First Report of Trametes hirsuta, Causal Agent White Rot in Avocado Trees Grown in the State of Michoacán, México
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Tree Selection and Sampling
2.3. Characterization of the Fungus
2.4. Data Analysis
2.5. Bioassays for Pathogenicity
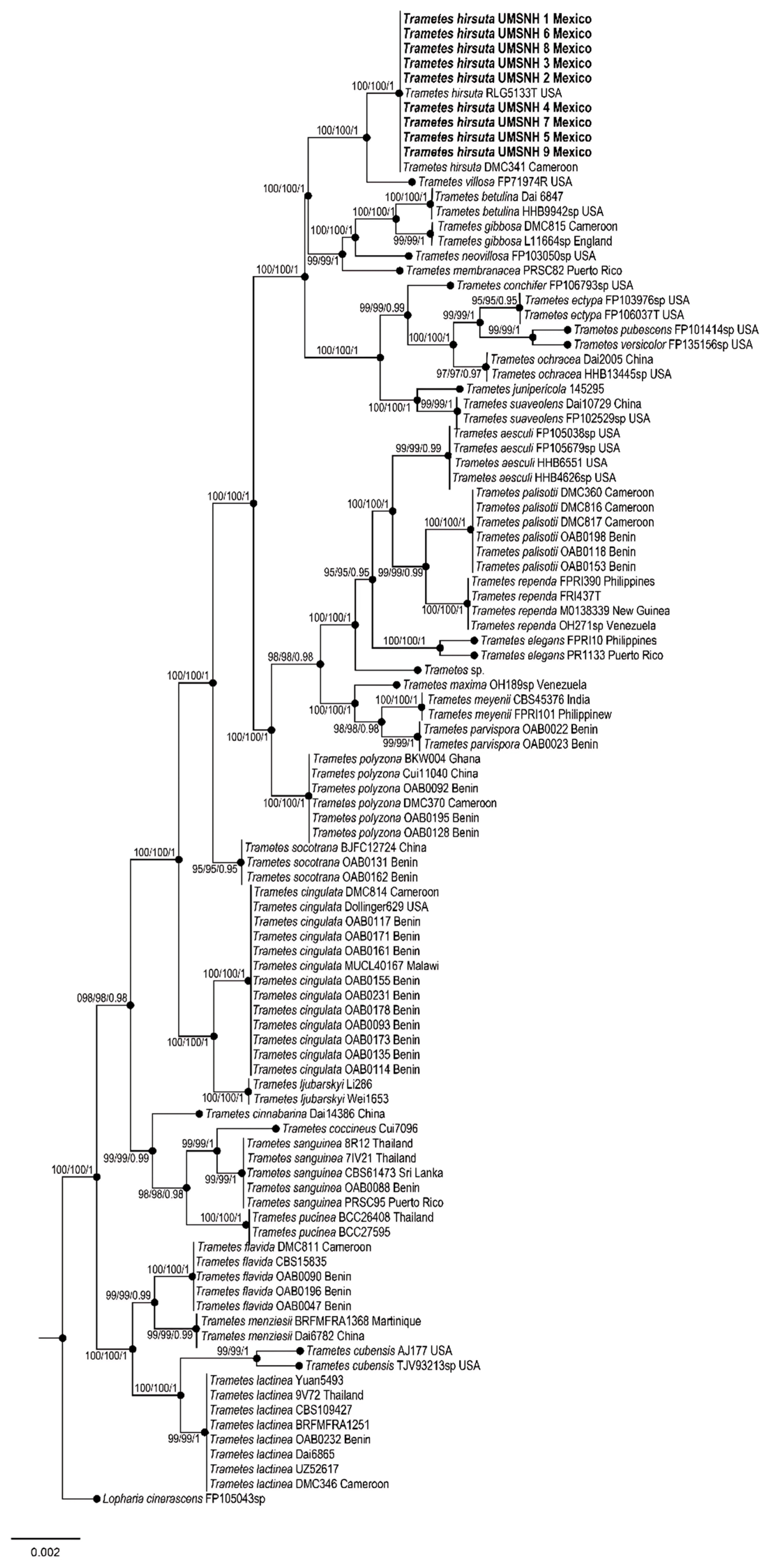
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbertson, R.L.; Ryvarden, L. North American Polypores: Megasporoporia-Wrightoporia; Fungiflora: Oslo, Norway, 1989; pp. 434–885. [Google Scholar]
- Ryvarden, L. Genera of polypores, nomenclature and taxonomy. In Synopsis Fungorum; Fungiflora: Oslo, Norway, 1991; p. 363. [Google Scholar]
- Carlson, A.; Justo, A.; Hibbett, D.S. Species delimitation in Trametes: A comparison of ITS, RPB1, RPB2 and TEF1 gene phylogenies. Mycologia 2014, 106, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Olou, B.A.; Krah, F.S.; Piepenbring, M.; Yorou, N.S.; Langer, E. Diversity of Trametes (Polyporales, Basidiomycota) in tropical Benin and description of new species Trametes parvispora. MycoKeys 2020, 65, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Castello, I.; Polizzi, G.; Vitale, A. Major Pathogens Affecting Carob in the Mediterranean Basin: Current Knowledge and Outlook in Italy. Pathogens 2023, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Tan, J.Y.; Xue, H.; Chow, M.L.; Ali, M.; Ng, A.; Leong, A.; Yeo, J.; Koh, S.M.; Tang, M.S.Y.; et al. A Metagenomic Survey of Wood Decay Fungi in the Urban Trees of Singapore. J. Fungi 2023, 9, 460. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.-D.; Wang, Y.-R.; Zhou, M.; Qiu, J.-Z.; Li, D.-W.; Vlasák, J.; Liu, H.-G.; Dai, Y.-C. Two new forest pathogens in Phaeolus (Polyporales, Basidiomycota) on Chinese coniferous trees were confirmed by molecular phylogeny. Front. Microbiol. 2022, 13, 942603. [Google Scholar] [CrossRef]
- Volobuev, S.V.; Bolshakov, S.; Shakhova, N.V. Synopsis of the macrofungi (Basidiomycota) on wood of fruit trees in the Central black Earth Region of Russia. S. Russ. Ecol. Dev. 2020, 15, 75–98. [Google Scholar] [CrossRef]
- Perdomo, O.P.; Lodge, J.; Ortega, D. Porus: Hongos Poriales de la República Dominicana; Sociedad Dominicana de Micología, Ed.; Búho: Santo Domingo, Dominican Republic, 2021; pp. 1–90. [Google Scholar]
- De Aza, M.J.; de la Rosa, J.G.; Santos, H.J. Nuevo récord de Trametes hirsuta (Wulfen) Lloyd (Basidiomycota) en refugio de vida silvestre laguna Mallén, San Pedro de Macorís-República Dominicana. Cienc. Ambiente Y Clima 2021, 4, 19–27. [Google Scholar] [CrossRef]
- Milenković, I.; Tomsǒvský, M.; Karadžićž, D.; Veselinović, M. Decline of Paulownia tomentosa caused by Trametes hirsuta in Serbia. For. Pathol. 2018, 48, e12438. [Google Scholar] [CrossRef]
- Marais, L.J. Avocado Diseases of Major Importance Worldwide and their Management. In Diseases of Fruits and Vegetables: Volume II; Naqvi, S.A.M.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar] [CrossRef]
