Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers of COVID-19 Disease Irrespective of Immunosuppression Status: A Case-Control Retrospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Population and Data Collection
2.3. Definitions
2.4. Outcomes
2.5. Statistical Analysis
2.6. Ethical Issues
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F., Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Petito, E.; Gresele, P. The role of platelets, neutrophils and endothelium in COVID-19 infection. Expert Rev. Hematol. 2022, 15, 727–745. [Google Scholar] [CrossRef]
- Liao, Y.C.; Liang, W.G.; Chen, F.W.; Hsu, J.H.; Yang, J.J.; Chang, M.S. IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha. J. Immunol. 2002, 169, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Asperges, E.; Albi, G.; Zuccaro, V.; Sambo, M.; Pieri, T.C.; Calia, M.; Colaneri, M.; Maiocchi, L.; Melazzini, F.; Lasagna, A.; et al. Dynamic NLR and PLR in Predicting COVID-19 Severity: A Retrospective Cohort Study. Infect. Dis. Ther. 2023, 12, 1625–1640. [Google Scholar] [CrossRef]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Jimeno, S.; Ventura, P.S.; Castellano, J.M.; García-Adasme, S.I.; Miranda, M.; Touza, P.; Lllana, I.; López-Escobar, A. Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur. J. Clin. Investig. 2021, 51, e13404. [Google Scholar] [CrossRef]
- Tadesse, Z.; Bekele Bayissa, A.; Diriba, T.; Chernet, N.; Tsegaye, S.; Tsega, M. Neutrophil-to-Lymphocyte Ratio and Cut-off Values as Predictor of Severity and Mortality in COVID-19 Patients in Millennium COVID-19 Care Center, Addis Ababa, Ethiopia. Int. J. Gen. Med. 2022, 15, 6739–6755. [Google Scholar] [CrossRef]
- Ali, H.S.; Ananthegowda, D.C.; Ebrahim, E.M.A.; Kannappilly, N.; Al Wraidat, M.; Mohamed, A.S.; Khatib, M.Y. Neutrophil-to-lymphocyte ratio as a predictor of clinical outcomes in critically ill COVID-19 patients: A retrospective observational study. Health Sci. Rep. 2022, 5, e844. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Khanna, P.; Singh, A.K. The impact of neutrophil-lymphocyte count ratio in COVID-19: A systematic review and meta-analysis. J. Intensive Care Med. 2022, 37, 857–869. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019, 39, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 483, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.; Genovese, C.; Fassio, F.; Canuti, M.; Giacomelli, A.; Ridolfo, A.L.; Asperges, E.; Albi, G.; Bruno, R.; Antinori, S.; et al. Prognostic Significance of NLR and PLR in COVID-19: A Multi-Cohort Validation Study. Infect. Dis. Ther. 2024, 13, 1147–1157. [Google Scholar] [CrossRef]
- Ulloque-Badaracco, J.R.; Ivan Salas-Tello, W.; Al-Kassab-Córdova, A.; Alarcón-Braga, E.A.; Benites-Zapata, V.A.; Maguiña, J.L.; Hernandez, A.V. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 patients: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14596. [Google Scholar] [CrossRef]
- Higaki, A.; Okayama, H.; Homma, Y.; Sano, T.; Kitai, T.; Yonetsu, T.; Torii, S.; Kohsaka, S.; Kuroda, S.; Node, K.; et al. Predictive value of neutrophil-to-lymphocyte ratio for the fatality of COVID-19 patients complicated with cardiovascular diseases and/or risk factors. Sci. Rep. 2022, 12, 13606. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kalemaki, D.; Tzagkarakis, E.; Lydakis, C. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect. Dis. 2018, 50, 163–174. [Google Scholar] [CrossRef]
- Nøst, T.H.; Alcala, K.; Urbarova, I.; Byrne, K.S.; Guida, F.; Sandanger, T.M.; Johansson, M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur. J. Epidemiol. 2021, 36, 841–848. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH) COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 22 March 2025).
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests; StatPearls Publishing, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489/?utm_source=chatgpt.com (accessed on 22 March 2025).
- Goyal, A.; Daneshpajouhnejad, P.; Hashmi, M.F.; Khalid, B. Acute Kidney Injury; StatPearls Publishing, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441896 (accessed on 22 March 2025).
