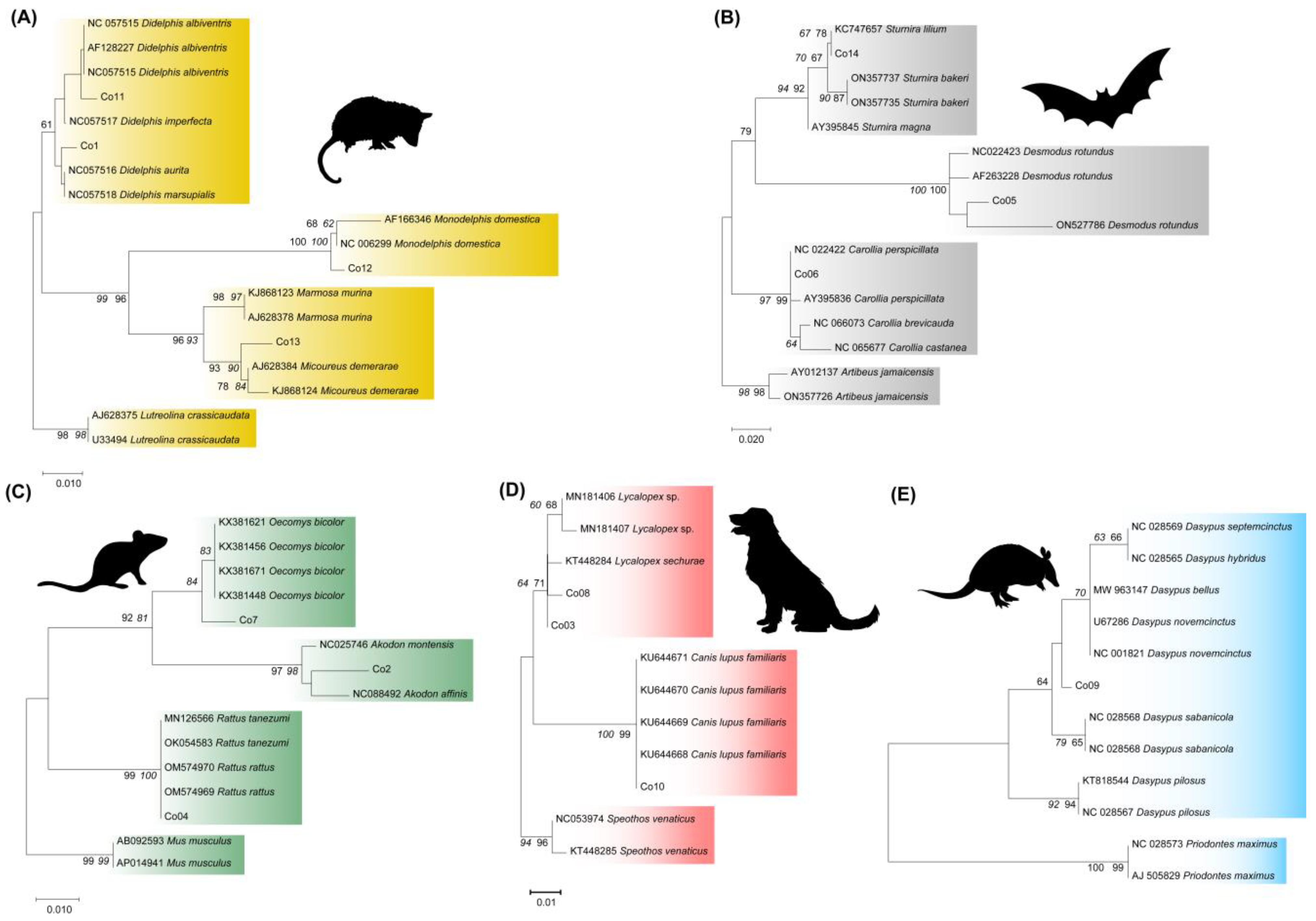
3.1. Evaluation of the Specificity of Mammalian Species Identification Using BMS Molecular Targets and T. cruzi Infection
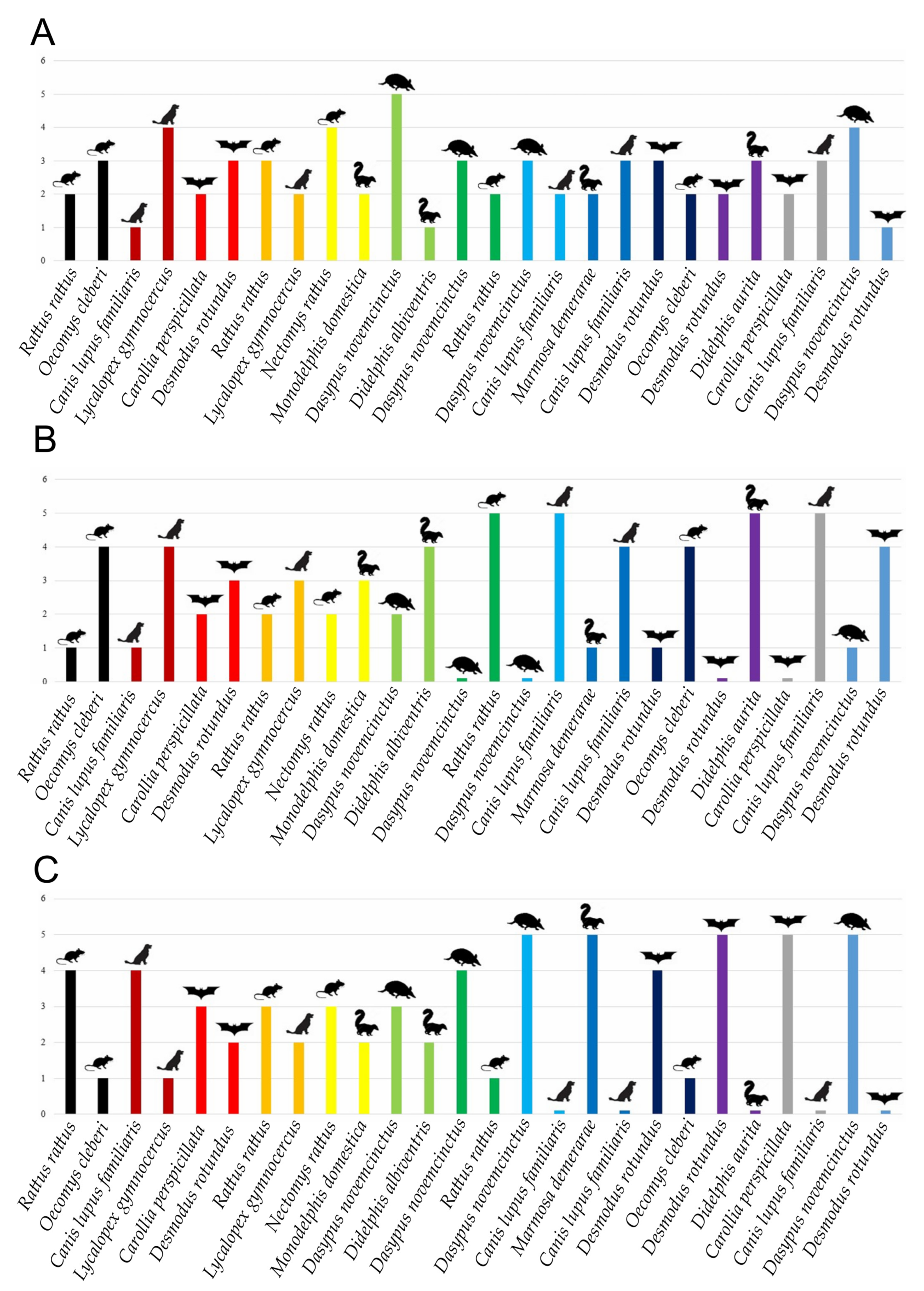
3.2. Sensitivity Assessment of Detecting Multiple Blood Clot DNAs
4. Discussion
4.1. Specificity of Molecular Species Identification
4.2. Sensitivity in Detecting Multiple DNA Samples from Blood Clots
4.3. The Importance of the Specificity and Sensitivity in Recovered Mammalian DNA for a Better Understanding of the Life Cycle of T. cruzi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, A.M.; Xavier, S.C.D.C.; Roque, A.L.R. Landmarks of the Knowledge and Trypanosoma cruzi Biology in the Wild Environment. Front. Cell. Infect. Microbiol. 2020, 6, 10. [Google Scholar] [CrossRef]
- Chagas, C. Descoberta do Tripanozoma cruzi e verificação da Tripanozomiase Americana: Retrospecto histórico. Mem. Inst. Oswaldo Cruz 1922, 15, 67–76. [Google Scholar] [CrossRef]
- WHO. Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease (accessed on 23 July 2024).
- Ministério da Saúde. Situação Epidemiológica. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/doenca-de-chagas/situacao-epidemiologica (accessed on 24 July 2024).
- Jansen, A.M.; Xavier, S.C.C.; Roque, A.L.R. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015, 151, 1–15. [Google Scholar] [CrossRef]
- Abreu, E.F.; Casali, D.; Costa-Araújo, R.; Garbino, G.S.T.; Libardi, G.S.; Loretto, D.; Loss, A.C.; Marmontel, M.; Moras, L.M.; Nascimento, M.C.; et al. Lista de Mamíferos do Brasil. Zenodo. 2022. Available online: https://zenodo.org/record/7469767 (accessed on 10 January 2025).
