The Effects of High-Dose Probiotic Supplementation on Immune Activation and Neurocognitive Disorders in People Living with HIV Undergoing Successful Antiretroviral Treatment: The Procog Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Plasma Markers of Immune Activation
2.3. Neuropsychological Testing and Definition of HAND
- (1)
- ANI, which involves at least two cognitive domains, is documented by performance at least 1 SD below the mean on NP tests, occurring without interference in everyday functioning. The asymptomatic impairment characteristics are defined by the short version of the Instrumental Activity of Daily Living battery and by patient questioning.
- (2)
- MND, characterized by involvement in at least two cognitive domains, is documented by performance of at least 1 SD below the mean on NP tests and is associated with mild interference in daily functioning.
- (3)
- HIV-associated dementia (HAD) is characterized by deficits in at least two cognitive domains, evidenced by performance at least two standard deviations below the normative mean on neuropsychological tests, leading to significant interference in daily functioning.
2.4. Randomization
- (1)
- Continuing ART unchanged;
- (2)
- Incorporating a 6-month course of high-dose oral probiotics into ART.
2.5. Demographic Parameters, Background Measurements, and Dietary Habits
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Patients at the Time of Inclusion and Trajectory of the Inflammatory Markers
3.2. Trends in Neuropsychological Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, A.; Zaongo, S.D.; Harypursat, V.; Wang, X.; Ouyang, J. HIV-associated neurocognitive disorder: Key implications of the microbiota-gut-brain axis. Front. Microbiol. 2024, 15, 1428239. [Google Scholar] [CrossRef] [PubMed]
- d’Arminio Monforte, A.; Cinque, P.; Mocroft, A.; Goebel, F.D.; Antunes, F.; Katlama, C.; Justesen, U.S.; Vella, S.; Kirk, O.; Lundgren, J. EuroSIDA Study Group Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann. Neurol. 2004, 55, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Mussini, C.; Antinori, A.; Walker, A.S.; Dorrucci, M.; Sabin, C.; Phillips, A.; Porter, K.; CASCADE Collaboration. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann. Neurol. 2008, 63, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lescure, F.X.; Omland, L.H.; Engsig, F.N.; Roed, C.; Gerstoft, J.; Pialoux, G.; Kronborg, G.; Larsen, C.S.; Obel, N. Incidence and impact on mortality of severe neurocognitive disorders in persons with and without HIV infection: A Danish nationwide cohort study. Clin. Infect. Dis. 2011, 52, 235–243. [Google Scholar] [CrossRef]