- Giacaman, A.; Henriquez, W.; Tolosa, G.; Prado, A.; Jerez, R.; Reveco, Y.; Martínez, C.; Baumert, C.; Rodríguez, B.; Sanhueza, B.; et al. Hematological abnormalities in immunosuppressed patients with COVID-19: Evidence from a single center. A cross-sectional study. J. Int. Immunopharmacol. 2022, 109, 108862. [Google Scholar] [CrossRef]
- Rahman, A.; Niloofa, R.; Jayarajah, U.; De Mel, S.; Abeysuriya, V.; Seneviratne, S.L. Hematological Abnormalities in COVID-19: A Narrative Review. Am. J. Trop. Med. Hyg. 2021, 104, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in immunocompromised populations: Implications for prognosis and repurposing of immunotherapies. J. Immunother. Cancer 2021, 9, e002630. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Kannan, S.; Khanna, P.; Singh, A.K. Role of platelet-to-lymphocyte count ratio (PLR), as a prognostic indicator in COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 211–221. [Google Scholar] [CrossRef]
- Madera-Sandoval, R.L.; Cérbulo-Vázquez, A.; Arriaga-Pizano, L.A.; Cabrera-Rivera, G.L.; Basilio-Gálvez, E.; Miranda-Cruz, P.E.; García de la Rosa, M.T.; Prieto-Chávez, J.L.; Rivero-Arredondo, S.V.; Cruz-Cruz, A.; et al. Potential biomarkers for fatal outcome prognosis in a cohort of hospitalized COVID-19 patients with pre-existing comorbidities. Clin. Transl. Sci. 2023, 16, 2687–2699. [Google Scholar] [CrossRef]
- Yildiz, H.; Castanares-Zapatero, D.; Pierman, G.; Pothen, L.; De Greef, J.; Aboubakar Nana, F.; Rodriguez-Villalobos, H.; Belkhir, L.; Yombi, J.C. Validation of Neutrophil-to-Lymphocyte Ratio Cut-off Value Associated with High In-Hospital Mortality in COVID-19 Patients. Int. J. Gen. Med. 2021, 14, 5111–5117. [Google Scholar] [CrossRef]
