Molecular Prevalence of Leishmania infantum Infection from Oral Swabs Collected from Dogs in Region of Northwestern Spain
Abstract
1. Introduction
2. Materials and Methods
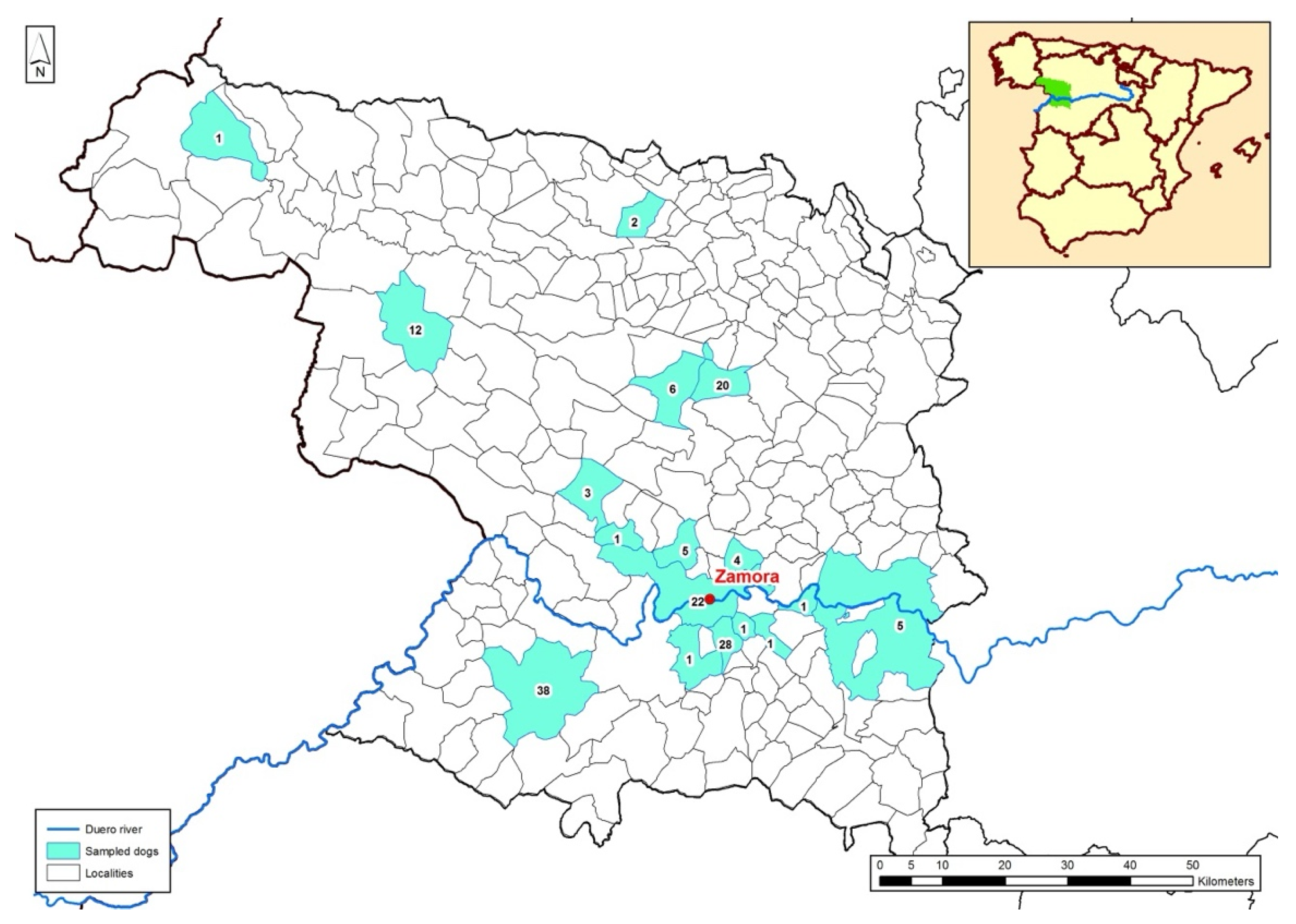
2.1. Animals Included in the Study and Geographical Setting
2.2. Sample Collection and Epidemiological Data
2.3. DNA Extraction
2.4. Real Time PCR (qPCR)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G. Leishmaniosis of companion animals in Europe: An update. Vet. Parasitol. 2015, 208, 35–47. [Google Scholar] [CrossRef]
- Vilas-Boas, D.F.; Nakasone, E.K.N.; Gonçalves, A.A.M.; Lair, D.F.; Oliveira, D.S.; Pereira, D.F.S.; Silva, G.G.; Conrado, I.D.S.S.; Resende, L.A.; Zaldívar, M.F.; et al. Global distribution of canine visceral leishmaniasis and the role of the dog in the epidemiology of the disease. Pathogens 2024, 13, 455. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing canine vector-borne diseases of zoonotic concern: Part one. Trends Parasitol. 2009, 25, 157–163. [Google Scholar] [CrossRef]
- Azami-Conesa, I.; Gómez-Muñoz, M.T.; Martínez-Díaz, R.A. A systematic review (1990–2021) of wild animals infected with zoonotic Leishmania. Microorganisms 2021, 9, 1101. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Control of Leishmaniasis. In Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniasis; WHO Technical Report Series, 949; WHO: Geneve, Switzerland, 2010. [Google Scholar]
- World Health Organization. Global Leishmaniasis update, 2006–2015: A turning point in Leishmaniasis surveillance. Wkly Epidemiol. Rec. 2017, 38, 557–572. [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease 2021: Findings from the GBD 2021 Study; IHME: Seattle, WA, USA, 2024; Available online: https://www.healthdata.org/sites/default/files/2024-05/GBD_2021_Booklet_FINAL_2024.05.16.pdf (accessed on 5 June 2025).
- Reguera, R.M.; Morán, M.; Pérez-Pertejo, Y.; García-Estrada, C.; Balaña-Fouce, R. Current status on prevention and treatment of canine leishmaniasis. Vet. Parasitol. 2016, 227, 98–114. [Google Scholar] [CrossRef]
- Ortega, M.V.; Moreno, I.; Domínguez, M.; de la Cruz, M.L.; Martín, A.B.; Rodríguez-Bertos, A.; López, R.; Navarro, A.; González, S.; Mazariegos, M.; et al. Application of a specific quantitative real-time PCR (qPCR) to identify Leishmania infantum DNA in spleen, skin and hair samples of wild Leporidae. Vet. Parasitol. 2017, 243, 92–99. [Google Scholar] [CrossRef]
- Francino, O.; Altet, L.; Sánchez-Robert, E.; Rodriguez, A.; Solano-Gallego, L.; Alberola, J.; Ferrer, L.; Sánchez, A.; Roura, X. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet. Parasitol. 2006, 137, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Aït-Oudhia, K.; Harrat, Z.; Benikhlef, R.; Dedet, J.P.; Pratlong, F. Canine Leishmania infantum enzymatic polymorphism: A review including 1023 strains of the Mediterranean area, with special reference to Algeria. Acta Trop. 2011, 118, 80–86. [Google Scholar] [CrossRef]