- Deane, M.P.; Lenzi, H.L.; Jansen, A. Trypanosoma cruzi: Vertebrate and invertebrate cycles in the same mammal host, the opossum Didelphis marsupialis. Mem. Inst. Oswaldo Cruz 1984, 79, 513–515. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.d.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasites Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Dario, M.A.; Furtado, C.; Lisboa, C.V.; De Oliveira, F.; Santos, F.M.; D’Andrea, P.S.; Roque, A.L.R.; Das Chagas Xavier, S.C.; Jansen, A.M. Trypanosomatid Richness Among Rats, Opossums, and Dogs in the Caatinga Biome, Northeast Brazil, a Former Endemic Area of Chagas Disease. Front. Cell. Infect. Microbiol. 2022, 12, 851903. [Google Scholar] [CrossRef]
- Carreira, J.C.A.; Jansen, A.M.; De Nazareth Meirelles, M.; Costa ESilva, F.; Lenzi, H.L. Trypanosoma cruzi in the Scent Glands of Didelphis marsupialis: The Kinetics of Colonization. Exp. Parasitol. 2001, 97, 129–140. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Cruickshank, C.; Stevens, J.R.; Teixeira, M.M.G.; Mathews, F. Parasites reveal movement of bats between the New and Old Worlds. Mol. Phylogenetics Evol. 2012, 63, 521–526. [Google Scholar] [CrossRef]
- Alves, F.M.; Rangel, D.A.; Vilar, E.M.; Pavan, M.G.; Moratelli, R.; Roque, A.L.R.; Jansen, A.M. Trypanosoma spp. Neobats: Insights about those poorly known trypanosomatids. Int. J. Parasitol. Parasites Wildl. 2021, 16, 145–152. [Google Scholar] [CrossRef]
- Dario, M.A.; Moratelli, R.; Schwabl, P.; Jansen, A.M.; Llewellyn, M.S. Small subunit ribosomal metabarcoding reveals extraordinary trypanosomatid diversity in Brazilian bats. PLoS Negl. Trop. Dis. 2017, 11, e0005790. [Google Scholar] [CrossRef]
- Torres, J.M.; De Oliveira, C.E.; Santos, F.M.; Sano, N.Y.; Martinez, É.V.; Alves, F.M.; Tavares, L.E.R.; Roque, A.L.R.; Jansen, A.M.; Herrera, H.M. Trypanosomatid diversity in a bat community of an urban area in Campo Grande, Mato Grosso do Sul, Brazil. Infect. Genet. Evol. 2024, 118, 105563. [Google Scholar] [CrossRef] [PubMed]
- Austen, J.M.; Barbosa, A.D. Diversity and Epidemiology of Bat Trypanosomes: A One Health Perspective. Pathogens 2021, 10, 1148. [Google Scholar] [CrossRef] [PubMed]
- Lilioso, M.; Reigada, C.; Pires-Silva, D.; Fontes Fvon, H.M.; Limeira, C.; Monsalve-Lara, J.; Folly-Ramos, E.; Harry, M.; Costa, J.; Almeida, C.E. Dynamics of food sources, ecotypic distribution and Trypanosoma cruzi infection in Triatoma brasiliensis from the northeast of Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0008735. [Google Scholar] [CrossRef]
- Santos, F.M.; Sano, N.Y.; Liberal, S.C.; Dario, M.A.; Nantes, W.A.G.; Alves, F.M.; da Silva, A.R.; De Oliveira, C.E.; Roque, A.L.R.; Herrera, H.M.; et al. Kinetoplastid Species Maintained by a Small Mammal Community in the Pantanal Biome. Pathogens 2022, 11, 1205. [Google Scholar] [CrossRef]
- Jansen, A.M.; Roque, A.L.R.; Xavier, S.C.C. Trypanosoma cruzi enzootic cycle. In American Trypanosomiasis Chagas Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–282. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128010297000125 (accessed on 30 January 2025).
- Urbano, P.; Hernández, C.; Ballesteros, N.; Vega, L.; Alvarado, M.; Velásquez-Ortiz, N.; Martínez, D.; Barragán, K.; Ramírez, A.; Páez-Triana, L.; et al. Exploring dietary differences among developmental stages of triatomines infected with Trypanosoma cruzi in different habitats. Int. J. Parasitol. 2024, 54, 559–568. [Google Scholar] [CrossRef]
- Urbano, P.; Hernández, C.; Velásquez-Ortiz, N.; Ballesteros, N.; Páez-Triana, L.; Vega, L.; Urrea, V.; Ramírez, A.; Muñoz, M.; Ibarra-Cerdeña, C.N.; et al. Transmission ecology of Trypanosoma cruzi by Rhodnius prolixus (Reduviidae: Triatominae) infesting palm-tree species in the Colombian Orinoco, indicates risks to human populations. PLoS Negl. Trop. Dis. 2024, 18, e0011981. [Google Scholar] [CrossRef]
- Almeida, C.E.; Máximo, M.M.; Pires-Silva, D.; Takiya, D.M.; Valença-Barbosa, C.; Viana, M.C.; Reigada, C.; Iñiguez, A.M.; Harry, M.; Folly-Ramos, E. From molecules to ecosystems: Insights into a network of interactions for a Chagas disease outbreak using Triatoma brasiliensis as natural samplers. Acta Trop. 2024, 251, 107107. [Google Scholar] [CrossRef]
- Da Silva Leal, A.R.; Da Silva Ferreira, A.L.; De Araujo-Pereira, T.; Torres De Sousa, R.L.; Furtado Campos, J.H.; Tavares Dos Reis, R.; Finamore-Araujo, P.; Diotaiuti, L.; Ferreira, F.C.; Mendonça, V.J.; et al. Eco-epidemiological aspects and risk factors associated with human Chagas disease in rural areas of the state of Piauí, Brazil. BMC Infect. Dis. 2024, 24, 1335. [Google Scholar] [CrossRef]