- Prozan, L.; Shusterman, E.; Ablin, J.; Mitelpunkt, A.; Weiss-Meilik, A.; Adler, A.; Choshen, G.; Kehat, O. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 compared with Influenza and respiratory syncytial virus infection. Sci. Rep. 2021, 11, 21519. [Google Scholar] [CrossRef]
- Qu, R.; Ling, Y.; Zhang, Y.H.; Wei, L.Y.; Chen, X.; Li, X.M.; Liu, X.Y.; Liu, H.M.; Guo, Z.; Ren, H.; et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020, 92, 1533–1541. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.; Ni, W.; Shen, H.; Qiu, M.; Zhao, Y. Peripheral blood inflammatory markers in predicting prognosis in patients with COVID-19. Some differences with influenza A. J. Clin. Lab. Anal. 2021, 35, e23657. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Mao, Z.; Xiao, M.; Wang, L.; Qi, S.; Zhou, F. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis. Crit. Care 2020, 24, 647. [Google Scholar] [CrossRef]
- Dymicka-Piekarska, V.; Dorf, J.; Milewska, A.; Łukaszyk, M.; Kosidło, J.W.; Kamińska, J.; Wolszczak-Biedrzycka, B.; Naumnik, W. Neutrophil/Lymphocyte Ratio (NLR) and Lymphocyte/Monocyte Ratio (LMR)—Risk of Death Inflammatory Biomarkers in Patients with COVID-19. J. Inflamm. Res. 2023, 16, 2209–2222. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
| Total Sample n = 393 | Immunocompromised | |||
|---|---|---|---|---|
| No (n = 262; 66.7%) | Yes (n = 131; 33.3%) | |||
| n (%) | n (%) | n (%) | p-Value | |
| Gender | ||||
| Male | 199 (50.6) | 139 (53.1) | 60 (45.8) | 0.175 + |
| Female | 194 (49.4) | 123 (46.9) | 71 (54.2) | |
| Age (years), mean (SD) | 64.7 (16.1) | 64.3 (16.3) | 65.4 (15.7) | 0.544 ‡ |
| Smoking | ||||
| No | 286 (73.7) | 201 (77.3) | 85 (66.4) | <0.001 + |
| Yes | 51 (13.1) | 41 (15.8) | 10 (7.8) | |
| In the past | 51 (13.1) | 18 (6.9) | 33 (25.8) | |
| Vaccination status | ||||
| Any vaccination against COVID-19 | 209 (53.9) | 124 (48.1) | 85 (65.4) | 0.001 + |
