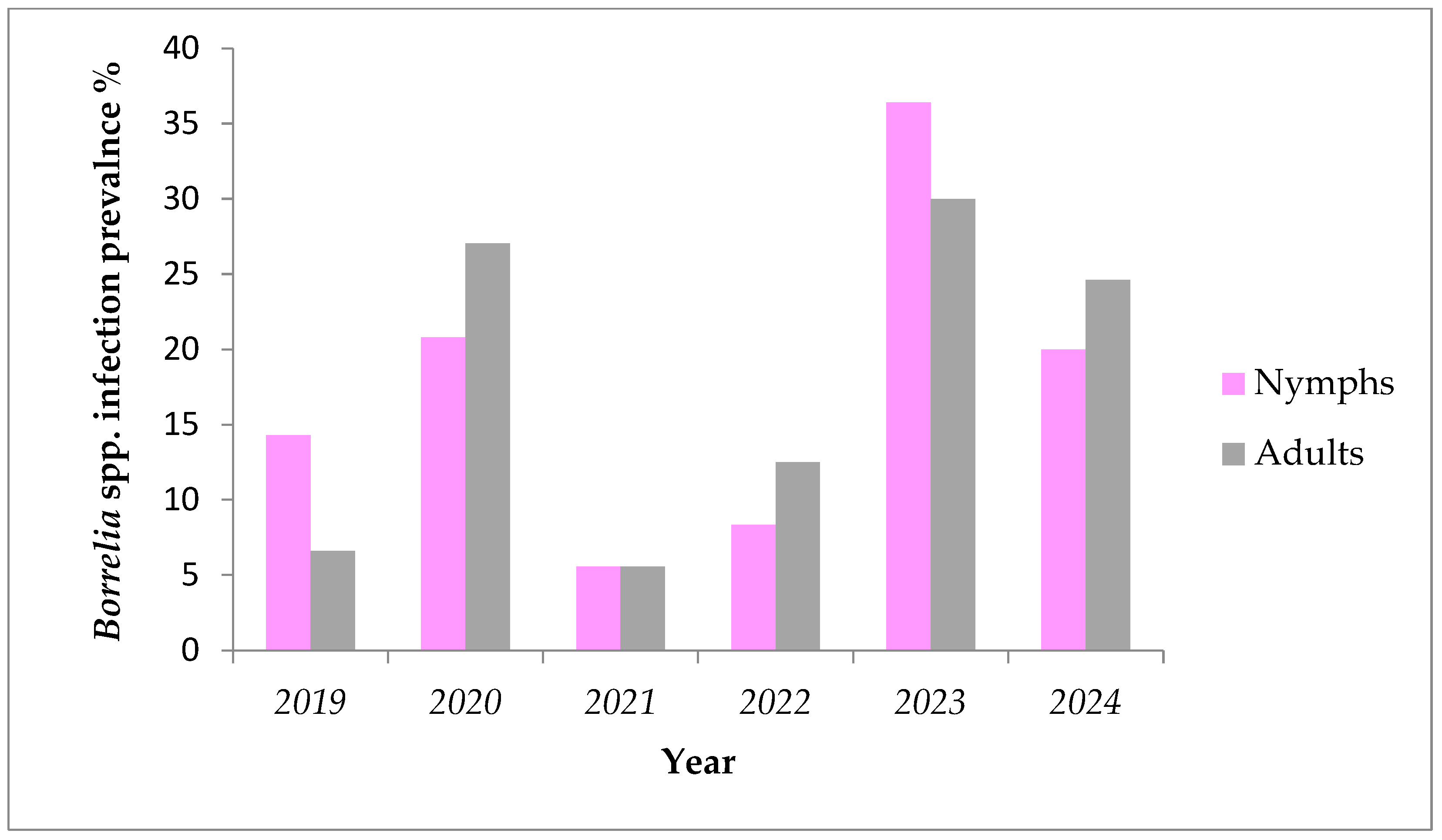Abstract
Ticks carry numerous pathogens that, if transmitted, can cause disease in humans and animals. Research on pathogens transmitted from ticks to humans is essential for improving public health strategies against tick-borne diseases (TBDs). In this study, Ixodes ricinus ticks found on humans were collected and examined between 2019 and 2024. This study is about the molecular characteristics of tick-borne pathogens (TBPs) in the region of northern Serbia, including Borrelia burgdorferi sensu lato (s.l.), Anaplasma phagocytophilum, and Coxiella burnetii. We identified B. burgdorferi s.l. as the most prevalent in ticks (20.45%). Molecular analysis identified two genospecies, B. afzelii and B. burgdorferi s.s., indicating genetic diversity among Borrelia spirochetes. A. phagocytophilum was detected in ticks with a prevalence of (1.62%), while C. burnetii was not found in any of the ticks. Our findings highlight the necessity of monitoring tick pathogens in ticks removed from humans. Serbia is an endemic region for some tick-borne diseases, such as Lyme disease. Regular surveillance of tick populations, with molecular identification of pathogens, offers insight into transmission dynamics, allowing for monitoring and public health interventions to be created if needed due to increased risk.