- Daflon-Teixeira, N.F.; Coutinho, C.; Gomes, T.F.; Toma, H.K.; Duarte, R.; Bóia, M.N.; Carvalho-Costa, F.A.; Almeida, C.E.; Lima, M.M. Multiple Approaches to Address Potential Risk Factors of Chagas Disease Transmission in Northeastern Brazil. Am. J. Trop. Med. Hyg. 2019, 100, 296–302. [Google Scholar] [CrossRef]
- De Siqueira, A.F. Estudos Sôbre a Reação Identificação de Sangue da Precipitina Aplicada à Ingerido por Triatomíneos. Rev. Inst. Med. Trop. São Paulo 1960, 2, 41–53. [Google Scholar]
- Silva, M.B.A.; de Menezes, K.R.; de Farias, M.C.G.; Andrade, M.S.; Victor, C.C.A.; Lorosa, E.S.; Jurberg, J. Description of the feeding preferences of triatominae in the Chagas disease surveillance study for the State of Pernambuco, Brazil (Hemiptera: Reduviidae). Rev. Soc. Bras. Med. Trop. 2017, 50, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Alencar, J.; De Mello, C.F.; Gil-Santana, H.R.; de Leão Giupponi, A.P.; Araújo, A.N.; Lorosa, E.S.; Guimarães, A.E.; dos Santos Silva, J. Feeding Patterns of Mosquitoes (Diptera: Culicidae) in the Atlantic Forest, Rio de Janeiro, Brazil. J. Med. Èntomol. 2015, 52, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Verly, T.; Costa, S.; Lima, N.; Mallet, J.; Odêncio, F.; Pereira, M.; Moreira, C.J.D.C.; Britto, C.; Pavan, M.G. Vector competence and feeding-excretion behavior of Triatoma rubrovaria (Blanchard, 1843) (Hemiptera: Reduviidae) infected with Trypanosoma cruzi TcVI. PLoS Negl. Trop. Dis. 2020, 14, e0008712. [Google Scholar] [CrossRef]
- Almeida, C.E.; Faucher, L.; Lavina, M.; Costa, J.; Harry, M. Molecular Individual-Based Approach on Triatoma brasiliensis: Inferences on Triatomine Foci, Trypanosoma cruzi Natural Infection Prevalence, Parasite Diversity and Feeding Sources. PLoS Negl. Trop. Dis. 2016, 10, e0004447. Available online: https://repositorio.unesp.br/handle/11449/172623 (accessed on 6 April 2019). [CrossRef]
- Valença-Barbosa, C.; Fernandes, F.A.; Santos, H.L.C.; Sarquis, O.; Harry, M.; Almeida, C.E.; Lima, M.M. Molecular Identification of Food Sources in Triatomines in the Brazilian Northeast: Roles of Goats and Rodents in Chagas Disease Epidemiology. Am. J. Trop. Med. Hyg. 2015, 93, 994–997. [Google Scholar] [CrossRef]
- Lilioso, M.; Pires-Silva, D.; Fontes, F.V.H.M.; Oliveira, J.; da Rosa, J.A.; Vilela, R.V.; Folly-Ramos, E.; Almeida, C.E. Triatoma petrocchiae (Hemiptera, Reduviidae, Triatominae): A Chagas disease vector of T. brasiliensis species complex associated to reptiles. Infect. Genet. Evol. 2020, 82, 104307. [Google Scholar] [CrossRef]
- Valença-Barbosa, C.; Finamore-Araujo, P.; Moreira, O.C.; Alvarez, M.V.N.; Borges-Veloso, A.; Barbosa, S.E.; Diotaiuti, L.; de Souza, R.d.C.M. High Parasitic Loads Quantified in Sylvatic Triatoma melanica, a Chagas Disease Vector. Pathogens 2022, 11, 1498. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Lima, L.; Xavier, S.C.D.C.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Int. J. Parasitol. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Kitano, T.; Umetsu, K.; Tian, W.; Osawa, M. Two universal primer sets for species identification among vertebrates. Int. J. Leg. Med. 2006, 121, 423–427. [Google Scholar] [CrossRef]
- Borghesan, T.C.; Ferreira, R.C.; Takata, C.S.; Campaner, M.; Borda, C.C.; Paiva, F.; Milder, R.V.; Teixeira, M.M.; Camargo, E.P. Molecular Phylogenetic Redefinition of Herpetomonas (Kinetoplastea, Trypanosomatidae), a Genus of Insect Parasites Associated with Flies. Protist 2013, 164, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Pessanha, T.S.; Herrera, H.M.; Jansen, A.M.; Iñiguez, A.M. “Mi Casa, Tu Casa”: The coati nest as a hub of Trypanosoma cruzi transmission in the southern Pantanal biome revealed by molecular blood meal source identification in triatomines. Parasites Vectors 2023, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Pessanha, T.S.; Pires, M.; Iñiguez, A.M. Molecular Detection of Blood Meal Source up to Three Months since the Last Meal: Experimental Starvation Resistance in Triatomines. Acta Trop. 2022, 232, 106507. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B. GeneDoc: A Tool for Editing and Annotating Multiple Sequence Alignments. 1997. Available online: https://api.semanticscholar.org/ (accessed on 31 May 2025).