- Mendoza-Churape, J.; Martínez-González, C.R.; Raymundo, T.; Valenzuela, R.; Barrios, P.A.; Sandoval, M.V.; Lara-Chávez, M.B.N. Occurrence and pathogenicity of the wood decay fungi Ganoderma australe and G. curtisii in avocado trees in Michoacán, Mexico. Nova Hedwig. 2024, 118, 133–156. [Google Scholar] [CrossRef]
- Çalış, Ö.; Çelik, S.; Fidan, H.; Tek, M.I.; Shah, M.; Tozlu, I.; Wani, S.H. Emerging pathogens and disease dynamics threatening avocado production in southern Türkiye. J. Plant Dis. Prot. 2024, 131, 1653–1663. [Google Scholar] [CrossRef]
- Cisneros-Zambrano, A.; Mendoza-Churape, A.; Contreras-Cornejo, H.A.; Raya Montaño, Y.A.; Martínez-González, C.R.; Raymundo, T.; Valenzuela, R.; Vargas-Sandoval, M.; Ruiz-Valencia, J.A.; Lara-Chávez, M.B.N. First report of Irpex rosettiformis causing white root rot in avocado trees in Michoacán, México. Plant Dis. 2024, 108, 805. [Google Scholar] [CrossRef] [PubMed]
- Largent, D.L. How to Identify Mushrooms to Genus I: Macroscopic Features; Eureka, Ed.; Mad River Press: Eureka, CA, USA, 1973; pp. 1–151. [Google Scholar]
- Jaeger, E.E.M.; Carroll, N.M.; Choudhury, S.; Dunlop, A.A.S.; Towler, H.M.A.; Matheson, M.M.; Adamson, P.; Okhravi, N.; Lightman, S. Rapid detection and identification of Candida, Aspergillus and Fusarium species in ocular samples using nested PCR. J. Clin. Microbiol. 2000, 38, 2902–2908. [Google Scholar] [CrossRef] [PubMed]
- White, T.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Sninsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenetics Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Zhao, C.; Fraczek, M.G.; Dineen, L.; Lebedinec, R.; Macheleidt, J.; Heinekamp, T.; Delneri, D.; Bowyer, P.; Brakhage, A.A.; Bromley, M. High-throughput gene replacement in Aspergillus fumigatus. Curr. Protoc. Microbiol. 2019, 54, e88. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017, 20, 1160–1166. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Müller, K.; Quandt, D.; Müller, J.; Neinhuis, C.; PhyDE®-Phylogenetic Data Editor. Program Distributed by the Authors, Version 10.0. 2005. Available online: https://www.phyde.de (accessed on 3 November 2022).
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. 2017. Available online: http://mesquiteproiect.org (accessed on 20 February 2024).
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Lanfear, R.; Calcott, B.; Kainer, D.; Mayer, C.; Stamatakis, A. Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evol. Biol. 2014, 14, 82. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, P.B.; Calcott, B.; Mayer, C.; Lanfear, R. Automatic selection of partitioning schemes for phylogenetic analyses using iterative k-means clustering of site rates. BMC Evol. Biol. 2015, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partition Finder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [PubMed]
- Rambaut, A. FigTree v1.4.2 [WWW Document]. 2016. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 17 April 2018).