1. Introduction
Ticks are blood-feeding ectoparasites and vectors of tick-borne pathogens (TBPs) that are important in human and veterinary medicine [1,2,3]. Questing ticks collected from natural habitats carry pathogens, but human-biting ticks offer an insight into the pathogens to which humans are directly exposed. Ixodes ricinus is the main vector of Lyme borreliosis in Europe but also can transmit other pathogens such as Anaplasma phagocytophilum and Coxiella burnetii. So far, we know that Serbia is an endemic area for some tick-borne infections [4]. The main factors influencing geographic spread of ticks can be classified as environmental (e.g., changes in micro- and macroclimate, migration of animals who are natural source of blood meal for ticks) and anthropogenic (e.g., professional and leisure activities, urbanization, traveling, etc.), leading to changes in the prevalence of tick-borne diseases (TBD) [5,6,7,8,9,10,11,12,13]. Influence of the named factors have, so far, contributed to an increased prevalence of TBDs in Serbia, where previous studies pointing out B. burgdorferi s.l. as a predominant TBP [14]. Those studies found I. ricinus ticks in Serbia infected with B. burgdorferi s.l., A. phagocytophilum, and Francisella tularensis in 26.7% of the infected ticks. Analyses of human-biting ticks present a possibility of determining tick-borne pathogens which could represent a risk for human health. Other studies have also found the infection rate of B. burgdorferi s.l. to be at 22.12% in ticks, with a seroprevalence of 25.81% seroprevalence in dogs from Serbia [15]. Further studies have confirmed this by identifying a variety of Borrelia genospecies such as B. afzelii, B. garinii, B. spielmanii, B. lusitaniae, B. valaisiana; B. miyamotoi was also identified later on, further emphasizing the genetic diversity of TBPs in the region [16,17]. However, the real prevalence and molecular characteristics of TBPs in ticks removed from humans are underexplored. In addition to Borrelia spp., other tick-borne pathogens that are present in Serbia and may represent a health risk are A.phagocytophilum and C. burnetii. Human anaplasmosis is not very often reported in Serbia, but studies have shown that people bitten by ticks are exposed to Anaplasma spp., with rare cases of subclinical bacteremia [9,17,18]. Coxiella burnetii has been detected in questing ticks and Q-fever disease remains a reportable disease in Serbia as endemic region. Human cases are reducing in incidence, but are still being reported, and animal cases are continually monitored [19,20]. According to the official report, “Annual report on infection diseases in the Republic of Serbia for 2021”, the incidence rate of Q-fever in humans in Serbia is on the decline, with the last cases reported in 2019, the incidence rate being 0.4/100,000 residents. The most widespread outbreak of Q-fever happened in 2013 when 89 cases were registered within four separate events [21]. All three mentioned diseases are constantly present among animals in Serbia, with or without clinical symptoms [15,20,22,23,24]. This means that the pathogens are circulating between hosts and vectors, representing a risk for possible infection.