- Steiner, C.; Tilak, M.-K.; Douzery, E.J.; Catzeflis, F.M. New DNA data from a transthyretin nuclear intron suggest an Oligocene to Miocene diversification of living South America opossums (Marsupialia: Didelphidae). Mol. Phylogenetics Evol. 2005, 35, 363–379. [Google Scholar] [CrossRef]
- Voss, R.S.; Jansa, S.A. Phylogenetic Relationships and Classification of Didelphid Marsupials, an Extant Radiation of New World Metatherian Mammals. Bull. Am. Mus. Nat. Hist. 2009, 322, 1–177. [Google Scholar] [CrossRef]
- Agrizzi, J. Molecular Diagnosis of Atlantic Forest Mammals Using Mitochondrial DNA Sequences: Didelphid Marsupials. TOZJ 2012, 5, 2–9. [Google Scholar] [CrossRef][Green Version]
- Fuentes-González, J.A.; Muñoz-Durán, J. Filogenia de los cánidos actuales (Carnivora: Canidae) mediante análisis de congruencia de caracteres bajo parsimonia. Actual. Biol. 2017, 34, 85–102. [Google Scholar] [CrossRef]
- Savolainen, P.; Zhang, Y.-P.; Luo, J.; Lundeberg, J.; Leitner, T. Genetic Evidence for an East Asian Origin of Domestic Dogs. Science 2002, 298, 1610–1613. [Google Scholar] [CrossRef]
- Vilà, C.; Savolainen, P.; Maldonado, J.E.; Amorim, I.R.; Rice, J.E.; Honeycutt, R.L.; Crandall, K.A.; Lundeberg, J.; Wayne, R.K. Multiple and Ancient Origins of the Domestic Dog. Science 1997, 276, 1687–1689. [Google Scholar] [CrossRef]
- Gentry, A. Mammal Species of the World, 3rd ed. In A Taxonomic and Geographic Reference; Don, E.W., DeeAnn, M.R., Eds.; Johns Hopkins University Press: Baltimore, ML, USA, 2005; Volume 2, 2142p, ISBN 0-8018-8221-4. A nomenclatural review. Bull. Zool. Nomencl. 2006, 63, 215–219. [Google Scholar]
- Asfora, P.H.; Palma, A.R.T.; Astúa, D.; Geise, L. Distribution of Oecomys catherinae Thomas, 1909 (Rodentia: Cricetidae) in northeastern Brazil with karyotypical and morphometrical notes. Biota Neotrop. 2011, 11, 415–424. [Google Scholar] [CrossRef]
- Mendes-Oliveira, A.C. Pequenos Mamíferos Não-Voadores da Amazônia Brasileira; Sociedade Brasileira de Mastozoologia: Rio de Janeiro, RJ, Brazil, 2014; Volume 1, 333p, ISBN 8563705024. [Google Scholar]
- Suárez-Villota, E.Y.; Carmignotto, A.P.; Brandão, M.V.; Percequillo, A.R.; Silva, M.J.D.J. Systematics of the genus Oecomys (Sigmodontinae: Oryzomyini): Molecular phylogenetic, cytogenetic and morphological approaches reveal cryptic species. Zool. J. Linn. Soc. 2017, 184, 182–210. [Google Scholar] [CrossRef]
- Rocha, R.G.; Justino, J.; Leite, Y.L.R.; Costa, L.P. DNA from owl pellet bones uncovers hidden biodiversity. Syst. Biodivers. 2015, 13, 403–412. [Google Scholar] [CrossRef]
- Kocher, A.; de Thoisy, B.; Catzeflis, F.; Huguin, M.; Valière, S.; Zinger, L.; Bañuls, A.; Murienne, J. Evaluation of short mitochondrial metabarcodes for the identification of Amazonian mammals. Methods Ecol. Evol. 2017, 8, 1276–1283. [Google Scholar] [CrossRef]
- Müller, L.; Gonçalves, G.L.; Cordeiro-Estrela, P.; Marinho, J.R.; Althoff, S.L.; Testoni, A.F. DNA Barcoding of Sigmodontine Rodents: Identifying Wildlife Reservoirs of Zoonoses. PLoS ONE 2013, 8, e80282. [Google Scholar] [CrossRef]
- FUNASA; Ministério da Saude. Manual de Controle de Roedores. In Assessoria de Comunicação e Educação em Saude; FUNASA: Tokyo, Japan, 2002. [Google Scholar]
- Vilela, J.F.; Mello, B.; Voloch, C.M.; Schrago, C.G. Sigmodontine rodents diversified in South America prior to the complete rise of the Panamanian Isthmus. J. Zool. Syst. Evol. Res. 2013, 52, 249–256. [Google Scholar] [CrossRef]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 2003, 55, 541–555. [Google Scholar] [CrossRef]
- Pacheco, V.; Patterson, B.D. Phylogenetic Relationships of the New World Bat Genus Sturnira (Chiroptera: Phyllostomidae). Bull. Am. Mus. Nat. Hist. 1991, 206, 101–121. [Google Scholar]
- Dinelli, L.L. Três Espécies Crípticas em Sturnira lilium (Chiroptera: Phyllostomidae): Evidências Baseadas em Genes Mitocondriais. Master’s Thesis, Universidade Federal do Espírito Santo, Centro de Ciências Humanas e Naturais, Vitória, Brazil, March 2014. Available online: http://repositorio.ufes.br/handle/10/3844 (accessed on 31 May 2025).