- Mukhtar, I.; Arredondo-Santoyo, M.; Vázquez-Garcidueñas, M.S.; Vázque-Marrufo, G. Isolation and molecular identification of laccase-producing saprophytic/phytopathogenic mushroom-forming from various ecosystems in Michoacán state, Mexico. Acta Mycol. 2019, 54, 1119. [Google Scholar] [CrossRef]
- Justo, A.; Hibbett, D.S. Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset. Taxon 2011, 60, 1567–1583. [Google Scholar] [CrossRef]
- Gafforov, Y.; Ordynets, A.; Langer, E.; Yarasheva, M.; Gugliotta, A.D.; Schigel, D. Species diversity with comprehensive annotations of wood-inhabiting poroid and corticioid fungi in Uzbekistan. Front. Microbiol. 2020, 11, 598321. [Google Scholar] [CrossRef]
- Valenzuela Garza, R. Las Familias Polyporaceae Sensu Stricto y Albatrellaceae en México; Informe Final SNIB-CONABIO Proyecto No. H201; Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional: México City, México, 1991. [Google Scholar]
- Ribeiro Tomé, L.; Dornelles Parise, M.T.; Parise, D.; de Carvalho Azevedo, V.A.; Brening, B.; Badotti, F.; Góes-Neto, A. Pure lignin induces overexpression of cytochrome P450 (CYP) encoding genes and brings insights into the lignocellulose depolymerization by Trametes villosa. Heliyon 2024, 10, e28449. [Google Scholar] [CrossRef]
- Barrasa, J.M.; Blanco, M.N.; Esteve-Ravento, F.; Altes, A.; Checa, J.; Martinez, A.T.; Ruiz-Dueñas, F.J. Wood and humus decay strategies by white-rot basidiomycetes correlate with two different dye decolorization and enzyme secretion patterns on agar plates. Fungal Genet. Biol. 2014, 72, 106–114. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Lagzian, A.; Vatankhah, M.; Kennedy, J.F. Modifying lignin: A promising strategy for plant disease control. Int. J. Biol. Macromol. 2024, 271, 132696. [Google Scholar]
- Bumpus, J.A.; Aust, S.D. Biodegradation of environmental pollutants by the white rot fungus Phanerochaete chrysosporium involvement of the lignin-degrading system. BioEssays 1987, 6, 166–170. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Moiseenko, K.V.; Glazunova, O.A.; Savinova, O.S.; Vasina, D.V.; Zherebker, A.Y.; Kulkova, N.A.; Nikolaev, E.N.; Fedorova, T.V. Relation between lignin molecular profile and fungal exo-proteome during kraft lignin modification by Trametes hirsuta LE-BIN 072. Bioresour. Technol. 2021, 335, 125229. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, A.; Milovanovic, I.; Stajic, M.; Vukojevic, J. Trametes species to degradate lignin. Int. Biodeterior. Biodegrad. 2013, 85, 52–56. [Google Scholar] [CrossRef]
- Ma, J.; Li, Q.; Wu, Y.; Yue, H.; Zhang, Y.; Zhang, J.; Shi, M.; Wang, S.; Liu, G.Q. Elucidation of ligninolysis mechanism of a newly isolated white-rot basidiomycete Trametes hirsuta X-13. Biotechnol. Biofuels 2021, 14, 189. [Google Scholar] [CrossRef]