- Makinson, A.; Dubois, J.; Eymard-Duvernay, S.; Leclercq, P.; Zaegel-Faucher, O.; Bernard, L.; Vassallo, M.; Barbuat, C.; Gény, C.; Thouvenot, E.; et al. Increased Prevalence of Neurocognitive Impairment in Aging People Living with Human Immunodeficiency Virus: The ANRS EP58 HAND 55–70 Study. Clin. Infect. Dis. 2019, 70, 2641–2648. [Google Scholar] [CrossRef]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.C.; Mpongo, N.; et al. Cognitive impairment in people living with HIV: Consensus recommendations for a new approach. Nat. Rev. Neurol. 2023, 19, 424–433. [Google Scholar] [CrossRef]
- Alford, K.; Daley, S.; Banerjee, S.; Vera, J.H. Quality of life in people living with HIV-associated neurocognitive disorder: A scoping review study. PLoS ONE 2021, 16, e0251944. [Google Scholar] [CrossRef]
- Elendu, C.; Aguocha, C.M.; Okeke, C.V.; Okoro, C.B.; Peterson, J.C. HIV-related neurocognitive disorders: Diagnosis, Treatment, and Mental Health Implications: A Review. Medicine 2023, 102, e35652. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; Leblanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef]
- Bandera, A.; Taramasso, L.; Bozzi, G.; Muscatello, A.; Robinson, J.A.; Burdo, T.H.; Gori, A. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front. Aging Neurosci. 2019, 11, 187. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Yan, J.; Zhou, X.; Isnard, S.; Harypursat, V.; Cui, H.; Routy, J.P.; Chen, Y. Relevance of biomarkers indicating gut damage and microbial translocation in people living with HIV. Front. Immunol. 2023, 14, 1173956. [Google Scholar] [CrossRef] [PubMed]
- MacCann, R.; Landay, A.L.; Mallon, P.W.G. HIV and comorbidities—The importance of gut inflammation and the kynurenine pathway. Curr. Opin. HIV AIDS 2023, 18, 102–110. [Google Scholar] [PubMed]
- Ceccarelli, G.; Brenchley, J.M.; Cavallari, E.N.; Scheri, G.C.; Fratino, M.; Pinacchio, C.; Schietroma, I.; Fard, S.N.; Scagnolari, C.; Mezzaroma, I.; et al. Impact of High-Dose Multi-Strain Probiotic Supplementation on Neurocognitive Performance and Central Nervous System Immune Activation of HIV-1 Infected Individuals. Nutrients 2017, 9, 1269. [Google Scholar] [CrossRef]
- Serrano-Villar, S.; Vázquez-Castellanos, J.F.; Vallejo, A.; Latorre, A.; Sainz, T.; Ferrando-Martínez, S.; Rojo, D.; Martínez-Botas, J.; Del Romero, J.; Madrid, N.; et al. The effects of prebiotics on microbial dysbiosis, butyrate production and immunity in HIV-infected subjects. Mucosal Immunol. 2017, 10, 1279–1293. [Google Scholar] [CrossRef]
- Fu, Y.S.; Chu, Q.S.; Ashuro, A.A.; Di, D.S.; Zhang, Q.; Liu, X.M.; Fan, Y.G. The Effect of Probiotics, Prebiotics, and Synbiotics on CD4 Counts in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2020, 2020, 7947342. [Google Scholar] [CrossRef]