- Miró, G.; Checa, R.; Montoya, A.; Hernández, L.; Dado, D.; Gálvez, R. Current situation of Leishmania infantum infection in shelter dogs in northern Spain. Parasit. Vectors 2012, 5, 60. [Google Scholar] [CrossRef]
- Alonso, F.; Giménez Font, P.; Manchón, M.; Ruiz de Ybáñez, R.; Segovia, M.; Berriatua, E. Geographical variation and factors associated to seroprevalence of canine leishmaniosis in an endemic Mediterranean area. Zoonoses Public Health 2010, 57, 318–328. [Google Scholar] [CrossRef]
- Rodríguez-Escolar, I.; Balmori-de la Puente, A.; Collado-Cuadrado, M.; Bravo-Barriga, D.; Delacour-Estrella, S.; Hernández-Lambraño, R.E.; Sánchez Agudo, J.Á.; Morchón, R. Analysis of the current risk of Leishmania infantum transmission for domestic dogs in Spain and Portugal and its future projection in climate change scenarios. Front. Vet. Sci. 2024, 11, 1399772. [Google Scholar] [CrossRef]
- Gálvez, R.; Montoya, A.; Cruz, I.; Fernández, C.; Martín, O.; Checa, R.; Chicharro, C.; Migueláñez, S.; Marino, V.; Miró, G. Latest trends in Leishmania infantum infection in dogs in Spain, Part I: Mapped seroprevalence and sand fly distributions. Parasit. Vectors 2020, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Reguera, R.; Balaña-Fouce, R.; Cubría, J.C.; Bujidos, M.L.A.; Ordoñez, D. Putrescine uptake inhibition by aromatic diamidines in Leishmania infantum promastigotes. Biochem. Pharmacol. 1994, 47, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Merino Goyenechea, J.; Castilla Gómez de Agüero, V.; Palacios Alberti, J.; Balaña-Fouce, R.; Martínez Valladares, M. Occurrence of leishmaniasis in Iberian wolves in northwestern Spain. Microorganisms 2023, 11, 1179. [Google Scholar] [CrossRef]
- Geisweid, K.; Weber, K.; Sauter-Louis, C.; Hartmann, K. Evaluation of a conjunctival swab polymerase chain reaction for the detection of Leishmania infantum in dogs in a non-endemic area. Vet. J. 2013, 198, 187–192. [Google Scholar] [CrossRef]
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine leishmaniasis: Update on epidemiology, diagnosis, treatment, and prevention. Vet. Sci. 2022, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Velez, R.; Ballart, C.; Domenech, E.; Abras, A.; Fernández-Arévalo, A.; Gómez, S.A.; Tebar, S.; Muñoz, C.; Cairó, J.; Gállego, M. Seroprevalence of canine Leishmania infantum infection in the Mediterranean region and identification of risk factors: The example of North-Eastern and Pyrenean areas of Spain. Prev. Vet. Med. 2019, 162, 67–75. [Google Scholar] [CrossRef]
- Morillas, F.; Sánchez Rabasco, F.; Ocaña, J.; Martín-Sánchez, J.; Ocana-Wihelmi, J.; Acedo, C.; Sanchís-Marín, M.C. Leishmaniosis in the focus of the Axarquia region, Malaga province, southern Spain: A survey of the human, dog, and vector. Parasitol. Res. 1996, 82, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Baxarias, M.; Mateu, C.; Miró, G.; Solano-Gallego, L. Serological survey of Leishmania infantum in apparently healthy dogs in different