This study aims to investigate the molecular characteristics of B. burgdorferi s.l., A. phagocytophilum, and C. burnetii in I. ricinus ticks removed from humans during the period of 2019–2024. By focusing on human-biting ticks, this research tries to provide information on the possible transmission of mentioned pathogens relevant for public health and possible risk.
2. Materials and Methods
2.1. Study Area and Tick Collection
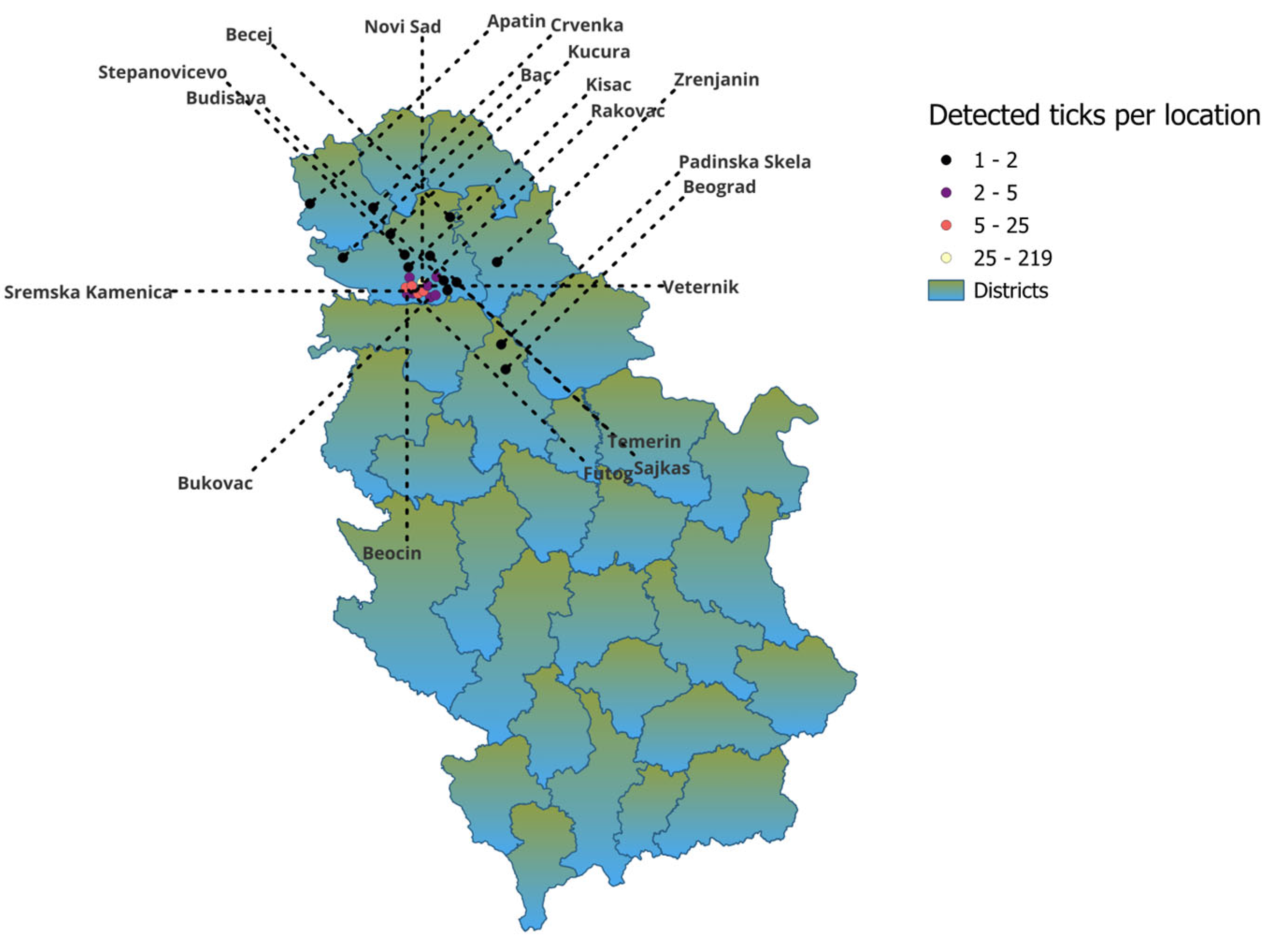
The study was conducted in the northern region of Serbia. Medical and veterinary institutions invited and encouraged people to report tick bites throughout the year. Human-biting Ixodes ricinus ticks were collected over a 6-year period (2019–2024) from patients seeking medical care in different healthcare facilities. Ticks were collected mostly from the city of Novi Sad area and from other 24 cities in the northern region of Serbia. Detailed distribution information is shown in Table 1 and Figure 1. Patients visited clinics after a tick bite and asked for a tick removal. After the removal of ticks by medical staff, they were placed in labeled plastic tubes or small bottles in medical institutions and then sent to the laboratory of the Scientific Veterinary Institute of “Novi Sad” for analysis.

Table 1.
Distribution of collected ticks.
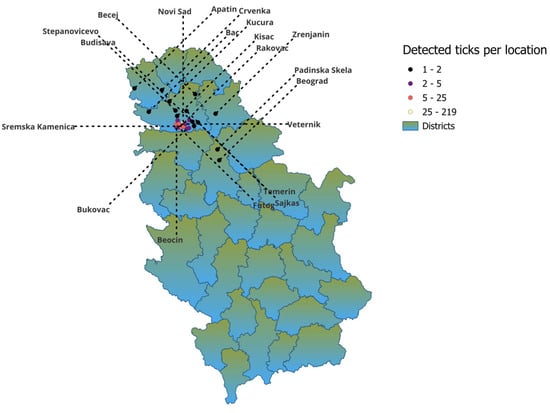
Figure 1.
Distribution of collected ticks per location.
2.2. Morphological Identification of Ticks
In the laboratory of the Scientific Veterinary Institute “Novi Sad”, ticks were identified by species, sex, and life stage based on morphological features using standard taxonomic keys described by Estrada-Pena et al. [25]. The collection locations and number of ticks were recorded. The total number of collected ticks was n = 308. Tick samples were stored at −20 °C prior to the molecular analysis.
2.3. Sample Preparation and DNA Extraction
Prior to DNA extraction, ticks were washed in 70% ethanol and distilled water. Ticks were then homogenized using the TissueLyser LT (Qiagen, Germantown, MD, USA)-50 Hz, for 3 min. DNA was extracted using the PureLink™ Genomic DNA Mini Kit (Invitrogen, Waltham, MA, USA, REF K182002), following the manufacturer’s instructions. During incubation at 56 °C, for a total of two hours, samples were processed in the TissueLyser LT every 30 min. Extracted DNA was either used immediately for real-time PCR or stored at −20 °C for later analysis.