- Kato, H.; Miura, D.; Kato, M.; Shimizu, M. Metabolic mechanism of lignin-derived aromatics in white-rot fungi. Appl. Microbiol. Biotechnol. 2024, 108, 108–532. [Google Scholar] [CrossRef]
- Vasina, D.V.; Pavlov, A.R.; Koroleva, O.V. Extracellular proteins of Trametes hirsuta st. 072 induced by copper ions and a lignocellulose substrate. BMC Microbiol. 2016, 16, 106. [Google Scholar] [CrossRef]
- Bhatt, I.M.; Pramod, S.; Koyani, R.D.; Rajput, K.S. Anatomical characterization of Eucalyptus globulus wood decay by two white rot species of Trametes. J. Plant Pathol. 2016, 98, 227–234. [Google Scholar]
- Fedorova, T.V.; Shakhova, N.V.; Klein, O.I.; Glazunova, O.A.; Maloshenok, L.G.; Kulikova, N.A.; Psurtseva, N.V.; Koroleva, O.V. Comparative analysis of the ligninolytic potential of basidiomycetes belonging to different taxonomic and ecological groups. Appl. Biochem. Microbiol. 2013, 49, 570–580. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Larsen, J.; Fernández-Pavía, S.P.; Oyama, K. Climate change, a booster of disease outbreaks by the plant pathogen Phytophthora in oak forests. Rhizosphere 2023, 27, 100719. [Google Scholar] [CrossRef]
- Arredondo-León, C.; Muñoz-Jiménez, J.; García-Romero, A. Recent changes in landscape-dynamics trends in tropical highlands, central Mexico. Interciencia 2008, 33, 569–577. [Google Scholar]
- Shabaev, A.V.; Fedorova, T.V. Influence of a wood substrate on the profile of volatile organic compounds produced by white rot fungus Trametes hirsuta LE-BIN072. Appl. Biochem. Microbiol. 2024, 60, 1240–1251. [Google Scholar] [CrossRef]
- Peng, M.; Bervoets, S.; Chin-A-Woeng, T.; Granchi, Z.; Hildén, K.; Mäkelä, M.R.; de Vries, R.P. The transcriptomic response of two basidiomycete fungi to plant biomass ins modulated by temperature to a different extent. Microbiol. Res. 2023, 270, 127333. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Butt, T.A.; Alam Naqvi, S.T.; Yousaf, S.; Qureshi, M.K.; Zafar, M.I.; Farooq, G.; Nawaz, I.; Iqbal, M. Lead tolerant endophyte Trametes hirsuta improved the growth and lead accumulation in the vegetative arts of Triticum aestivum L. Heliyon 2020, 6, e04188. [Google Scholar] [CrossRef] [PubMed]
- Tomsǒvský, M.; Kolaří, M.; Pañoutová, S.; Homolka, L. Molecular phylogeny of European Trametes (Basidiomycetes, Polyporales) species based on LSU and ITS (nrDNA) sequences. Nova Hedwig. 2006, 82, 269–280. [Google Scholar] [CrossRef]
- Horisawa, S.; Yoshida, M.; Umezawa, K.; Wada, T.; Abe, H.; Doi, S.; Samejima, M.; Momohara, I. Diversity and community structure of wood-inhabiting fungi found in Japanese wooden houses analyzed by the next-generation sequencing. J. Wood Sci. 2017, 63, 369–378. [Google Scholar] [CrossRef]
- Liers, C.; Ullrich, R.; Pecyna, M.; Schlosser, D.; Hofrichter, M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzym. Microb. Technol. 2007, 41, 785–793. [Google Scholar] [CrossRef]
- Hincal, S.; Yalcin, M. Biological control of some wood-decay fungi with antagonistic fungi. Biodegradation 2023, 34, 597–607. [Google Scholar] [CrossRef]