| Partially vaccinated (≤2 doses) | 63 (16.0) | 57 (21.8) | 6 (4.6) | <0.001 + |
| Fully vaccinated (3 doses) | 116 (29.5) | 57 (21.8) | 59 (45.0) | <0.001 + |
| Boosted vaccinated (>3 doses) | 30 (7.6) | 10 (3.8) | 20 (15.3) | <0.001 + |
| Duration from last vaccine dose to hospitalization (days), median (IQR) | 9 (0–174) | 0 (0–171) | 69 (0–185) | 0.049 ‡ |
| COVID-19 disease | ||||
| Mild/Moderate | 180 (46) | 127 (48.8) | 53 (40.5) | 0.116 + |
| Severe | 211 (54) | 133 (51.2) | 78 (59.5) | |
| Variant | ||||
| Delta | 141 (35.9) | 98 (37.4) | 43 (32.8) | 0.372 + |
| Omicron | 252 (64.1) | 164 (62.6) | 88 (67.2) | |
| Anti-CD 20 monotherapy | 5 (1.3) | 0 (0) | 5 (3.8) | 0.004 ++ |
| COVID-19 treatment | ||||
| Duration of Remdesivir (days), median (IQR) | 5 (5–5) | 5 (5–5) | 5 (5–10) | <0.001 ‡ |
| Tocilizumab | 54 (13.7) | 46 (17.5) | 8 (6.1) | 0.002 + |
| Baricitinib | 23 (5.9) | 18 (6.9) | 5 (3.8) | 0.221 + |
| Anakinra | 25 (6.4) | 15 (5.7) | 10 (7.6) | 0.471 + |
| Steroids | 173 (44.0) | 123 (47.0) | 50 (38.2) | 0.063 + |
| Long duration on steroid therapy | 49 (12.5) | 20 (7.6) | 29 (22.1) | <0.001 + |
| Concomitant disease | 306 (77.9) | 175 (66.8) | 131 (100) | <0.001 + |
| Secondary infections | 94 | 0 | 94 | <0.001 ++ |
| Baseline WHO stage | ||||
| 3 | 177 (45.0) | 124 (47.3) | 53 (40.5) | 0.581 ++ |
| 4 | 196 (49.9) | 124 (47.3) | 72 (54.9) | |
| 5 | 17 (4.3) | 12 (4.6) | 5 (3.8) | |
| 6 | 3 (0.7) | 2 (0.7) | 1 (0.8) | |
| Maximum oxygen therapy | ||||
| No | 117 (29.8) | 69 (26.3) | 48 (36.6) | 0.091 ++ |
| Nasal cannula | 128 (32.6) | 88 (33.6) | 40 (30.5) | |
| Venturi Mask | 65 (16.5) | 46 (17.6) | 19 (14.5) | |
| HFNC | 30 (7.6) | 25 (9.5) | 5 (3.8) | |
| Non-rebreather mask | 5 (1.2) | 2 (0.8) | 3 (2.3) | |
| Intubation | 44 (11.2) | 29 (11.7) | 15 (11.5) | |