2.4. Molecular Analysis
2.4.1. Real-Time PCR
All ticks were tested for Borrelia burgdorferi s.l., Anaplasma phagocytophilum, and Coxiella burnetii using real-time PCR (Mx3005P® QPCR System, Agilent, Santa Clara, CA, USA). The detection of target pathogens was performed using the following commercial kits: BactoReal® Kit Borrelia burgdorferi s.l. (Ingenetix GmbH, Vienna, Austria), A. phagocytophilum Genesig Standard Kit (Primerdesign Ltd., Eastleigh, UK), and BactoReal® Kit C. burnetii (Ingenetix GmbH, Vienna, Austria).
2.4.2. Nested PCR for Molecular Identification of Borrelia spp.
Samples that tested positive for Borrelia in real-time PCR were further analyzed at the Laboratory for Diagnostics of Borreliosis and Leptospirosis, Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Slovenia. The analysis was conducted for conformation, using the LightMix Kit Borrelia spp. EC (TIB MOLBIOL, Berlin, Germany), which detects and identifies Borrelia genomic DNA by amplifying a 160 bp fragment of the ospA gene. Species differentiation was performed through melting curve analysis on a LightCycler480 II (Roche, Basel, Switzerland).
2.5. Molecular Typing of Borrelia spp.
Molecular typing was performed on Borrelia-positive samples using the ospC gene, located on a plasmid. A 617 bp region of the ospC gene was amplified using primers OC6(+) and OC623 [26,27] with the Q5® High-Fidelity PCR Kit (New England BioLabs, Ipswich, MA, USA), annealing at 54 °C. Primer sequences and annealing temperatures are shown in Table 2. Amplified products were sequenced using Sanger sequencing (Macrogen Europe, Amsterdam, The Netherlands). Sequences were processed using the Staden package [28] and submitted to GenBank (accession numbers: OR735158–OR735165).

Table 2.
Primers for ospC PCR Amplification.
2.6. Phylogenetic Analysis
To assess the identity and genetic diversity of the Borrelia species identified in this study, obtained ospC sequences were analyzed using the Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 3 March 2025). In the next step, sequences were aligned using the MUSCLE algorithm. Based on the lowest Bayesian Information Criterion and corrected Akaike Information Criterion, General Time Reversible + Gamma + Invariant Sites (GTR + G + I) model was used to construct a phylogenetic tree. An unrooted phylogenetic tree was constructed to assess the genetic relatedness and genotype clustering of Borrelia sp. as the analysis focused on grouping species rather than evolutionary direction. The evolutionary history was inferred using the Maximum Likelihood (ML) method with complete deletion option and bootstrap set at 1000. These analyses and the phylogenetic tree construction were performed using the MEGA 11 software [29].
2.7. Statistical Analysis
The Kruskal–Wallis test was used to evaluate differences in pathogen prevalence across the study period. Results were considered statistically significant at p < 0.05.
3. Results
Out of 308 I. ricinus ticks removed from humans, 111/308 were nymphs (36.04%) and 197/308 were adult females (63.96%), while no larvae were detected. Borrelia spp. was detected in 56 ticks (17.8%) by real-time PCR. Molecular identification using the LightMix Kit Borrelia spp. EC (TIB MOLBIOL) was used to confirm the presence of B. afzelii in samples. Borrelia afzelii was identified as a sole genospecies in one group. Borrelia burgdorferi s.s. was also confirmed by ospC genotyping. Genotyping revealed seven successful partial ospC gene sequences of B. afzelii and B. burgdorferi s.s.
Anaplasma phagocytophilum was present in only five samples (1.62%; one nymph and four adult females), while C. burnetii was not found in any of the ticks. No co-infections were found among the ticks tested.
During a six-year period (2019–2024) fluctuations were found in prevalence of Borrelia spp. in nymphs and adult females removed from humans (Figure 2 and Table 3). The lowest prevalence for both nymphs and adults was registered in the year 2018 (3.44% and 7.69%, respectively), while highest values were observed in 2020 (25% and 33%, respectively). These variations were not statistically significant (Kruskal–Wallis, p > 0.05). The numbers of ticks positive for A. phagocytophilum over the years are also shown in Table 3.
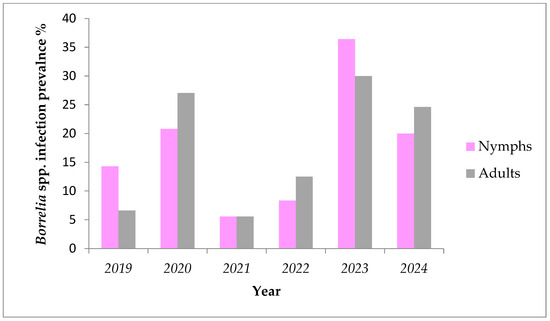
Figure 2.
Prevalence of Borrelia spp. in ticks removed from humans by development stage during the period of 2019–2024.

Table 3.
Number of positive ticks removed from humans by development stage by year.