- Vasilchenko, A.V.; Poshvina, D.V.; Semenov, M.V.; Timofeev, V.N.; Iashnikov, A.V.; Stepanov, A.A.; Pervushina, A.N.; Vasilchenko, A.S. Triazoles and Strobilurin Mixture Affects Soil Microbial Community and Incidences of Wheat Diseases. Plants 2023, 12, 660. [Google Scholar] [CrossRef]





| Municipality | Altitude | Precipitation | Coordinates | Incidence of Diseases by Location | Strain/Isolates |
|---|---|---|---|---|---|
| Ziracuaretiro | 1571 | 1200 | 19.48°70′34″ N; −101.96°31′72″ W | 1.71% | UMSNH 6 |
| 1571 | 1200 | 19.46°16′25″ N; −101.94°46′33″ W | 1.71% | UMSNH8 | |
| Tacámbaro | 1654 | 1172 | 19.36°63′89″ N; −101.44°91′97″ W | 3.42% | UMSNH1 |
| Timgambato | 1982 | 1100 | 19.50°11′47″ N; −101.89°14′25″ W | 1.14% | UMSNH5 |
| Tancítaro | 2079 | 1800 | 19.33°87′34″ N; −102.34°54′09″ W | 1.4% | UMSNH9 |
| Pátzcuaro | 2329 | 1200 | 19.46°80′28″ N; −101.69°85′30″ W | 2.28% | UMSNH3 |
| Turicato | 1891 | 849 | 19.14°07′43″ N; −101.59°46′08″ W | 1.71% | UMSNH2 |
| Salvador Escalante | 2200 | 1600 | 19.42°01′74″ N; −101.76°63′53″ W | 2.57% | UMSNH8 |
| Nuevo Parangaricutiro | 1965 | 1800 | 19.43°67′40″ N; −102.18°15′93″ W | 2.28% | UMSNH7 |
| Uruapan | 1620 | 1600 | 19.44°30′28″ N; −102.02°15′48″ W | 4.57% | UMSNH4 |
| GenBank Accesions | |||||||
|---|---|---|---|---|---|---|---|
| Species Name | Isolate | Origin | ITS | LSU | rpb1 | rpb2 | tef1 |
| Trametes aesculi | HHB4626sp | USA | JN164950 | ----- | KF573173 | KF573134 | KF573083 |
| Trametes aesculi | FP105679sp | USA | JN164944 | JN164799 | JN164833 | JN164861 | JN164899 |
| Trametes aesculi | HHB6551sp | USA | JN164938 | ----- | KF573172 | KF573163 | KF573082 |
| Trametes aesculi | FP105038sp | USA | JN164951 | ----- | KF573174 | KF573135 | KF573081 |
| Trametes betulina | HHB9942sp | USA | JN164983 | JN164794 | ----- | JN164860 | ----- |
| Trametes betulina | Dai6847 | KC848305 | KC848390 | ----- | ----- | ----- | |
| Trametes cingulata | MUCL: 40167 | Malawi | JN645075 | ----- | ----- | ----- | ----- |
| Trametes cingulata | Dollinger 629 | USA | KY264043 | ----- | ----- | ----- | ----- |
| Trametes cingulata | DMC814 | Cameroon | KC589133 | KC589159 | ----- | ----- | ----- |
| Trametes cingulata | OAB0135 | Benin | MK736973 | ----- | ----- | ----- | ----- |
| Trametes cingulata | OAB0117 | Benin | MK736972 | ----- | ----- | ----- | ----- |
| Trametes cingulata | OAB0093 | Benin | MK736970 | ----- | ----- | ----- | ----- |
| Trametes cingulata | OAB0114 | Benin | MK736971 | MK736950 | ----- | ----- | ----- |
| Trametes cingulata | OAB0161 | Benin | MK736975 | MK736951 | ----- | ----- | ----- |
| Trametes cingulata | OAB0155 | Benin | MK736974 | ----- | ----- | ----- | ----- |
| Trametes cingulata | OAB0171 | Benin | MK736976 | MK736952 | ----- | ----- | ----- |
| Trametes cingulata | OAB0173 | Benin | MK736977 | MK736953 | ----- | ----- | ----- |
| Trametes cingulata | OAB0178 | Benin | MK736978 | MK736954 | ----- | ----- | ----- |