| Discharge oxygen therapy | ||||
| No oxygen need | 324 (82.4) | 214 (81.7) | 110 (84.0) | 0.013 ++ |
| Oxygen supply | 36 (9.2) | 20 (7.6) | 16 (12.2) | |
| Duration of hospitalization (days), median (IQR) | 9 (6–14) | 8 (5–14) | 10 (7–16) | 0.036 ‡‡ |
| Duration of intubation (days), median (IQR) | 12 (6–29) | 12 (7–31) | 10 (3–23) | 0.386 ‡‡ |
| Intubation | 44 (11.2) | 29 (11.7) | 15 (11.5) | 0.972 |
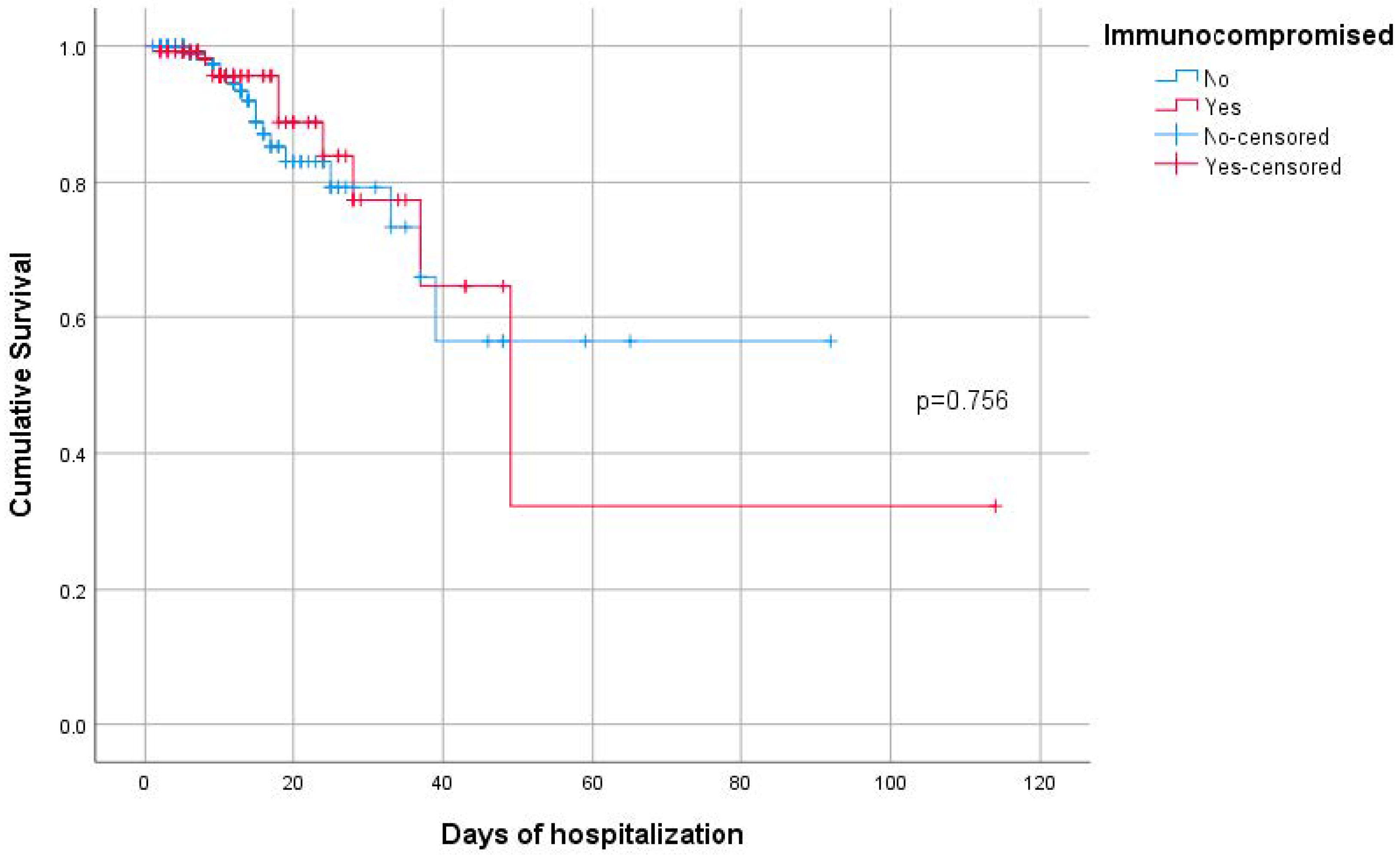
| Death | 30 (7.6) | 20 (7.6) | 10 (7.6) | >0.999 + |
| Total Sample n = 393 | Immunocompromised | |||
|---|---|---|---|---|
| No (n = 262; 66.7%) | Yes (n = 131; 33.3%) | |||
| n (%) | n (%) | n (%) | p-Value | |
| NLR (day 1), median (IQR) | 3.83 (2.24–6.89) | 3.7 (2.34–6.48) | 4.04 (1.86–8.53) | 0.812 ‡‡ |
| NLR (day 4), median (IQR) | 2.5 (1.5–5.5) | 2.62 (1.62–5.75) | 2.3 (1.41–5.24) | 0.275 ‡‡ |
| PLR (day 1), median (IQR) | 175.3 (114.7–269.3) | 173.5 (114.7–256.5) | 180 (114.6–296.6) | 0.464 ‡‡ |
| PLR (day 4), median (IQR) | 153.9 (97.7–245.2) | 158.5 (103.9–246.7) | 148.3 (83.4–244) | 0.284 ‡‡ |
| D-DIM (day 1) | ||||
| <1 | 236 (68.4) | 172 (71.7) | 64 (61) | 0.049 + |
| ≥1 | 109 (31.6) | 68 (28.3) | 41 (39) | |
| D-DIM (day 4) | ||||
| <1 | 77 (64.2) | 53 (63.1) | 24 (66.7) | 0.708 + |
| ≥1 | 43 (35.8) | 31 (36.9) | 12 (33.3) | |
| D-DIM (day 1) | ||||
| <0.5 | 130 (37.7) | 96 (40) | 34 (32.4) | 0.179 + |
| >0.5 | 215 (62.3) | 144 (60) | 71 (67.6) | |
| D-DIM (day 4) | ||||
| <0.5 | 48 (40) | 35 (41.7) | 13 (36.1) | 0.569 + |
| >0.5 | 72 (60) | 49 (58.3) | 23 (63.9) | |
| PCT (day 1) | ||||
| <0.25 | 147 (91.3) | 104 (93.7) | 43 (86) | 0.133 ++ |
| >0.25 | 14 (8.7) | 7 (6.3) | 7 (14) | |
| CRP (day 1) | ||||
| ≤10 | 311 (81) | 208 (81.9) | 103 (79.2) | 0.530 + |
| >10 | 73 (19) | 46 (18.1) | 27 (20.8) | |
| CRP (day 4) | ||||