areas of Spain. Vet. Med. Sci. 2023, 9, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Olmeda, P.; Díaz-Regañón, D.; Villaescusa, A.; Amusategui, I.; García, A.; Herrero, F.; Tesouro, M.A.; Rodríguez-Franco, F.; García-Sancho, M.; Martín-Fraile, D.; et al. Twenty-year evolution of Leishmania infantum infection in dogs in Valdeorras (Galicia, Northwestern Spain): Implication of climatic factors and preventive measures. Parasites Vectors 2024, 17, 281. [Google Scholar] [CrossRef]
- Peris, M.P.; Esteban-Gil, A.; Ortega-Hernández, P.; Morales, M.; Halaihel, N.; Castillo, J.A. Comparative study of Real-Time PCR (TaqMan Probe and Sybr Green), serological techniques (ELISA, IFA and DAT) and clinical signs evaluation, for the diagnosis of canine leishmaniasis in experimentally infected dogs. Microorganisms 2021, 9, 2627. [Google Scholar] [CrossRef]
- Quaresma, P.F.; Murta, S.M.; Ferreira Ede, C.; da Rocha-Lima, A.C.; Xavier, A.A.; Gontijo, C.M. Molecular diagnosis of canine visceral leishmaniasis: Identification of Leishmania species by PCR-RFLP and quantification of parasite DNA by real-time PCR. Acta Trop. 2009, 111, 289–294. [Google Scholar] [CrossRef]
- Castelli, G.; Bruno, F.; Reale, S.; Catanzaro, S.; Valenza, V.; Vitale, F. Molecular diagnosis of Leishmaniasis: Quantification of parasite load by a Real-Time PCR assay with high sensitivity. Pathogens 2021, 10, 865. [Google Scholar] [CrossRef]
- Belinchón-Lorenzo, S.; Parejo, J.C.; Iniesta, V.; Fernández-Cotrina, J.; Muñoz-Madrid, R.; Monroy, I.; Baz, V.; Gómez-Luque, A.; Serrano-Aguilera, F.J.; Barneto, J.L.; et al. First detection of Leishmania kDNA in canine cerumen samples by qPCR. Vet. Parasitol. 2016, 228, 65–68. [Google Scholar] [CrossRef]
- Corpas-López, V.; Merino-Espinosa, G.; Acedo-Sánchez, C.; Díaz-Sáez, V.; Morillas-Márquez, F.; Martín-Sánchez, J. Hair parasite load as a new biomarker for monitoring treatment response in canine leishmaniasis. Vet. Parasitol. 2016, 223, 20–25. [Google Scholar] [CrossRef]
- Cantos-Barreda, A.; Escribano, D.; Siriyasatien, P.; Cerón, J.J.; Thomas, M.C.; Afonso-Lehmann, R.N.; López, M.C.; Bernal, L.J.; Phumee, A.; Lubas, G.; et al. Detection of Leishmania infantum DNA by real-time PCR in saliva of dogs. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101542. [Google Scholar] [CrossRef]
- Aschar, M.; de Oliveira, E.T.; Laurenti, M.D.; Marcondes, M.; Tolezano, J.E.; Hiramoto, R.M.; Corbett, C.E.; da Matta, V.L. Value of the oral swab for the molecular diagnosis of dogs in different stages of infection with Leishmania infantum. Vet. Parasitol. 2016, 225, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Gradoni, L.; Ferroglio, E.; Zanet, S.; Mignone, W.; Venco, L.; Bongiorno, G.; Fiorentino, E.; Cassini, R.; Grillini, M.; Simonato, G.; et al. Monitoring and detection of new endemic foci of canine leishmaniosis in northern continental Italy: An update from a study involving five regions (2018–2019). Vet. Parasitol. Reg. Stud. Rep. 2022, 27, 100676. [Google Scholar] [CrossRef]