| Trametes cingulata | OAB0231 | Benin | MK736979 | MK736955 | ----- | ----- | ----- |
| Trametes cinnabarina | Dai 14386 | China | KX880629 | KX880667 | KX880818 | KX880854 | ----- |
| Trametes coccinea | Cui 7096 | KC848330 | KC848414 | ----- | ----- | ----- | |
| Trametes conchifer | FP106793sp | USA | JN164924 | JN164797 | JN164823 | JN164849 | ----- |
| Trametes cubensis | TJV93-213sp | USA | JN164923 | JN164798 | JN164834 | JN164865 | ----- |
| Trametes cubensis | AJ177 | USA | JN164905 | ----- | ----- | ----- | ----- |
| Trametes cubensis | UZ526-17 | Malasya | MF363158 | ----- | ----- | ----- | ----- |
| Trametes ectypa | FP10397sp | USA | JN164961 | ----- | ----- | ----- | ----- |
| Trametes ectypa | FP106037T | USA | JN164929 | JN164803 | JN164824 | JN164848 | ----- |
| Trametes elegans | PR1133 | Puerto Rico | JN164937 | ----- | KF573178 | KF573139 | KF573075 |
| Trametes elegans | FPRI10 | Philippines | JN164973 | ----- | ----- | KF573138 | KF573074 |
| Trametes elegans | FP150762 | Belize | JN164928 | ----- | ----- | KF573137 | KF573076 |
| Trametes flavida | OAB0047 | Benin | MK736966 | MK736946 | ----- | ----- | ----- |
| Trametes flavida | OAB0090 | Benin | MK736967 | ----- | ----- | ----- | ----- |
| Trametes flavida | OAB0196 | Benin | MK736968 | MK736947 | ----- | ----- | ----- |
| Trametes flavida | DMC811 | Cameroon | KC589130 | KC589156 | ----- | ----- | ----- |
| Trametes flavida | CBS 15835 | MH855616 | MH867126 | ----- | ----- | ----- | |
| Trametes gibbosa | DMC815 | Cameroon | KC589144 | KC589164 | ----- | ----- | ----- |
| Trametes gibbosa | L11664sp | England | JN164943 | JN164800 | JN164831 | JN164859 | ----- |
| Trametes hirsuta | DMC341 | Cameroon | KC589146 | KC589166 | ----- | ----- | ----- |
| Trametes hirsuta | RLG5133T | USA | JN164941 | JN164801 | JN164829 | JN164854 | ----- |
| Trametes hirsuta | UMSNH 1 | Mexico | OR492495 | OR492546 | PQ961250 | PQ972541 | PQ978426 |
| Trametes hirsuta | UMSNH 2 | Mexico | OR492496 | OR492547 | PQ961251 | PQ972542 | PQ978427 |
| Trametes hirsuta | UMSNH 3 | Mexico | OR492497 | OR492548 | PQ961252 | PQ972543 | PQ978428 |
| Trametes hirsuta | UMSNH 4 | Mexico | OR492498 | OR492549 | PQ961253 | PQ972544 | PQ978429 |
| Trametes hirsuta | UMSNH 5 | Mexico | OR492499 | OR492550 | PQ961254 | PQ972545 | PQ978430 |
| Trametes hirsuta | UMSNH 6 | Mexico | OR492500 | OR492550 | PQ961255 | PQ972546 | PQ978431 |
| Trametes hirsuta | UMSNH 7 | Mexico | OR492501 | OR492552 | PQ961256 | PQ972547 | PQ978432 |
| Trametes hirsuta | UMSNH 8 | Mexico | OR492502 | OR492553 | PQ961257 | PQ972548 | PQ978433 |
| Trametes hirsuta | UMSNH 9 | Mexico | OR492503 | OR492554 | PQ961258 | PQ972549 | PQ978434 |
| Trametes junipericola | 145295 (O) | KC017758 | KC017763 | ----- | ----- | ----- | |
| Trametes lactinea | DMC346 | Cameroon | KC589126 | KC589152 | ----- | ----- | ----- |