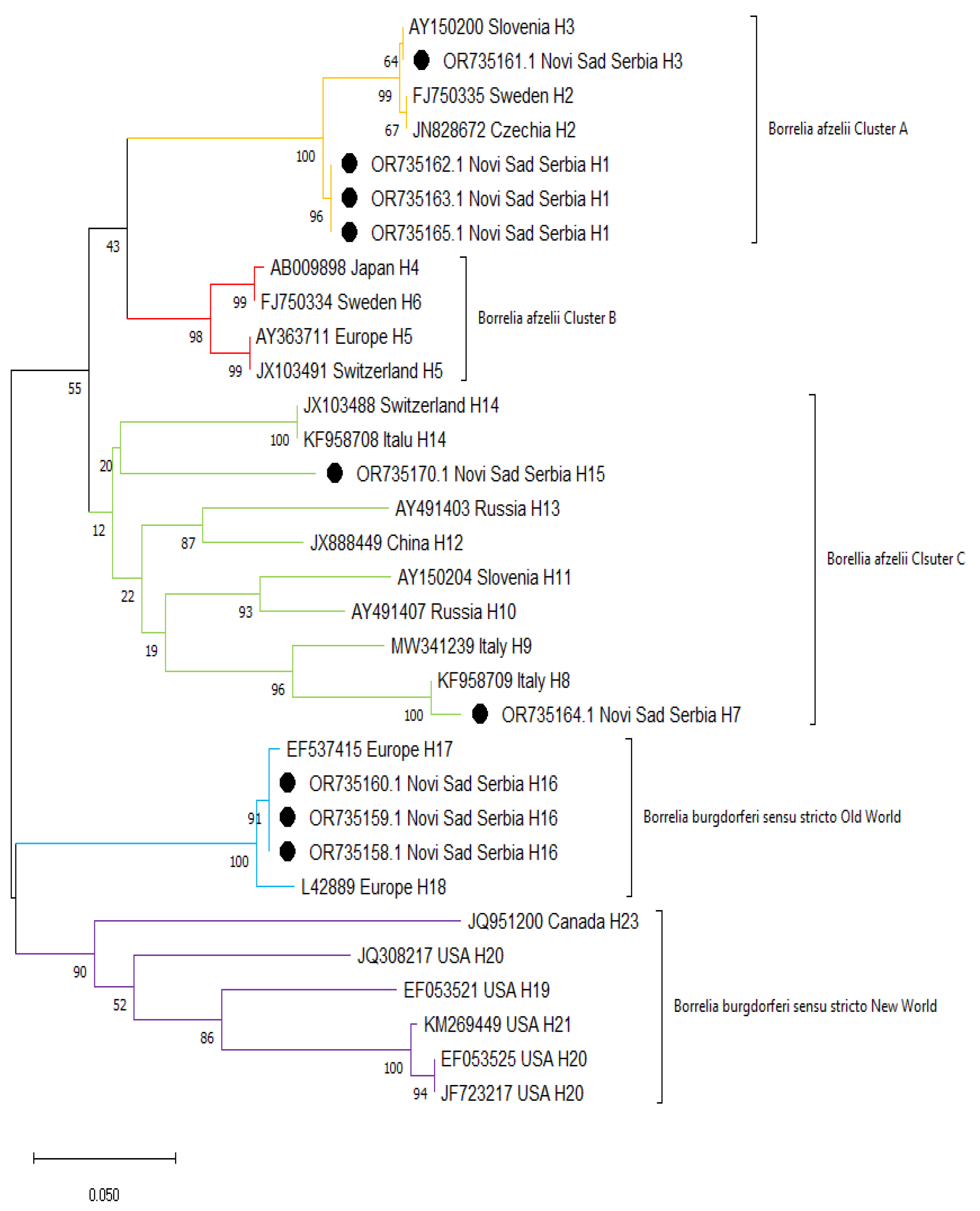
The results of sequencing of ospC gene confirmed species affiliation of the tested samples to B. burgdorferi s.s. and B. afzelii. The B. afzelii sequences obtained in the current study clustered in 2 groups (clusters A and C) together with other sequences reported previously, mainly from Europe (Figure 3). Similarly, B. burgdorferi s.s. sequences from Serbia obtained in this study formed a cluster with other European sequences (Old World), whereas North American sequences formed a separate cluster (New World).
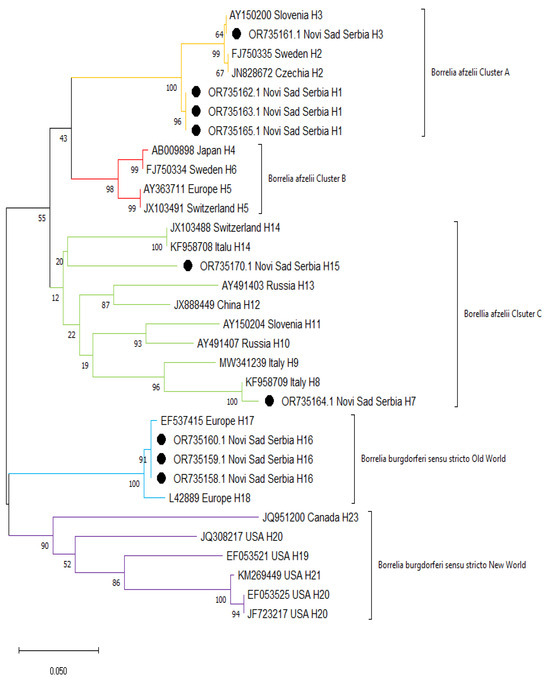
Figure 3.