- Berrahal, F.; Mary, C.; Roze, M.; Berenger, A.; Escoffier, K.; Lamoroux, D.; Dunan, S. Canine leishmaniasis: Identification of asymptomatic carriers by polymerase chain reaction and immunoblotting. Am. J. Trop. Med. Hyg. 1996, 55, 273–277. [Google Scholar] [CrossRef]
- Cabral, M.; O’Grady, J.E.; Gomes, S.; Sousa, J.C.; Thompson, H.; Alexander, J. The immunology of canine leishmaniosis: Strong evidence for a developing disease spectrum from asymptomatic dogs. Vet. Parasitol. 1998, 76, 173–180. [Google Scholar] [CrossRef]
- Bravo-Barriga, D.; Ruiz-Arrondo, I.; Peña, R.E.; Lucientes, J.; Delacour-Estrella, S. Phlebotomine sand flies (Diptera, Psychodidae) from Spain: An updated checklist and extended distributions. Zookeys 2022, 1106, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Baneth, G.; Koutinas, A.F.; Solano-Gallego, L.; Bourdeau, P.; Ferrer, L. Canine leishmaniosis – new concepts and insights on an expanding zoonosis: Part one. Trends Parasitol. 2008, 24, 324–330. [Google Scholar] [CrossRef]
- Baneth, G.; Solano-Gallego, L. Leishmaniasis. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; Rabanal, R.; Fondevila, D.; Ramos, J.A.; Domingo, M. Skin lesions in canine leishmaniasis. J. Small Anim. Pract. 1988, 29, 381–388. [Google Scholar] [CrossRef]
- Martín-Sánchez, J.; Morales-Yuste, M.; Acedo-Sánchez, C.; Barón, S.; Díaz, V.; Morillas-Márquez, F. Leishmaniasis in Southeastern Spain. Emerg. Infect. Dis. 2009, 15, 795–798. [Google Scholar] [CrossRef]
- Rombolà, P.; Barlozzari, G.; Carvelli, A.; Scarpulla, M.; Iacoponi, F.; Macrì, G. Seroprevalence and risk factors associated with exposure to Leishmania infantum in dogs, in an endemic Mediterranean region. PLoS ONE 2021, 16, e0244923. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, R.; Miró, G.; Descalzo, M.A.; Nieto, J.; Dado, D.; Martín, O.; Cubero, E.; Molina, R. Emerging trends in the seroprevalence of canine leishmaniasis in the Madrid region (central Spain). Vet. Parasitol. 2010, 169, 327–334. [Google Scholar] [CrossRef]
- Carvalho, F.S.; Wenceslau, A.A.; Albuquerque, G.R.; Munhoz, A.D.; Gross, E.; Carneiro, P.L.; Oliveira, H.C.; Rocha, J.M.; Santos, I.A.; Rezende, R.P. Leishmania (Viannia) braziliensis in dogs in Brazil: Epidemiology, co-infection, and clinical aspects. Genet. Mol. Res. 2015, 14, 12062–12073. [Google Scholar] [CrossRef]
- Miranda, S.; Roura, X.; Picado, A.; Ferrer, L.; Ramis, A. Characterization of sex, age, and breed for a population of canine leishmaniosis diseased dogs. Res. Vet. Sci. 2008, 85, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J. Ageing, immunosenescence and inflammageing in the dog and cat. J. Comp. Pathol. 2010, 142, S60–S69. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, L.; Marín-García, P.J.; Llobat, L. Serum levels and genetic variations of cytokines in two canine breeds (Ibizan hound and boxer) in the Mediterranean region, in terms of Leishmania infantum infection. Comp. Immunol. Microbiol. Infect. Dis. 2022, 90–91, 101908. [Google Scholar] [CrossRef]