| Trametes lactinea | CBS 109427 | Taiwan | MH862825 | ----- | ----- | ----- | ----- |
| Trametes lactinea | LIP: GUY09 | French Guiana | JN645069 | ----- | ----- | ----- | ----- |
| Trametes lactinea | Dai6865 | KC848327 | KC848411 | ----- | ----- | ----- | |
| Trametes lactinea | OAB0232 | Benin | MK736983 | MK736948 | ----- | ----- | ----- |
| Trametes lactinea | BCC 33266 | Thailand | GQ982888 | GQ982881 | ----- | ----- | ----- |
| Trametes lactinea | Yuan 5493 | KC848320 | KC848404 | ----- | ----- | ----- | |
| Trametes ljubarskyi | Wei1653 | KC848332 | KC848416 | ----- | ----- | ----- | |
| Trametes ljubarskyi | Li286 | KC848331 | KC848415 | ----- | ----- | ----- | |
| Trametes maxima | OH189sp | Venezuela | JN164957 | JN164804 | JN164816 | JN164864 | ----- |
| Trametes membranacea | PRSC82 | Puerto Rico | JN164945 | JN164805 | JN164832 | JN164857 | ----- |
| Trametes menziesii | BRFM FRA | Martinique | JN645103 | ----- | ----- | ----- | ----- |
| Trametes menziesii | Dai6782 | KC848289 | KC848374 | ----- | ----- | ----- | |
| Trametes meyenii | Philippines | JN164933 | ----- | KF573179 | KF573145 | ----- | |
| Trametes meyenii | CBS45376 | India | MH860991 | MH872762 | ----- | ----- | ----- |
| Trametes neovillosa | FP103050sp | USA | JN164958 | JN164806 | JN164835 | JN164862 | ----- |
| Trametes ochracea | HHB1344sp | USA | JN164954 | JN164812 | JN164826 | JN164852 | ----- |
| Trametes ochracea | Dai2005 | China | KC848272 | KC848357 | ----- | ----- | ----- |
| Trametes palisotii | OAB0118 | Benin | MK736980 | MK736956 | MK802884 | MK802882 | MK802886 |
| Trametes palisotii | OAD0153 | Benin | MK736981 | MK736957 | MK802885 | MK802883 | MK802887 |
| Trametes palisotii | OAD0198 | Benin | MK736982 | MK736958 | ----- | ----- | MK802888 |
| Trametes palisotii | DMC360 | Cameroon | KC589139 | KC589160 | ----- | ----- | ----- |
| Trametes palisotii | DMC817 | Cameroon | KC589142 | KC589163 | ----- | ----- | ----- |
| Trametes palisotii | DMC816 | Cameroon | KC589141 | KC589162 | ----- | ----- | ----- |
| Trametes parvispora | OAB002 | Benin | MK736989 | MK736964 | ----- | MN127965 | ----- |
| Trametes parvispora | OAB0023 | Benin | MK736990 | MK736965 | ----- | MN127964 | ----- |
| Trametes polyzona | DMC370 | Cameroon | KC589125 | KC589151 | ----- | ----- | ----- |
| Trametes polyzona | Cui 11040 | China | KX880647 | KX880689 | KX880836 | KR610849 | ----- |
| Trametes polyzona | BKW004 | Ghana | JN164978 | JN164790 | ----- | ----- | ----- |
| Trametes polyzona | OAB0092 | Benin | MK736984 | MK736959 | ----- | ----- | ----- |
| Trametes polyzona | OAB0128 | Benin | MK736985 | MK736960 | ----- | ----- | ----- |
| Trametes polyzona | OAB0195 | Benin | MK736986 | MK736961 | ----- | ----- | ----- |
| Trametes pubescens | FP101414sp | USA | JN164963 | JN164811 | JN164827 | JN164851 | ----- |
| Trametes punicea | BCC26408 | Thailand | JF372685 | FJ372707 | ----- | ----- | ----- |
| Trametes punicea | BCC27595 | FJ372686 | FJ372708 | ----- | ----- | ----- | |