| ≤10 | 293 (87.2) | 201 (89.3) | 92 (82.9) | 0.096 + |
| >10 | 43 (12.8) | 24 (10.7) | 19 (17.1) | |
| Fibr (day 1) | ||||
| ≤400 | 19 (19.0) | 10 (14.7) | 9 (28.1) | 0.111 + |
| >400 | 81 (81.0) | 58 (85.3) | 23 (71.9) | |
| Fibr (day 4) | ||||
| ≤400 | 26 (24.5) | 14 (18.9) | 12 (37.5) | 0.041 + |
| >400 | 80 (75.5) | 60 (81.1) | 20 (62.5) | |
| Fibr (day 1) | ||||
| ≤600 | 71 (71) | 45 (66.2) | 26 (81.3) | 0.121 + |
| >600 | 29 (29) | 23 (33.8) | 6 (18.8) | |
| Fibr (day 4) | ||||
| ≤600 | 87 (82.1) | 60 (81.1) | 27 (84.4) | 0.685 + |
| >600 | 19 (17.9) | 14 (18.9) | 5 (15.6) | |
| Liver dysfunction | 60 (15.5) | 48 (18.8) | 12 (9.2) | 0.014 + |
| Acute kidney injury | 62 (16.1) | 50 (19.7) | 12 (9.2) | 0.008 + |
| Thrombocytopenia | 102 (26.5) | 58 (22.6) | 44 (34.4) | 0.013 + |
| Median (IQR) | Median (IQR) | p-Value | |
|---|---|---|---|
| Variant: | Delta | Omicron | |
| NLR (day 1) | 3.08 (1.95–5.59) | 4.14 (2.47–7.95) | 0.003 |
| NLR (day 4) | 2.43 (1.51–4.94) | 2.52 (1.58–5.77) | 0.772 |
| PLR (day 1) | 180.0 (120.6–244.8) | 173.9(114.0–291.1) | 0.648 |
| PLR (day 4) | 162.8 (108.8–244.0) | 147.8 (92.8–246.3) | 0.380 |
| COVID-19 disease: | Mild/Moderate | Severe | |
| NLR (day 1) | 2.99 (2.05–5.01) | 4.68 (2.62–9.44) | <0.001 |
| NLR (day 4) | 1.84 (1.38–3.25) | 3.46 (1.93–6.47) | <0.001 |
| PLR (day 1) | 157.8 (111.4–215.1) | 207.3 (134.2–314.4) | <0.001 |
| PLR (day 4) | 127.4 (91.2–204.3) | 174.1 (108.6–306.7) | <0.001 |
| Intubation | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | ||||||||
| Median (IQR) | Median (IQR) | AUC (95% CI) | Optimal Cut-Off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||
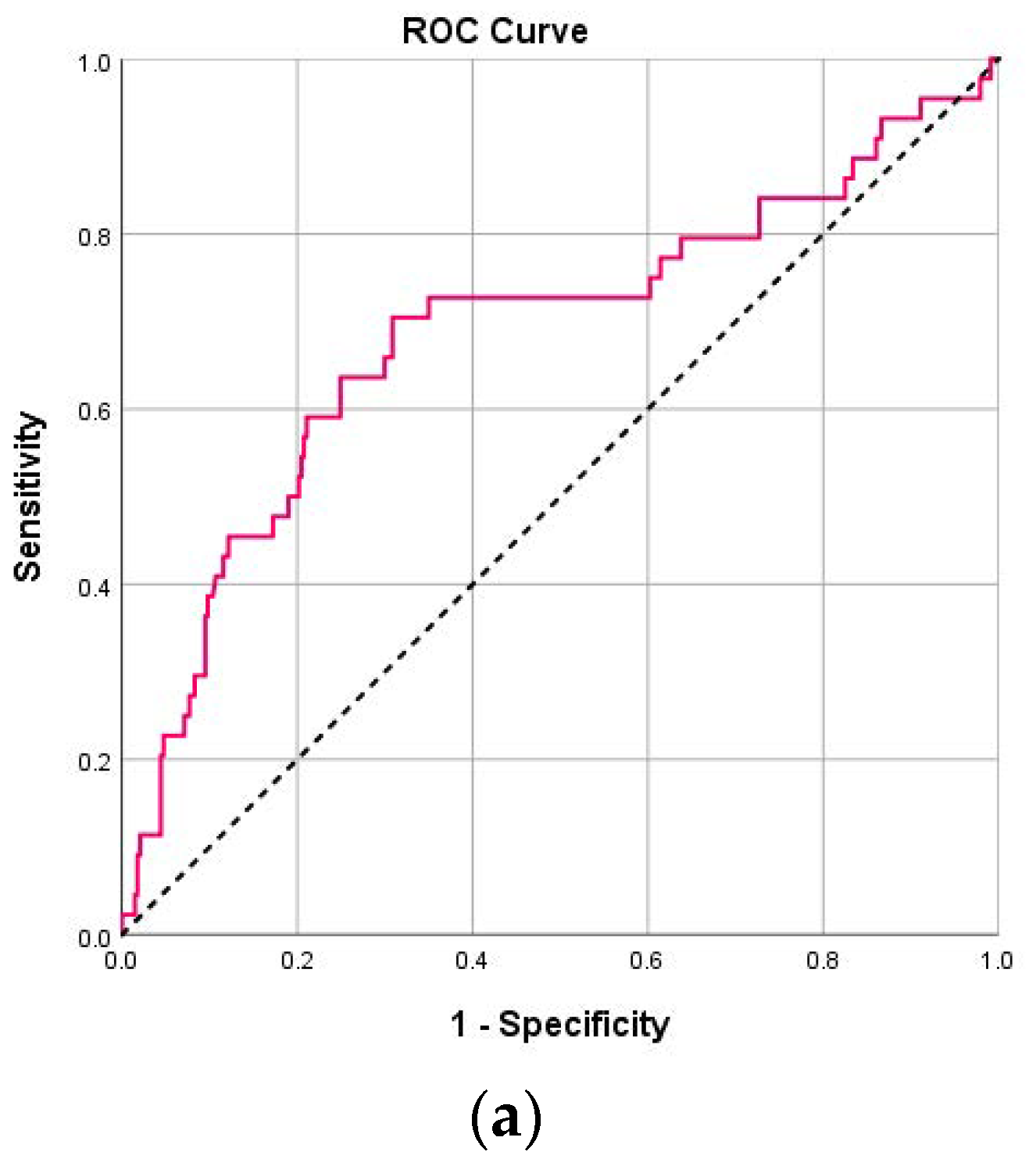
| NLR (day 1) | 3.58 (2.21–6.08) | 7.29 (2.90–11.95) | <0.001 | 0.69 (0.59–0.78) | 5.06 | 70.5 | 68.8 | 22.8 | 94.7 |