- Oliveira, T.N.; Guedes, P.E.; Souza, G.B.; Carvalho, F.S.; Alberto Carlos, R.S.; Albuquerque, G.R.; Munhoz, A.D.; Silva, F.L. Diagnosis and epidemiology of canine leishmaniasis in southeastern Bahia, Brazil. Genet. Mol. Res. 2016, 15, 210. [Google Scholar] [CrossRef]
- Ballart, C.; Alcover, M.M.; Picado, A.; Nieto, J.; Castillejo, S.; Portús, M.; Gállego, M. First survey on canine leishmaniasis in a non-classical area of the disease in Spain (Lleida, Catalonia) based on a veterinary questionnaire and a cross-sectional study. Prev. Vet. Med. 2013, 109, 116–127. [Google Scholar] [CrossRef]
- Cabezón, O.; Martínez-Orellana, P.; Ribas, M.P.; Baptista, C.J.; Gassó, D.; Velarde, R.; Aguilar, X.F.; Solano-Gallego, L. Leishmania infection in wild lagomorphs and domestic dogs in north-east Spain. Animals 2024, 14, 1080. [Google Scholar] [CrossRef]
- Queiroz, P.V.; Monteiro, G.R.; Macedo, V.P.; Rocha, M.A.; Batista, L.M.; Queiroz, J.W.; Jerônimo, S.M.; Ximenes, M.F. Canine visceral leishmaniasis in urban and rural areas of Northeast Brazil. Res. Vet. Sci. 2009, 86, 267–273. [Google Scholar] [CrossRef]
| Factors associated with the dog | Age | Months |
| Hair length | Short: 1; Medium: 2; Long: 3 | |
| Sex | Male or Female | |
| Weight | kg | |
| Breed | Name of the breed | |
| Aptitude | Company: 1; Shepherd: 2; Hunting: 3 | |
| Factors associated with location | Sampling location | Name of the location |
| Most frequent habitat | Inside a house: 1; Outdoors (in a kennel): 2 | |
| Geographical environment | Urban: 1; Peri-urban: 2; Rural: 3 | |
| Geographical area relief | Plain: 1; Hill: 2; Mountain: 3 | |
| Overnight stay | Inside: 1; Outside: 2 | |
| Factors associated with possible clinical signs related to leishmaniasis | Presence or absence | Yes/No |
| Type of clinical signs | Swollen lymph nodes: 1 | |
| Dermatitis: 2 | ||
| Alopecia: 3 | ||
| Ulcers: 4 | ||
| Onychogryphosis: 5 | ||
| Epistaxis: 6 | ||
| Mucous membranes pallor: 7 | ||
| Ocular lesions: 8 | ||
| Weight loss: 9 | ||
| Intensity of clinical signs | Mild: 1; Moderate: 2; Severe: 3 |
| Dog Breed | N | Positive by qPCR |
|---|---|---|
| Spanish Alano | 2 | 1 |
| Blue Griffon of Gascony | 4 | 2 |
| Brittany Fawn Basset | 2 | 1 |
| Beagle | 2 | - |
| Bichon Maltes | 3 | 1 |
| Andalusian Buzzard | 2 | 1 |
| Boxer | 1 | 1 |
| German Barco | 1 | - |
| French Bulldog | 1 | - |
| Deutsch Drahthaar | 1 | - |
| German Pug or Great Dane | 1 | - |
| Epagneul Breton | 21 | 8 |
| Fox Terrier | 1 | - |
| Spanish Galgo | 13 | 2 |
| Golden Retriever | 4 | 3 |
| Spanish Mastin | 5 | 1 |
| Crossbreed or Mongrel Dog | 23 | 6 |
| Pachon Navarro Dog | 1 | - |
| German Sheperd | 1 | - |
| Brie Shepherd | 1 | - |
| Burgos Retriever | 2 | - |
| Petit Basset | 1 | - |
| Warren Hound | 25 | 10 |
| Spanish Bloodhound | 3 | 1 |
| Miniature Schnauzer | 2 | - |
| English Setter | 26 | 7 |