- Carter, G.M.; Esmaeili, A.; Shah, H.; Indyk, D.; Johnson, M.; Andreae, M.; Sacks, H.S. Probiotics in Human Immunodeficiency Virus Infection: A Systematic Review and Evidence Synthesis of Benefits and Risks. Open Forum Infect. Dis. 2016, 3, ofw164. [Google Scholar] [CrossRef]
- Hunt, P.W. Soluble CD163 and Clinical Outcomes in Treated HIV Infection: Insights into Mechanisms. J. Infect. Dis. 2016, 214, 1132–1133. [Google Scholar] [CrossRef]
- Lv, T.; Cao, W.; Li, T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. J. Immunol. Res. 2021, 2021, 7316456. [Google Scholar] [CrossRef]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Pasquereau, S.; Kumar, A.; Herbein, G. Targeting TNF and TNF Receptor Pathway in HIV-1 Infection: From Immune Activation to Viral Reservoirs. Viruses 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, M.; Videloup, L.; Lobbedez, T.; Lebreuilly, J.; Morello, R. Thuillier Lecouf A Improved Detection and Evaluation of Depression in Patients with Chronic Kidney Disease: Validity and Reliability of Screening (PHQ-2) and Diagnostic (BDI-FS-Fr) Tests of Depression in Chronic Kidney Disease. Kidney Dis. 2019, 5, 228–238. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Chatzinikolaou, A.; Tzikas, S.; Lavdaniti, M. Assessment of Quality of Life in Patients With Cardiovascular Disease Using the SF-36, MacNew, and EQ-5D-5L Questionnaires. Cureus 2021, 13, e17982. [Google Scholar] [CrossRef]
- Carey, C.L.; Woods, S.P.; Gonzalez, R.; Conover, E.; Marcotte, T.D.; Grant, I.; Heaton, R.K. HNRC Group Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J. Clin. Exp. Neuropsychol. 2004, 26, 307–319. [Google Scholar] [CrossRef]
- Blackstone, K.; Moore, D.J.; Franklin, D.R.; Clifford, D.B.; Collier, A.C.; Marra, C.M.; Gelman, B.B.; McArthur, J.C.; Morgello, S.; Simpson, D.M.; et al. Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. Clin. Neuropsychol. 2012, 26, 894–908. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Van der Linden, M.; Coyette, F.; Poitrenaud, J.; Kalafat, M.; Calicis, F.; Wyns, C.; Adam, S. L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16). In L’évaluation des Troubles de la Mémoire: Présentation de Quatre Tests de Mémoire Épisodique (avec leur Étalonnage); Solal: Marseille, France, 2004. [Google Scholar]
- Sander, K.; Roth, P.; Scheich, H. Left-lateralized fMRI activation in the temporal lobe of high repressive women during the identification of sad prosodies. Brain Res. Cogn. Brain Res. 2003, 16, 441–456. [Google Scholar] [CrossRef]
- Fastenau, P.S.; Denburg, N.L.; Hufford, B.J. Adult norms for the Rey-Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the Extended Complex Figure Test. Clin. Neuropsychol. 1999, 13, 30–47. [Google Scholar] [CrossRef]