Maximum likelihood phylogenetic tree of ospC sequences from Borrelia spp. Sequences from this study are marked with black dots.
4. Discussion
Prevalence of B. burgdorferi s.l., was reported earlier throughout Serbia as 25–28% in questing I. ricinus ticks, collected from vegetation and dogs [30,31]. Also, the similar percentage was found in ticks collected from vegetation and dogs in different parts of a northern Serbian province (Vojvodina) [4,15]. Exposure to B. burdgorferi was reported in dogs from the northern part of Serbia. Also, exposure to B. burgdorferi s.l., and development of Lyme disease is described in Serbian residents [18,32]. These findings show that both dogs and humans in Serbia have been in contact with the pathogen, with confirmed cases of Lyme borreliosis, indicating that this region is an endemic region for Lyme borreliosis [15,18,33]. There is no official estimation of the national Lyme disease incidence, because the disease stopped being reportable in 2016, regardless of the endemic nature of the disease. In our study, B. burgdorferi s.l. was detected in 17.8% of I. ricinus ticks removed from humans, which is consistent with reports from 2020 and 2021 [9,18]. Interestingly, higher prevalence rates were recorded in 2019, reaching 33.3% [34]. This might be due to a dynamic pattern of pathogen circulation, influenced by environmental factors, tick population dynamics or host availability. Fluctuations in tick pathogen presence in ticks could not be compared to fluctuations of Lyme borreliosis cases in humans, due to the fact that Lyme borreliosis has been an unreportable disease in Serbia since 2016. That is also one of the reasons for this study—to highlight the importance of disease reporting in following up on the occurrence and potential risk at present and in the future. Genotyping analysis confirmed the presence of both B. afzelii and B. burgdorferi sensu stricto (s.s.) in the collected ticks. Borrelia afzelii was found to be the sole genospecies in certain samples. Phylogenetic analysis further demonstrated that Serbian B. burgdorferi s.s. sequences clustered with other European strains, forming a group that is distinct from North American variants. This highlights regional genetic similarities that may have implications for Lyme borreliosis epidemiology and pathogenicity. In addition to Borrelia, our study detected Anaplasma phagocytophilum in 1.62% of ticks taken from humans. This finding aligns with previous studies in Serbia, where A. phagocytophilum prevalence in ticks taken off humans, ranged from 6% in 2019 to 9.68% in 2020 and 3.95% in 2021 [9,17,18]. While reports of canine Anaplasma infections in Serbia remain scarce [17], seroprevalence studies have detected Anaplasma-reactive IgG in 2.45% of cattle (with immunofluorescence test) [23] and 26.1% of dogs (with immunochromatography test) [22]. The presence of A. phagocytophilum in Serbian ticks and animals suggests that this pathogen is actively circulating among local vectors and animal hosts. Unlike previous study on questing ticks from Serbia [19], our study did not detect Coxiella burnetii in any ticks removed from humans. It could be because the tick bites occurred in urban areas or out-of-the-city green areas, and not on pastures or places near cattle/sheep. The absence of C. burnetii in tick samples suggests that while the disease is circulating in the environment, tick infections remain rare or sporadic, and Q-fever is transferred among animals in other ways of infection. Q-fever is definitely present in Serbia among ruminants and sheep [20,24] but as there are other ways of spreading infection apart from tick bites, it could be concluded that the disease among humans is not—or is rarely—spread by ticks. Maybe the result would be different if the ticks were taken off sheep or cattle. The prevalence of B. burgdorferi s.l. in ticks that bite humans shows significant variation across the region. In Romania, studies have found B. burgdorferi s.l. in 3% of ticks and A. phagocytophilum in 1.3% [35]. Similar results were obtained in a study carried out in Cluj-Napoca—a region known for Lyme disease. The authors of this study reported a B. burgdorferi s.l. prevalence of 12.64% [36,37]. Higher prevalence rates were observed in Sarajevo Canton, Bosnia and Herzegovina, where B. spielmanii and B. lusitaniae were found in 66.7% of ticks that infest humans, as determined by nested PCR [38]. These differences across the region may be due to variations in tick density, the availability of hosts, climate conditions, and diagnostic methods. These results highlight the need for coordinated surveillance strategies throughout Southeastern Europe. A key limitation of our study is that samples are mainly from the northern part of Serbia, which may not accurately represent pathogen distribution across the entire country, but does represent the endemic region in Serbia. Additionally, we only checked ticks for Borrelia, Anaplasma, and Coxiella, excluding other tick-borne pathogens such as Rickettsia and Babesia. These three pathogens were the focus of this study, because of their presence in animals (and humans). Future research that includes sampling in different regions of Serbia and whole-genome sequencing could offer a more comprehensive understanding of pathogen diversity and evolution. The variety of Borrelia strains and the presence of A. phagocytophilum underscore the importance of continued surveillance and public health awareness.