| Irish Setter | 2 | - |
| Variable | N | Positive (%) | N | Positive (%) | N | Positive (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | 80 | 27 (33.8%) | Female | 71 | 16 (22.5%) | - | - | - |
| Hair length | Short | 66 | 21 (31.8%) | Medium | 47 | 13 (27.7%) | Long | 38 | 11 (28.9%) |
| Aptitude | Company | 17 | 6 (35.3%) | Shepherd | 11 | 1 (9.1%) | Hunting | 123 | 38 (30.9%) |
| Population | Samples Collected | Positive by PCR | Percentage (%) |
|---|---|---|---|
| Arcenillas | 1 | 0 | - |
| Bermillo de Sayago | 38 | 18 | 11.92 |
| Carbajales de Alba | 3 | 0 | - |
| Zamora | 15 | 0 | - |
| Carrascal | 7 | 1 | 0.6 |
| Granja de Moreruela | 20 | 4 | 2.6 |
| Granucillo de Vidriales | 2 | 0 | - |
| La Inhiesta | 5 | 0 | - |
| Monfarracinos | 4 | 3 | 1.99 |
| Moraleja del Vino | 1 | 0 | - |
| Morales del Vino | 28 | 10 | 6.62 |
| El Perdigón | 1 | 1 | 0.6 |
| Pobladura de Aliste | 12 | 5 | 3.31 |
| Ribadelago | 1 | 0 | - |
| San Pedro de la Nave | 1 | 0 | - |
| Santa Eulalia de Tábara | 6 | 3 | 1.99 |
| Toro | 5 | 0 | - |
| Villalazán | 1 | 0 | |
| Total | 151 | 45 | 29.8 |
| Variable | N | Positive (%) | N | Positive (%) | N | Positive (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Most frequent habitat during the day | Inside a house | 6 | 1 (16.7%) | Outdoors | 142 | 44 (31.0%) | - | - | - |
| Geographical environment | Urban | 6 | 2 (33.3%) | Peri-urban | 59 | 13 (22.0%) | Rural | 83 | 30 (36.1%) |
| Geographical area relief | Plain | 109 | 36 (33.0%) | Hill | 39 | 9 (23.1%) | Mountain | 0 | 0 (0.00%) |
| Overnight stay | Inside | 138 | 42 (30.4%) | Outside | 11 | 3 (27.3%) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino-Goyenechea, J.; Valderas-García, E.; Castilla Gómez de Agüero, V.; Balaña-Fouce, R.; Martínez-Valladares, M. Molecular Prevalence of Leishmania infantum Infection from Oral Swabs Collected from Dogs in Region of Northwestern Spain. Pathogens 2025, 14, 569. https://doi.org/10.3390/pathogens14060569
Merino-Goyenechea J, Valderas-García E, Castilla Gómez de Agüero V, Balaña-Fouce R, Martínez-Valladares M. Molecular Prevalence of Leishmania infantum Infection from Oral Swabs Collected from Dogs in Region of Northwestern Spain. Pathogens. 2025; 14(6):569. https://doi.org/10.3390/pathogens14060569
Chicago/Turabian StyleMerino-Goyenechea, Javier, Elora Valderas-García, Verónica Castilla Gómez de Agüero, Rafael Balaña-Fouce, and María Martínez-Valladares. 2025. "Molecular Prevalence of Leishmania infantum Infection from Oral Swabs Collected from Dogs in Region of Northwestern Spain" Pathogens 14, no. 6: 569. https://doi.org/10.3390/pathogens14060569
APA StyleMerino-Goyenechea, J., Valderas-García, E., Castilla Gómez de Agüero, V., Balaña-Fouce, R., & Martínez-Valladares, M. (2025). Molecular Prevalence of Leishmania infantum Infection from Oral Swabs Collected from Dogs in Region of Northwestern Spain. Pathogens, 14(6), 569. https://doi.org/10.3390/pathogens14060569