- Villalobos, F.; Valerio, A.A. The phylogenetic relationships of the bat genus Sturnira Gray, 1842 (Chiroptera: Phyllostomidae). Mamm. Biol. 2002, 67, 268–275. [Google Scholar] [CrossRef]
- Lemos, T.H.; Da Cunha Tavares, V.; Moras, L.M. Character variation and taxonomy of short-tailed fruit bats from Carollia in Brazil. Zoologia 2020, 37, e34587. [Google Scholar] [CrossRef]
- De Machado, J.C.A. Biogeografia Cladística e Sistemática Molecular de Tatus do Gênero Dasypus e Uma Abordagem Filogeográfica da Espécie D. novemcinctus (Mammalia, Cingulata, Dasypodidae). Master’s Thesis, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil, 2017. Available online: http://www.bdtd.uerj.br/handle/1/5874 (accessed on 31 May 2025).
- Feijó, A.; Cordeiro-Estrela, P. The Correct Name of the Endemic Dasypus (Cingulata: Dasypodidae) from North western Argentina. Zootaxa 2014, 3887, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, F.; Oria-Martínez, B.; Rendón-Franco, E.; Villalobos, G.; Muñoz-García, C.I. Trypanosoma cruzi, beyond the dogma of non-infection in birds. Infect. Genet. Evol. 2022, 99, 105239. [Google Scholar] [CrossRef] [PubMed]
- Botto-Mahan, C.; Correa, J.P.; Araya-Donoso, R.; Farías, F.; San Juan, E.; Quiroga, N.; Campos-Soto, R.; Reyes-Olivares, C.; González-Acuña, D. Lizards as Silent Hosts of Trypanosoma cruzi. Emerg. Infect. Dis. 2022, 28, 1250–1253. Available online: https://wwwnc.cdc.gov/eid/article/28/6/22-0079_article.htm (accessed on 1 August 2023). [CrossRef]
- Stevens, L.; Dorn, P.L.; Hobson, J.; de la Rua, N.M.; Lucero, D.E.; Klotz, J.H.; Schmidt, J.O.; Klotz, S.A. Vector Blood Meals and Chagas Disease Transmission Potential, United States. Emerg. Infect. Dis. 2012, 18, 646–649. [Google Scholar] [CrossRef]
- Stevens, L.; Monroy, M.C.; Rodas, A.G.; Dorn, P.L. Hunting, Swimming, and Worshiping: Human Cultural Practices Illuminate the Blood Meal Sources of Cave Dwelling Chagas Vectors (Triatoma dimidiata) in Guatemala and Belize. PLoS Negl. Trop. Dis. 2014, 8, e3047. [Google Scholar] [CrossRef]
- Arias-Giraldo, L.M.; Muñoz, M.; Hernández, C.; Herrera, G.; Velásquez-Ortiz, N.; Cantillo-Barraza, O.; Urbano, P.; Cuervo, A.; Ramírez, J.D. Identification of blood-feeding sources in Panstrongylus, Psammolestes, Rhodnius and Triatoma using amplicon-based next-generation sequencing. Parasites Vectors 2020, 13, 434. [Google Scholar] [CrossRef]
- Legey, A.P.; Pinho, A.P.; Xavier, S.C.; Marchevsky, R.; Carreira, J.C.; Leon, L.L.; Jansen, A.M. Trypanosoma cruzi in marsupial didelphids (Philander frenata and Didelphis marsupialis): Differences in the humoral immune response in natural and experimental infections. Rev. Soc. Bras. Med. Trop. 2003, 36, 241–248. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Xavier, S.C.C.; da Rocha, M.G.; Duarte, A.C.M.; D’andrea, P.S.; Jansen, A.M. Trypanosoma cruzi Transmission Cycle Among Wild and Domestic Mammals in Three Areas of Orally Transmitted Chagas Disease Outbreaks. Am. J. Trop. Med. Hyg. 2008, 79, 742–749. [Google Scholar] [CrossRef]
- Herrera, L.; D’Andrea, P.S.; Xavier, S.C.C.; Mangia, R.H.; Fernandes, O.; Jansen, A.M. Trypanosoma cruzi infection in wild mammals of the National Park ‘Serra da Capivara’ and its surroundings (Piauí, Brazil), an area endemic for Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 379–388. [Google Scholar] [CrossRef]
- Raccurt, C.P. Trypanosoma cruzi in French Guinea: Review of accumulated data since 1940. Med. Trop. 1996, 56, 79–87. [Google Scholar]