5. Conclusions
The presence of tick-borne pathogens in ticks depends on different factors, such as environmental factors, vector distribution, human exposure patterns, etc. Our findings confirm a notable prevalence of Borrelia spp. in human-biting ticks, with fluctuations observed over the study period, though without statistical significance. Molecular analysis identified B. afzelii and B. burgdorferi s.s., indicating genetic diversity among Borrelia strains in Serbia. Anaplasma phagocytophilum was detected at a low frequency meaning that further research is needed and collection of ticks from different kinds of animals including wild animals. Coxiella burnetii was absent, potentially due to its low presence in ticks, and also due to it rarely being transferred by ticks to either humans or animals.
These results reinforce previous reports that Serbia is an endemic region for some tick-borne diseases, highlighting the need for public health measures. The absence of standardized case definitions and diagnostic guidelines in combination with not reporting of the disease, further complicates disease surveillance and management. These results also show how collaboration between environmental, medical and veterinary disciplines can contribute to greater results and knowledge, packed into a One Health approach.
This study provides valuable molecular insights into pathogens in ticks removed from humans, despite limited sample not fully capturing the epidemiological landscape across Serbia. Expanding surveillance efforts, integrating molecular and epidemiological data, and developing targeted control strategies will be essential for looking at the growing public health problem with tick-borne diseases in Serbia.
Author Contributions
Conceptualization, S.S. and M.Ž.; methodology, M.Ž., V.C.-Š. and T.C.K.; investigation, S.S. M.Ž. and A.P.; writing—original draft preparation S.S., M.Ž., A.P. and V.G.; writing—review and editing, V.C.-Š., E.R.-S. and T.C.K.; supervision S.S., E.R.-S., A.P. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of Republic of Serbia by the Contract of implementation and funding of research work of NIV-NS in 2024, Contract No: 451-03-66/2024-03/200031. The research was performed during the bilateral collaboration between Serbia and Slovenia, within the duration of the project: “Lyme borreliosis and leptospirosis pathogen determination and developing methods for identification and conformation of infection in Serbia and Slovenia from a One Health perspective”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We especially acknowledge the help and guidance from Zbigniew Zając with the writing of the manuscript. We are thankful to Nevenka Aleksić, a holder of Cambridge CPE, for text editing and proofreading. AI-based software ChatGPT 4o mini was utilized for proofreading and spell-checking to enhance the clarity and accuracy of the text. The authors retain full responsibility for the content and interpretation of the findings presented in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Migné, C.V.; Hönig, V.; Bonnet, S.I.; Palus, M.; Rakotobe, S.; Galon, C.; Heckmann, A.; Vyletova, E.; Devillers, E.; Attoui, H.; et al. Evaluation of two artificial infection methods of live ticks as tools for studying interactions between tick-borne viruses and their tick vectors. Sci. Rep. 2022, 12, 491. [Google Scholar] [CrossRef]
- Potkonjak, A.; Gutiérrez, R.; Savić, S.; Vračar, V.; Nachum-Biala, Y.; Jurišić, A.; Kleinerman, G.; Rojas, A.; Petrović, A.; Baneth, G.; et al. Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick-Borne Dis. 2016, 7, 199–203. [Google Scholar] [CrossRef]
- Michael, L. Focus on Common Small Animal Vector-Borne Diseases in Central and Southeastern Europe. Acta Vet. Sciendo 2020, 70, 147–169. [Google Scholar] [CrossRef]
- Potkonjak, A.; Zekic-Stosic, M. Tick-borne infections of dogs in Serbia: A review of research. Vet. Glas. 2020, 74, 107–124. [Google Scholar] [CrossRef]
- Ivanović, I.; Stošić, M.Ž.; Sabljić, E.R.; Kišek, T.C.; Špik, V.C.; Popović, A.; Savić, S. Ecology and prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus (Acari: Ixodidae) ticks. Acta Vet. Hung. 2022, 70, 15–23. [Google Scholar] [CrossRef]
- Audino, T.; Pautasso, A.; Bellavia, V.; Carta, V.; Ferrari, A.; Verna, F.; Grattarola, C.; Iulini, B.; Pintore, M.D.; Bardelli, M.; et al. Ticks infesting humans and associated pathogens: A cross-sectional study in a 3-year period (2017–2019) in northwest Italy. Parasites Vectors 2021, 14, 136. [Google Scholar] [CrossRef]