| Trametes rependa | FRI437T | JN164985 | ----- | KF573177 | KF573142 | KF573080 | |
| Trametes rependa | FPRI390 | Philippines | JN164921 | ----- | KF573175 | KF573141 | KF573077 |
| Trametes rependa | OH271sp | Venezuela | JN164936 | ----- | KF573176 | KF573143 | KF573079 |
| Trametes rependa | M0138339 | Papua New | KF573029 | ----- | ----- | KF573140 | KF573078 |
| Trametes sanguinea | OAB0088 | Benin | MK736969 | MK736949 | ----- | ----- | ----- |
| Trametes sanguínea | PRSC95 | Puerto Rico | JN164982 | JN164795 | JN164842 | JN164858 | ----- |
| Trametes sanguínea | BCC 36861 | Thailand | GQ982885 | GQ982878 | ----- | ----- | ----- |
| Trametes sanguínea | 8R12 | Thailand | FJ372672 | FJ372694 | ----- | ----- | ----- |
| Trametes sanguinea | CBS61473 | Sri Lanka | MH860781 | MH872513 | ----- | ----- | ----- |
| Trametes socotrana | BJFC12724 | China | KC848313 | KC848397 | ----- | ----- | ----- |
| Trametes sp. | BC1 | Finland | KT896651 | ----- | ----- | ----- | ----- |
| Trametes suaveolens | FP102529sp | USA | JN164966 | JN164807 | JN164828 | JN164853 | ----- |
| Trametes suaveolens | Dai 10729 | China | JN048770 | JN048789 | ----- | ----- | ----- |
| Trametes versicolor | FP13515sp | USA | JN164919 | JN164809 | JN164825 | JN164850 | ----- |
| Trametes villosa | FP71974R | USA | JN164969 | JN164810 | JN164830 | JN164855 | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Churape, J.; Lara-Chávez, M.B.N.; Ramírez-Mendoza, R.; Martínez-González, C.R.; Contreras-Cornejo, H.A.; Raya-Montaño, Y.A.; Ávila-Val, T.d.C.; Vargas-Sandoval, M. First Report of Trametes hirsuta, Causal Agent White Rot in Avocado Trees Grown in the State of Michoacán, México. Pathogens 2025, 14, 532. https://doi.org/10.3390/pathogens14060532
Mendoza-Churape J, Lara-Chávez MBN, Ramírez-Mendoza R, Martínez-González CR, Contreras-Cornejo HA, Raya-Montaño YA, Ávila-Val TdC, Vargas-Sandoval M. First Report of Trametes hirsuta, Causal Agent White Rot in Avocado Trees Grown in the State of Michoacán, México. Pathogens. 2025; 14(6):532. https://doi.org/10.3390/pathogens14060532
Chicago/Turabian StyleMendoza-Churape, Juan, Ma. Blanca Nieves Lara-Chávez, Rosario Ramírez-Mendoza, César Ramiro Martínez-González, Hexon Angel Contreras-Cornejo, Yurixhi Atenea Raya-Montaño, Teresita del Carmen Ávila-Val, and Margarita Vargas-Sandoval. 2025. "First Report of Trametes hirsuta, Causal Agent White Rot in Avocado Trees Grown in the State of Michoacán, México" Pathogens 14, no. 6: 532. https://doi.org/10.3390/pathogens14060532
APA StyleMendoza-Churape, J., Lara-Chávez, M. B. N., Ramírez-Mendoza, R., Martínez-González, C. R., Contreras-Cornejo, H. A., Raya-Montaño, Y. A., Ávila-Val, T. d. C., & Vargas-Sandoval, M. (2025). First Report of Trametes hirsuta, Causal Agent White Rot in Avocado Trees Grown in the State of Michoacán, México. Pathogens, 14(6), 532. https://doi.org/10.3390/pathogens14060532