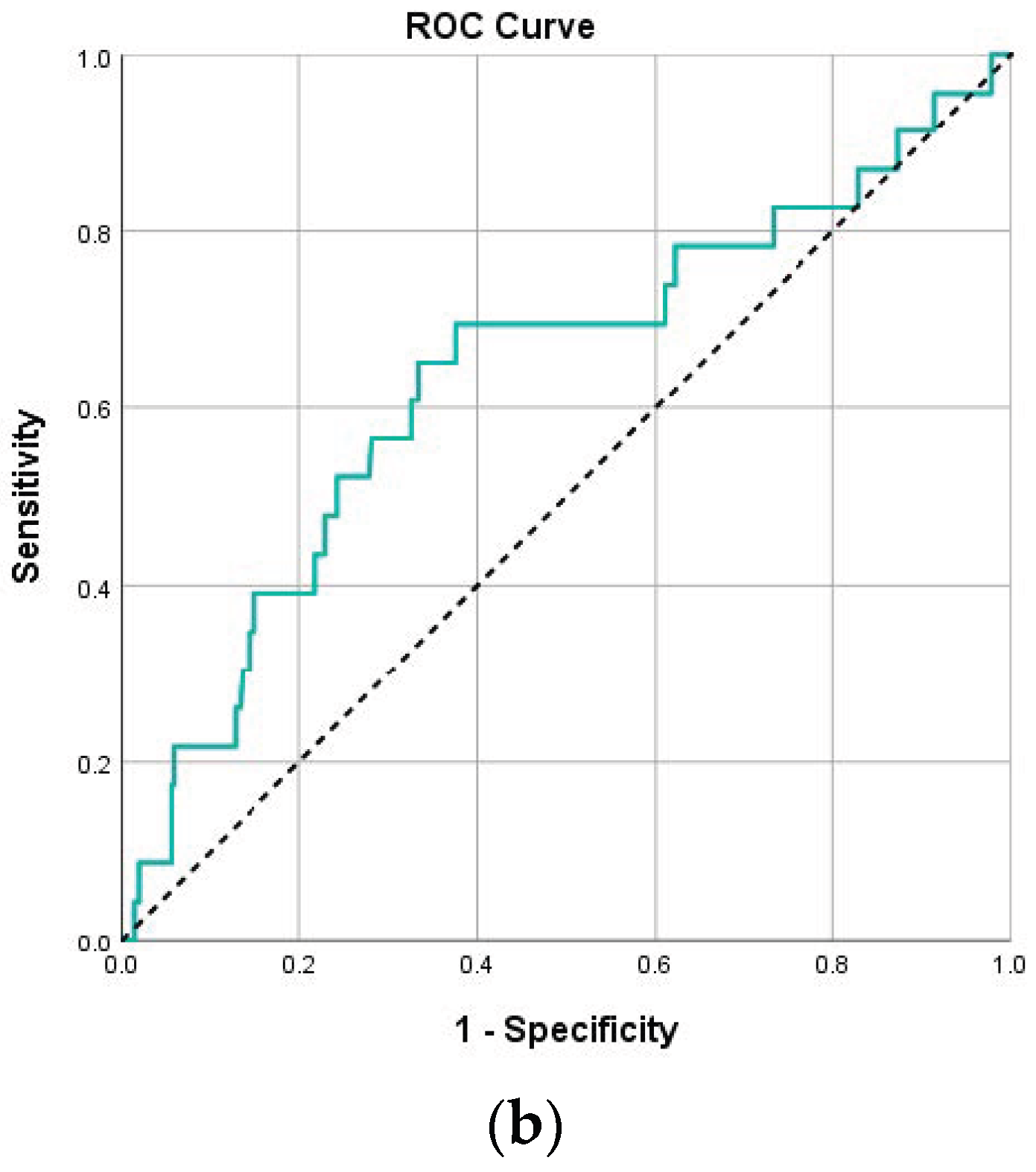
| NLR (day 4) | 2.28 (1.51–4.59) | 6.81 (2.55–12.55) | <0.001 | 0.74 (0.64–0.85) | 6.40 | 60.0 | 86.7 | 34.4 | 94.9 |
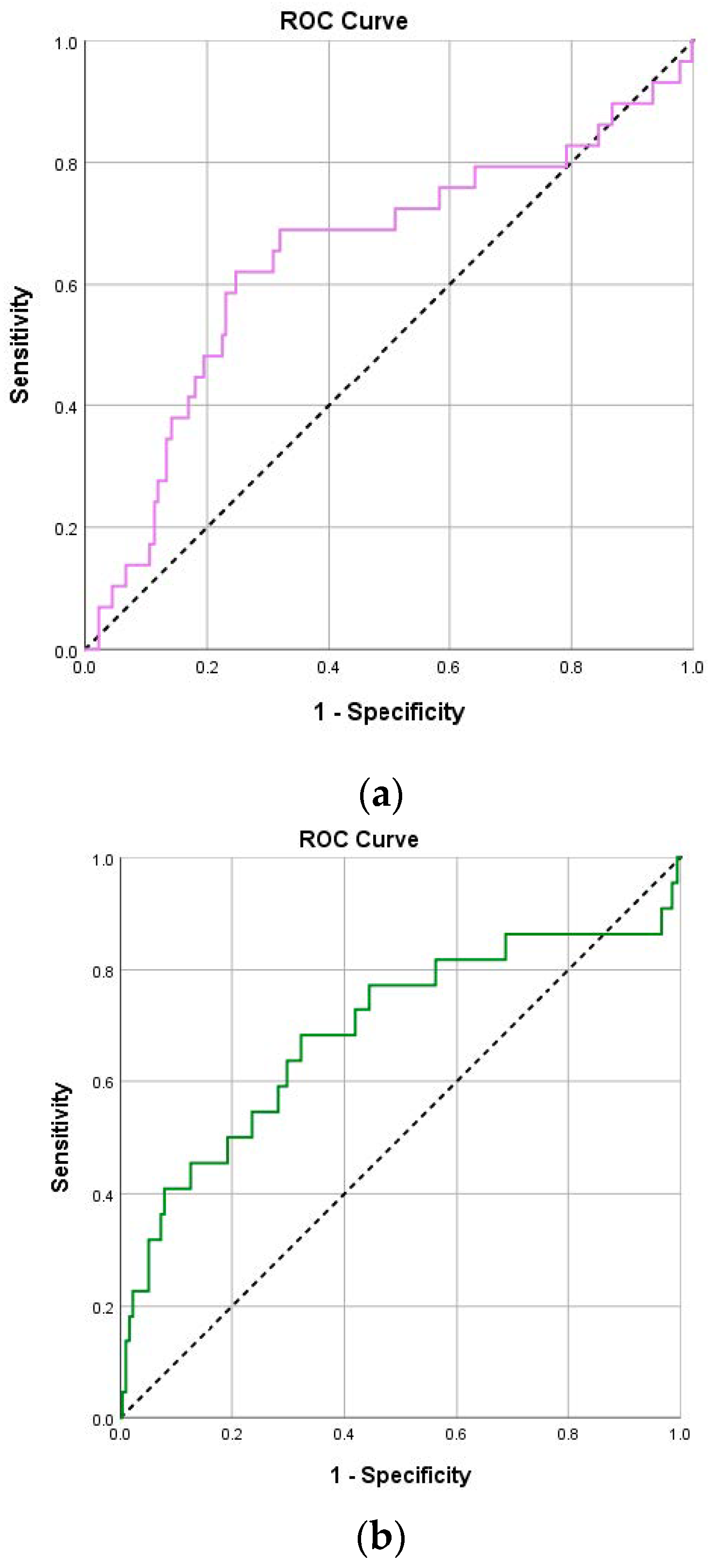
| PLR (day 1) | 172.6 (114.6–250.4) | 268.4 (149.4–369.6) | 0.003 | 0.64 (0.54 – 0.74) | 262.2 | 59.1 | 76.9 | 25.0 | 93.5 |
| PLR (day 4) | 145.7 (95.6–227.0) | 241.3 (120.1–485.0) | 0.003 | 0.65 (0.54–0.77) | 217.3 | 61.1 | 72.1 | 20.8 | 93.9 |
| Death | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | ||||||||
| Median (IQR) | Median (IQR) | AUC (95% CI) | Optimal Cut-Off | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | ||
| NLR (day 1) | 3.6 (2.2–6.5) | 7.5 (4.8–11.8) | <0.001 | 0.70 (0.59–0.81) | 4.82 | 75.9 | 65 | 14.9 | 97.1 |
| NLR (day 4) | 2.4 (1.5–5) | 9.3 (2.4–18.9) | <0.001 | 0.73 (0.59–0.87) | 6.41 | 68.2 | 84.1 | 22.7 | 97.5 |
| PLR (day 1) | 172.7 (114.1–261.9) | 270 (156–367.3) | 0.009 | 0.65 (0.53–0.76) | 229 | 69 | 68.1 | 14.8 | 96.5 |
| PLR (day 4) | 147.8 (96.8–233.4) | 255.3 (165.7–541) | 0.003 | 0.69 (0.55–0.83) | 205.4 | 68.2 | 67.7 | 12.6 | 96.9 |
| Dependent Variable | Independent Variable | OR (95% CI) + | p-Value |
|---|---|---|---|
| Intubation | COVID-19 disease (Severe vs. Mild/Moderate) | 6.15 (2.31–16.34) | <0.001 |
| Immunocompromised (Yes vs. No) | 0.74 (0.36–1.5) | 0.400 | |
| NLR (day 1) (>5.06 vs. <5.06) | 2.86 (1.27–6.44) | 0.011 | |
| PLR (day 1) (>262.2 vs. < 262.2) | 2.22 (1.02–4.85) | 0.044 | |
| Death | COVID-19 disease (Severe vs. Mild/Moderate) | 5.65 (1.17–27.25) | 0.031 |
| Age (years) | 1.08 (1.04–1.13) | <0.001 | |
| Smoking | |||
| Yes vs. No | 3.88 (1.01–16.85) | 0.049 | |
| In the past vs. No | 2.96 (0.79–11.15) | 0.108 | |
| Vaccination against COVID-19 (Yes vs. No) | 0.33 (0.12–0.9) | 0.031 | |