- Beard, C.B.; Pye, G.; Steurer, F.J.; Rodriguez, R.; Campman, R.; Peterson, A.T.; Ramsey, J.; Wirtz, R.A.; Robinson, L.E. Chagas Disease in a Domestic Transmission Cycle in Southern Texas, USA. Emerg. Infect. Dis. 2003, 9, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, M.V.; Lauricella, M.A.; Ceballos, L.A.; Lanati, L.; Marcet, P.L.; Levin, M.J.; Kitron, U.; Gürtler, R.E.; Schijman, A.G. Molecular epidemiology of domestic and sylvatic Trypanosoma cruzi infection in rural northwestern Argentina. Int. J. Parasitol. 2008, 38, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.E.; Cimino, R.; Mackern-Oberti, J.P.; Martín, C.M.-S.; Cattan, P.E.; Superina, M. Eco-epidemiological Survey of Trypanosoma cruzi in Dogs from Mendoza, Argentina. Ecohealth 2025, 22, 79–90. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Turriago, B.; Tapia-Calle, G.; Guhl, F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Veter. Parasitol. 2013, 196, 216–219. [Google Scholar] [CrossRef]
- Rocha, F.L.; Roque, A.L.R.; De Lima, J.S.; Cheida, C.C.; Lemos, F.G.; de Azevedo, F.C.; Arrais, R.C.; Bilac, D.; Herrera, H.M.; Mourão, G.; et al. Trypanosoma cruzi Infection in Neotropical Wild Carnivores (Mammalia: Carnivora): At the Top of the T. cruzi Transmission Chain. PLoS ONE 2013, 8, e67463. [Google Scholar] [CrossRef]
- Sillero-Zubiri, C. Family Canidae (dogs). In Handbook Mammals of the World; Lynx Ediciones: Barcelona, Spain, 2009; pp. 352–447. [Google Scholar]
- Butler, J.; du Toit, J.; Bingham, J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: Threats of competition and disease to large wild carnivores. Biol. Conserv. 2004, 115, 369–378. [Google Scholar] [CrossRef]
- Pinto, C.M.; Ocaña-Mayorga, S.; Lascano, M.S.; Grijalva, M.J. Infection by trypanosomes in marsupials and rodents associated with human dwellings in Ecuador. J. Parasitol. 2006, 92, 1251–1255. [Google Scholar] [CrossRef]
- Cab-Romero, M.; da Costa, A.P.; Costa, J.O.J.; de Oliveira Manhães, I.B.; da Silva, R.E.; Ruiz-Piña, H.A.; Reyes-Novelo, E.A.; Escobedo-Ortegona, J.; Tonhosolo, R.; Marcili, A. Synanthropic mammals in transmission cycle of Trypanosoma cruzi in Yucatán, Mexico. Braz. J. Glob. Health 2020, 1, 21–28. [Google Scholar] [CrossRef]
- Brandão, E.M.V.; Xavier, S.C.C.; Carvalhaes, J.G.; D’Andrea, P.S.; Lemos, F.G.; Azevedo, F.C.; Cássia-Pires, R.; Jansen, A.M.; Roque, A.L.R. Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens 2019, 8, 190. [Google Scholar] [CrossRef]
- Hernández, C.; Salazar, C.; Brochero, H.; Teherán, A.; Buitrago, L.S.; Vera, M.; Soto, H.; Florez-Rivadeneira, Z.; Ardila, S.; Parra-Henao, G.; et al. Untangling the transmission dynamics of primary and secondary vectors of Trypanosoma cruzi in Colombia: Parasite infection, feeding sources and discrete typing units. Parasites Vectors 2016, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.M.; Ocaña-Mayorga, S.; Tapia, E.E.; Lobos, S.E.; Zurita, A.P.; Aguirre-Villacís, F.; MacDonald, A.; Villacís, A.G.; Lima, L.; Teixeira, M.M.G.; et al. Bats, Trypanosomes, and Triatomines in Ecuador: New Insights into the Diversity, Transmission, and Origins of Trypanosoma cruzi and Chagas Disease. PLoS ONE 2015, 10, e0139999. [Google Scholar] [CrossRef] [PubMed]
- Argibay, H.D.; Orozco, M.M.; Cardinal, M.V.; Rinas, M.A.; Arnaiz, M.; Segura, C.M.; Gürtler, R.E. First finding of Trypanosoma cruzi II in vampire bats from a district free of domestic vector-borne transmission in Northeastern Argentina. Parasitology 2016, 143, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Añez, N.; Crisante, G.; Soriano, P.J. Trypanosoma cruzi congenital transmission in wild bats. Acta Trop. 2009, 109, 78–80. [Google Scholar] [CrossRef]
| Mammalian Species | Common Name in Brazil | Collection Site 1 | Brazilian Biome |
|---|---|---|---|
| Canis lupus familiaris | Domestic dog | Mangaratiba-RJ | Atlantic Forest |
| Cerdocyon thous | Bush dog | Mamamguape-PB | Atlantic Forest |