- Casarotti, A.; Papagno, C.; Zarino, B. Modified Taylor Complex Figure: Normative data from 290 adults. J. Neuropsychol. 2014, 8, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Forn, C.; Belenguer, A.; Parcet-Ibars, M.A.; Avila, C. Information-processing speed is the primary deficit underlying the poor performance of multiple sclerosis patients in the Paced Auditory Serial Addition Test (PASAT). J. Clin. Exp. Neuropsychol. 2008, 30, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Cardebat, D.; Doyon, B.; Puel, M.; Goulet, P.; Joanette, Y. Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol. Belg. 1990, 90, 207–217. [Google Scholar] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Sun, M.F.; Shen, Y.Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Younas, M.; Psomas, C.; Reynes, J.; Corbeau, P. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med. 2016, 17, 89–105. [Google Scholar] [CrossRef]
- Li, S.X.; Armstrong, A.; Neff, C.P.; Shaffer, M.; Lozupone, C.A.; Palmer, B.E. Complexities of Gut Microbiome Dysbiosis in the Context of HIV Infection and Antiretroviral Therapy. Clin. Pharmacol. Ther. 2016, 99, 600–611. [Google Scholar] [CrossRef]
- Subra, C.; Trautmann, L. Role of T Lymphocytes in HIV Neuropathogenesis. Curr. HIV/AIDS Rep. 2019, 16, 236–243. [Google Scholar] [CrossRef]
- Joseph, J.; Colosi, D.A.; Rao, V.R. HIV-1 Induced CNS Dysfunction: Current Overview and Research Priorities. Curr. HIV Res. 2016, 14, 389–399. [Google Scholar] [CrossRef]
- Clayton, K.L.; Collins, D.R.; Lengieza, J.; Ghebremichael, M.; Dotiwala, F.; Lieberman, J.; Walker, B.D. Resistance of HIV-infected macrophages to CD8+ T lymphocyte-mediated killing drives activation of the immune system. Nat. Immunol. 2018, 19, 475–486. [Google Scholar] [CrossRef]
- Weber, M.T.; Finkelstein, A.; Uddin, M.N.; Reddy, E.A.; Arduino, R.C.; Wang, L.; Tivarus, M.E.; Zhong, J.; Qiu, X.; Schifitto, G. Longitudinal Effects of Combination Antiretroviral Therapy on Cognition and Neuroimaging Biomarkers in Treatment-Naive People With HIV. Neurology 2022, 99, e1045–e1055. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.M.; Zhao, Y.; Clifford, D.B.; Letendre, S.; Evans, S.; Henry, K.; Ellis, R.J.; Rodriguez, B.; Coombs, R.W.; Schifitto, G.; et al. AIDSClinical Trials Group 736 Study Team Impact of combination antiretroviral therapy on cerebrospinal fluid HIVRNA, neurocognitive performance. Aids 2009, 23, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Letendre, S.; Vaida, F.; Haubrich, R.; Heaton, R.K.; Sacktor, N.; Clifford, D.B.; Best, B.M.; May, S.; Umlauf, A.; et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin. Infect. Dis. 2014, 58, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Ownby, R.L.; Acevedo, A. A pilot study of cognitive training with and without transcranial direct current stimulation to improve cognition in older persons with HIV-related cognitive impairment. Neuropsychiatr. Dis. Treat. 2016, 12, 2745–2754. [Google Scholar] [CrossRef]