| Anti-CD 20 monotherapy (RITUXIMAB) (Yes vs. No) | 220.28 (13.07–3713.87) | <0.001 | |
| CRP (day 1) (>10 vs. <10) | 2.61 (1.01–6.78) | 0.049 | |
| NLR (day 1) (>4.82 vs. <4.82) | 3.92 (1.37–11.2) | 0.011 | |
| Immunocompromised (Yes vs. No) | 0.54 (0.17–1.73) | 0.301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolopoulou, A.; Rapti, V.; Alexiou, P.; Charalampous, C.M.; Livanou, M.E.; Sakka, V.; Syrigos, K.N.; Poulakou, G. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers of COVID-19 Disease Irrespective of Immunosuppression Status: A Case-Control Retrospective Single-Center Study. Pathogens 2025, 14, 550. https://doi.org/10.3390/pathogens14060550
Papanikolopoulou A, Rapti V, Alexiou P, Charalampous CM, Livanou ME, Sakka V, Syrigos KN, Poulakou G. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers of COVID-19 Disease Irrespective of Immunosuppression Status: A Case-Control Retrospective Single-Center Study. Pathogens. 2025; 14(6):550. https://doi.org/10.3390/pathogens14060550
Chicago/Turabian StylePapanikolopoulou, Amalia, Vasiliki Rapti, Polyxeni Alexiou, Charalampos M. Charalampous, Maria Effrosyni Livanou, Vissaria Sakka, Konstantinos N. Syrigos, and Garyfallia Poulakou. 2025. "Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers of COVID-19 Disease Irrespective of Immunosuppression Status: A Case-Control Retrospective Single-Center Study" Pathogens 14, no. 6: 550. https://doi.org/10.3390/pathogens14060550
APA StylePapanikolopoulou, A., Rapti, V., Alexiou, P., Charalampous, C. M., Livanou, M. E., Sakka, V., Syrigos, K. N., & Poulakou, G. (2025). Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as Prognostic Markers of COVID-19 Disease Irrespective of Immunosuppression Status: A Case-Control Retrospective Single-Center Study. Pathogens, 14(6), 550. https://doi.org/10.3390/pathogens14060550