| Lycalopex gymnocercus | Field fox | Alegrete-RS | Pampa |
| Oecomys cleberi | Tree mouse | Cumari-GO | Cerrado |
| Necromys lasiurus | Pixuna | Mamamguape-PB | Atlantic Forest |
| Rattus rattus | Rat | Paraty-RJ | Atlantic Forest |
| Desmodus rotundus | Vampire bat | Mamamguape-PB | Atlantic Forest |
| Sturnira lilium | Fruit bat | Mamamguape-PB | Atlantic Forest |
| Carollia perspicillata | Short-tail bat | Mamamguape-PB | Atlantic Forest |
| Didelphis albiventris | White-ear possum | Mamamguape-PB | Atlantic Forest |
| Monodelphis domestica | Short-tailed cuica | Mamamguape-PB | Atlantic Forest |
| Marmosa (Micoureus) demerarae | Cuica | Mamamguape-PB | Atlantic Forest |
| Didelphis aurita | Black-ear possum | Mamamguape-PB | Atlantic Forest |
| Dasypus novemcinctus | Armadillo | Mamamguape-PB | Atlantic Forest |
| Mammal Species | Blast/NCBI Genetic Identity Result | Maximum Genetic Identity | GenBank Accession Number | Blast/NCBI Genetic Identity Result | Maximum Genetic Identity | GenBank Accession Number |
|---|---|---|---|---|---|---|
| Molecular Target 12S rDNA | Molecular Target cytb | |||||
| Canis lupus familiaris | Canis lupus familiaris | 100% | KU291093 | Canis lupus familiaris | 99% | DQ309764 |
| Lycalopex gymnocercus | Lycalopex sechurae | 98.63% | KT228284 | Lycalopex sechurae | 99.06% | KT447686 |
| Cerdocyon thous | Lycalopex sechurae | 98.00% | KT448284 | ND | ND | ND |
| Oecomys cleberi | Oecomys bicolor | 98.92% | JF693852.1 | Oecomys cleberi | 98.88% | KR190443 |
| Necromys lasiurus | Akodon montensis | 97.70% | KF769456 | Bolomys lasiurus | 98.57% | KR190443 |
| Rattus rattus | Rattus rattus | 100% | OM574970.1 | Rattus rattus | 99.00% | KT232247 |
| Desmodus rotundus | Desmodus rotundus | 97.00% | HG003310 | Desmodus rotundus | 99.00% | HG003310 |
| Sturnira lilium | Sturnira lilium | 100% | KC747657 | Sturnira lilium | 100% | KC747657 |
| Carollia perspicillata | Carollia perspicillata | 100% | HG003309 | Carollia perspicillata | 100% | FJ589715.1 |
| Didelphis albiventris | Didelphis albiventris | 99.36% | NC057515.1 | Didelphis albiventris | 99.85% | AF089802 |
| Monodelphis domestica | Monodelphis domestica | 99.53% | AJ508398 | Monodelphis domestica | 100% | KM071418 |
| Marmosa (Micoureus) demerarae | Marmosa (Micoureus) demerarae | 99.05% | AJ628384.1 | Marmosa (Micoureus) demerarae | 98.80% | AJ487006 |
| Didelphis aurita | Didelphis aurita | 99.08% | NC057518.1 | Didelphis aurita | 99.40% | GU112886 |
| Dasypus novemcinctus | Dasypus yesepi | 100% | NC028570 | Dasypus novemcinctus | 99.00% | KU253494 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, Q.M.; Pessanha, T.S.; Iñiguez, A.M. Genetic Identification of Brazilian Mammalian Hosts of Trypanosoma cruzi: Improving Blood Meal Source Discrimination in Vector-Borne Transmission. Pathogens 2025, 14, 579. https://doi.org/10.3390/pathogens14060579
Oliveira QM, Pessanha TS, Iñiguez AM. Genetic Identification of Brazilian Mammalian Hosts of Trypanosoma cruzi: Improving Blood Meal Source Discrimination in Vector-Borne Transmission. Pathogens. 2025; 14(6):579. https://doi.org/10.3390/pathogens14060579
Chicago/Turabian StyleOliveira, Quezia Moura, Thaíla Santos Pessanha, and Alena Mayo Iñiguez. 2025. "Genetic Identification of Brazilian Mammalian Hosts of Trypanosoma cruzi: Improving Blood Meal Source Discrimination in Vector-Borne Transmission" Pathogens 14, no. 6: 579. https://doi.org/10.3390/pathogens14060579
APA StyleOliveira, Q. M., Pessanha, T. S., & Iñiguez, A. M. (2025). Genetic Identification of Brazilian Mammalian Hosts of Trypanosoma cruzi: Improving Blood Meal Source Discrimination in Vector-Borne Transmission. Pathogens, 14(6), 579. https://doi.org/10.3390/pathogens14060579