- Livelli, A.; Orofino, G.C.; Calcagno, A.; Farenga, M.; Penoncelli, D.; Guastavigna, M.; Carosella, S.; Caramello, P.; Pia, L. Evaluation of a Cognitive Rehabilitation Protocol in HIV Patients with Associated Neurocognitive Disorders: Efficacy and Stability Over Time. Front. Behav. Neurosci. 2015, 9, 306. [Google Scholar] [CrossRef]
- Ruet, A.; Deloire, M.S.; Charré-Morin, J.; Hamel, D.; Brochet, B. A new computerised cognitive test for the detection of information processing speed impairment in multiple sclerosis. Mult. Scler. 2013, 19, 1665–1672. [Google Scholar] [CrossRef]
- Reitan, R.M. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955, 19, 393–394. [Google Scholar] [CrossRef]
- Ruchinskas, R. Wechsler adult intelligence scale-4th edition digit span performance in subjective cognitive complaints, amnestic mild cognitive impairment, and probable dementia of the Alzheimer type. Clin. Neuropsychol. 2019, 33, 1436–1444. [Google Scholar] [CrossRef]
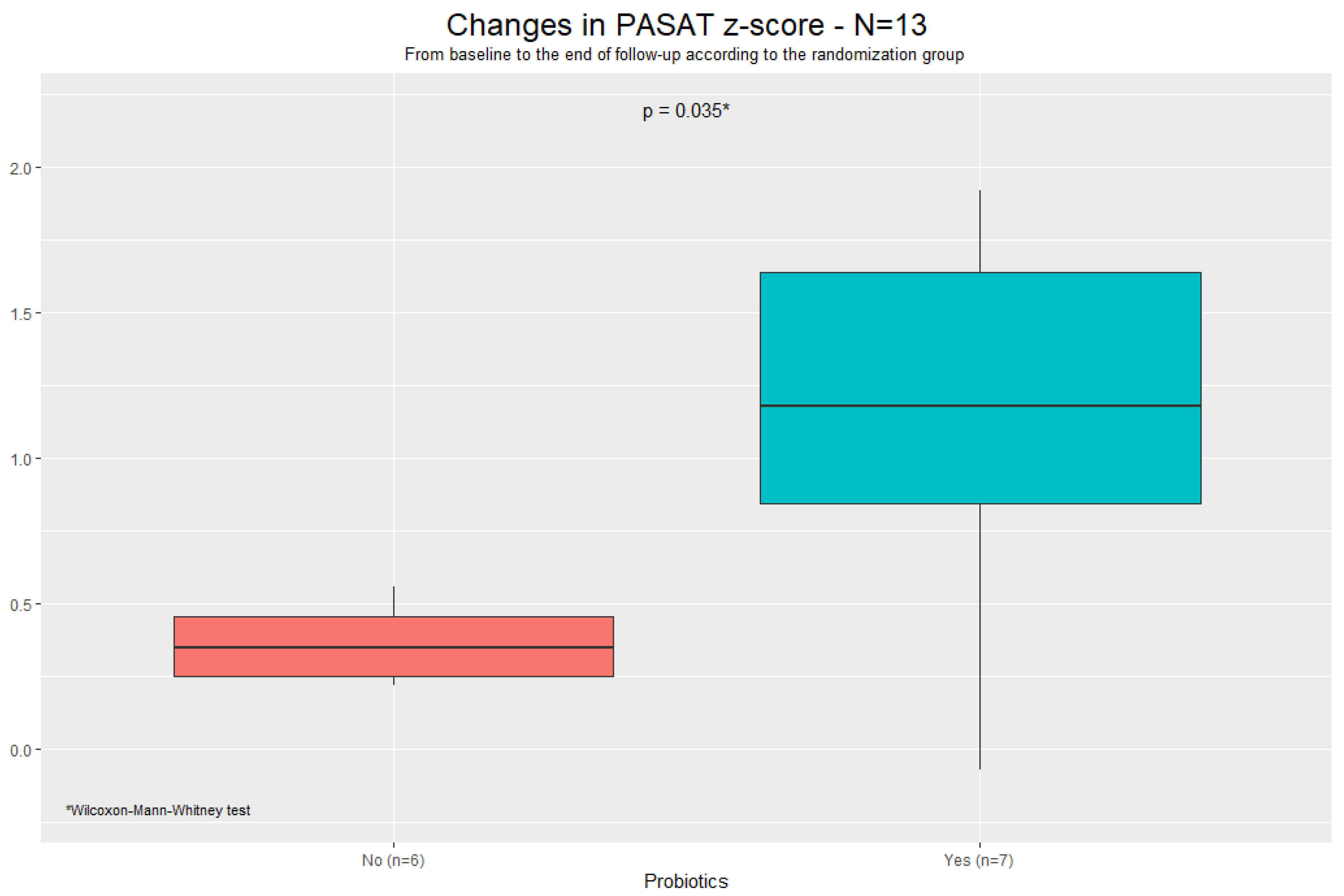
| Total—N = 31 | Randomization | |||||||
|---|---|---|---|---|---|---|---|---|
| No Probiotics—n = 15 | Probiotics—n = 16 | |||||||
| n | Median | [Q1; Q3] | Median | [Q1; Q3] | Median | [Q1; Q3] | p-Value * | |
| Age at inclusion (years) | 31 | 62.0 | [57.0; 71.0] | 63.0 | [56.0; 73.0] | 60.5 | [57.0; 66.3] | 0.553 |
| Years of study (n) | 30 | 9.0 | [9.0; 12.0] | 9.0 | [8.5; 10.5] | 11.0 | [9.0; 12.0] | 0.191 |
| CD4 count at inclusion (cc/mm3) | 31 | 744.0 | [558.5; 816.0] | 612.0 | [506.0; 803.5] | 773.0 | [662.3; 880.5] | 0.188 |
| CD8 count at inclusion (cc/mm3) | 31 | 840.0 | [541.5; 1105.5] | 733.0 | [486.5; 1105.5] | 891.5 | [604.3; 1072.3] | 0.892 |
| Nadir CD4 (cc/mm3) | 30 | 246.0 | [133.3; 396.3] | 184.0 | [47.5; 372.0] | 252.0 | [189.0; 460.0] | 0.161 |
| Viral load peak (cp/mL) | 21 | 82,640.0 | [8100.0; 150,000.0] | 91,000.0 | [12,200.0; 100,500.0] | 53,835.0 | [11,250.0; 197,250.0] | 0.973 |
| Years on ART (n) | 23 | 19.0 | [16.0; 26.0] | 18.5 | [17.0; 24.8] | 24.0 | [15.0; 26.0] | 1.000 |
| ART regimens received (n) | 22 | 7.0 | [4.3; 12.0] | 8.0 | [4.5; 10.8] | 6.5 | [4.5; 12.8] | 0.921 |
| Months on last ART (n) | 30 | 25.0 | [13.8; 38.8] | 25.0 | [16.5; 49.5] | 24.0 | [11.0; 31.0] | 0.443 |
| Daily consumption of yogurt (n) | 24 | 1.0 | [1.0; 2.0] | 1.0 | [1.0; 2.0] | 2.0 | [1.0; 2.0] | 0.657 |
| Daily consumption of cheese (n) | 24 | 1.0 | [1.0; 2.0] | 1.0 | [1.0; 2.0] | 1.0 | [1.0; 2.0] | 0.898 |
| Daily consumption of milk (centiliters) | 25 | 0.0 | [0.0; 100.0] | 0.0 | [0.0; 0.0] | 60.0 | [0.0; 162.5] | 0.027 |
| n | n | (%) | n | (%) | n | (%) | p-Value ** | |
| Sex | 31 | 1.000 | ||||||
| Female | 8 | (25.8) | 4 | (50.0) | 4 | (50.0) | ||
| Men | 23 | (74.2) | 11 | (47.8) | 12 | (52.2) | ||
| AIDS | 31 | 0.220 | ||||||
| No | 24 | (77.4) | 10 | (41.7) | 14 | (58.3) | ||
| Yes | 7 | (22.6) | 5 | (71.4) | 2 | (28.6) | ||
| Vegetarian diet | 31 | - | ||||||
| No | 28 | (100.0) | 14 | (50.0) | 14 | (50.0) | ||
| Missing | 3 | 1 | 2 | |||||
| Vegetalian diet | 31 | - | ||||||
| No | 29 | (100.0) | 14 | (48.3) | 15 | (51.7) | ||
| Missing | 2 | 1 | 1 | |||||
| Total—N = 28 | Probiotics | ||||||
|---|---|---|---|---|---|---|---|
| No—n = 14 | Yes—n = 14 | ||||||
| Median | [Q1; Q3] | Median | [Q1; Q3] | Median | [Q1; Q3] | p-Value * | |
| Il6 (pg/mL) | 2.0 | [1.5; 2.8] | 2.6 | [1.5; 4.0] | 1.7 | [1.5; 2.3] | 0.188 |
| hCRP (mg/L) | 1.2 | [0.5; 2.0] | 1.6 | [0.9; 2.4] | 0.7 | [0.4; 1.4] | 0.135 |
| sCD14 (ng/mL) | 2473.8 | [2104.3; 3473.4] | 2473.8 | [2137.3; 3104.1] | 2657.7 | [2056.4; 3518.9] | 0.910 |
| sCD163 (pg/mL) | 386.7 | [300.5; 491.4] | 376.8 | [307.7; 415.3] | 386.7 | [295.1; 505.9] | 0.839 |
| MCP-1 (pg/mL) | 329.9 | [273.7; 382.7] | 340.9 | [275.0; 381.0] | 326.8 | [281.8; 371.5] | 0.667 |
| sTNFR1 (pg/mL) | 1182.8 | [1003.7; 1671.8] | 1182.8 | [1054.0; 1736.3] | 1249.5 | [819.4; 1524.4] | 0.482 |
| sTNFR2 (pg/mL) | 2267.6 | [1809.8; 3068.0] | 2271.5 | [1892.6; 2642.7] | 2267.6 | [1581.2; 3200.4] | 0.701 |
| Probiotics | |||||
|---|---|---|---|---|---|
| No—n = 14 | Yes—n = 14 | ||||
| Median | [Q1; Q3] | Median | [Q1; Q3] | p-Value * | |
| Differences M6-D0 | |||||
| Il-6 (pg/mL) | −0.1 | [−1.1; 0.0] | 0.0 | [−0.2; 1.1] | 0.114 |
| hCRP (mg/L) | −0.4 | [−0.8; −0.0] | 0.3 | [−0.3; 2.3] | 0.031 |
| sCD14 (ng/mL) | −216.4 | [−748.1; 259.1] | −38.9 | [−751.9; 365.5] | 0.804 |
| sCD163 (pg/mL) | −1.2 | [−23.1; 112.4] | 54.8 | [31.8; 131.4] | 0.194 |
| MCP-1 (pg/mL) | −5.0 | [−51.0; 29.4] | −3.6 | [−62.0; 26.1] | 1.000 |
| sTNFR1 (pg/mL) | −70.6 | [−351.7; −25.4] | 133.3 | [−115.6; 431.4] | 0.094 |
| sTNFR2 (pg/mL) | −88.1 | [−393.8; 236.0] | 190.7 | [−156.9; 630.1] | 0.125 |
| Probiotics | ||||||
|---|---|---|---|---|---|---|
| No—n = 14 | Yes—n = 14 | |||||
| n | Median | [Q1; Q3] | Median | [Q1; Q3] | p-Value * | |
| Trajectory from baseline to month 6 | ||||||
| Language DS | 28 | 0.0 | [−0.8; 0.0] | −0.5 | [−0.5; 0.0] | 0.230 |
| Attention/working memory DS | 20 | 0.0 | [−0.2; 0.3] | −0.2 | [−0.4; 0.0] | 0.079 |
| Executive function DS | 28 | −0.1 | [−0.3; 0.4] | 0.0 | [−0.3; 0.0] | 0.944 |
| Learning DS | 28 | 0.0 | [−0.1; 0.4] | −0.1 | [−0.4; 0.3] | 0.366 |
| Memory DS | 20 | −0.1 | [−0.4; 0.0] | −0.3 | [−0.6; 0.2] | 0.711 |
| Information processing speed DS | 28 | 0.0 | [−0.8; 1.0] | 0.0 | [−1.0; 0.0] | 0.199 |
| GDS | 28 | 0.0 | [−0.2; 0.1] | −0.3 | [−0.4; −0.1] | 0.048 |
| IADL score | 28 | 0.0 | [−0.8; 0.8] | 0.0 | [0.0; 0.0] | 0.936 |
| BDI-FS score | 28 | −0.5 | [−3.8; 0.8] | −0.5 | [−2.8; 0.0] | 0.871 |
| SF-36 | ||||||
| Physical component summary | 28 | −0.3 | [−6.9; 6.1] | −3.8 | [−9.8; 2.2] | 0.748 |
| Mental component summary | 28 | 1.2 | [−3.2; 12.1] | 1.05 | [−12.5; 6.0] | 0.667 |
| n | n | (%) | n | (%) | p-Value ** | |
| GDS | 28 | 0.023 | ||||
| ≤−0.137 (median) | 4 | (28.6) | 10 | (71.4) | ||
| >−0.137 (median) | 10 | (71.4) | 4 | (28.6) | ||
| Finger tapping M6 | 28 | 0.445 | ||||
| <4 | 5 | (35.7) | 7 | (50.0) | ||
| 4 | 9 | (64.3) | 7 | (50.0) | ||
| Luria M6 | 28 | 0.705 | ||||
| <4 | 8 | (57.1) | 7 | (50.0) | ||
| 4 | 6 | (42.9) | 7 | (50.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassallo, M.; Zerlini, M.; Fabre, R.; Joly, H.; Durant, J.; Makinson, A.; Mauries, A.; Capeau, J.; Fellahi, S.; Bastard, J.-P.; et al. The Effects of High-Dose Probiotic Supplementation on Immune Activation and Neurocognitive Disorders in People Living with HIV Undergoing Successful Antiretroviral Treatment: The Procog Study. Pathogens 2025, 14, 568. https://doi.org/10.3390/pathogens14060568
Vassallo M, Zerlini M, Fabre R, Joly H, Durant J, Makinson A, Mauries A, Capeau J, Fellahi S, Bastard J-P, et al. The Effects of High-Dose Probiotic Supplementation on Immune Activation and Neurocognitive Disorders in People Living with HIV Undergoing Successful Antiretroviral Treatment: The Procog Study. Pathogens. 2025; 14(6):568. https://doi.org/10.3390/pathogens14060568
Chicago/Turabian StyleVassallo, Matteo, Margaux Zerlini, Roxane Fabre, Heloise Joly, Jacques Durant, Alain Makinson, Amandine Mauries, Jacqueline Capeau, Soraya Fellahi, Jean-Philippe Bastard, and et al. 2025. "The Effects of High-Dose Probiotic Supplementation on Immune Activation and Neurocognitive Disorders in People Living with HIV Undergoing Successful Antiretroviral Treatment: The Procog Study" Pathogens 14, no. 6: 568. https://doi.org/10.3390/pathogens14060568
APA StyleVassallo, M., Zerlini, M., Fabre, R., Joly, H., Durant, J., Makinson, A., Mauries, A., Capeau, J., Fellahi, S., Bastard, J.-P., Pradier, C., & Lebrun-Frenay, C. (2025). The Effects of High-Dose Probiotic Supplementation on Immune Activation and Neurocognitive Disorders in People Living with HIV Undergoing Successful Antiretroviral Treatment: The Procog Study. Pathogens, 14(6), 568. https://doi.org/10.3390/pathogens14060568