- Banović, P.; Piloto-Sardiñas, E.; Mijatović, D.; Foucault-Simonin, A.; Simin, V.; Bogdan, I.; Obregón, D.; Mateos-Hernández, L.; Moutailler, S.; Cabezas-Cruz, A. Differential detection of tick-borne pathogens in human platelets and whole blood using microfluidic PCR. Acta Trop. 2023, 238, 106756. [Google Scholar] [CrossRef]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef]
- Korotkov, Y.; Kozlova, T.; Kozlovskaya, L. Observations on changes in abundance of questing Ixodes ricinus, castor bean tick, over a 35-year period in the eastern part of its range (Russia, Tula region). Med. Vet. Èntomol. 2015, 29, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Voyiatzaki, C.; Papailia, S.I.; Venetikou, M.S.; Pouris, J.; Tsoumani, M.E.; Papageorgiou, E.G. Climate Changes Exacerbate the Spread of Ixodes ricinus and the Occurrence of Lyme Borreliosis and Tick-Borne Encephalitis in Europe—How Climate Models Are Used as a Risk Assessment Approach for Tick-Borne Diseases. Int. J. Environ. Res. Public. Heal. 2022, 19, 6516. [Google Scholar] [CrossRef]
- Tomanović, S.; Radulović, Ž.; Masuzawa, T.; Milutinović, M. Coexistence of emerging bacterial pathogens in Ixodes ricinusticks in Serbia. Parasite 2010, 17, 211–217. [Google Scholar] [CrossRef]
- Savić, S.; Vidić, B.; Lazić, S.; Lako, B.; Potkonjak, A.; Lepšanović, Z. Borrelia burgdorferi in ticks and dogs in the province of Vojvodina, Serbia. Parasite 2010, 17, 357–361. [Google Scholar] [CrossRef]
- Potkonjak, A.; Kleinerman, G.; Gutiérrez, R.; Savić, S.; Vračar, V.; Nachum-Biala, Y.; Jurišić, A.; Rojas, A.; Petrović, A.; Ivanović, I.; et al. Occurrence of Borrelia burgdorferi Sensu Lato in Ixodes ricinus Ticks with First Identification of Borrelia miyamotoi in Vojvodina, Serbia. Vector-Borne Zoonotic Dis. 2016, 16, 631–635. [Google Scholar] [CrossRef]
- Banović, P.; Díaz-Sánchez, A.A.; Galon, C.; Simin, V.; Mijatović, D.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Humans infested with Ixodes ricinus are exposed to a diverse array of tick-borne pathogens in Serbia. Ticks Tick Borne Dis. 2021, 12, 101609. [Google Scholar] [CrossRef]
- Banović, P.; Díaz-Sánchez, A.A.; Simin, V.; Foucault-Simonin, A.; Galon, C.; Wu-Chuang, A.; Mijatović, D.; Obregón, D.; Moutailler, S.; Cabezas-Cruz, A. Clinical Aspects and Detection of Emerging Rickettsial Pathogens: A “One Health” Approach Study in Serbia, 2020. Front. Microbiol. 2022, 12, 797399. [Google Scholar] [CrossRef] [PubMed]
- Tomanović, S.; Chochlakis, D.; Radulović, Ž.; Milutinović, M.; Ćakić, S.; Mihaljica, D.; Tselentis, Y.; Psaroulaki, A. Analysis of pathogen co-occurrence in host-seeking adult hard ticks from Serbia. Exp. Appl. Acarol. 2013, 59, 367–376. [Google Scholar] [CrossRef]
- Debeljak, Z.; Medić, S.; Baralić, M.; Andrić, A.; Tomić, A.; Vidanović, D.; Šekler, M.; Matović, K.; Vasković, N. Clinical, epidemiological and epizootic features of a Q fever outbreak in the border region between Serbia and Montenegro. J. Infect. Dev. Ctries. 2018, 12, 290–296. [Google Scholar] [CrossRef]
- Jovanović, V. Annual Report on Infectious Diseases in the Republic of Serbia for 2021. Belgrade. 2021. Available online: https://www.batut.org.rs/index.php?content=2523 (accessed on 10 March 2025).
- Filipović, M.M.K.; Beletić, A.D.; Božović, A.V.I.; Milanović, Z.; Tyrrell, P.; Buch, J.; Breitschwerdt, E.B.; Birkenheuer, A.J.; Chandrashekar, R. Molecular and Serological Prevalence of Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeenses, E. ewingii, Borrelia burgdorferi, Babesia canis, B. gibsoni and B. vogeli among Clinically Healthy Outdoor Dogs in Serbia. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 117–122. [Google Scholar] [CrossRef]
- Vasić, A.; Nieder, M.; Zdravković, N.; Bojkovski, J.; Bugarski, D.; Pavlović, I.; Silaghi, C. Tick infestation and occurrence of Anaplasma phagocytophilum and piroplasms in cattle in the Republic of Serbia. Parasitol. Res. 2018, 117, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Zutic, J.; Vojinovic, D.; Stanojevic, S.; Kureljusic, B.; Milicevic, V.; Kureljusic, J.; Spalevic, L. Seroprevalence of Coxiella burnetii in cattle in the Belgrade epizootiological area. Biotechnol. Anim. Husb. 2020, 36, 359–369. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Bouattour, A.J.L.C.; Camicas, J.L.; Walker, A.R. Ticks of Domestic Animals in the Mediterranean Region. In A Guide to Identification of Species; University of Zaragoza: Zaragoza, Spain, 2004. [Google Scholar]
- Wang, I.-N.; E Dykhuizen, D.; Qiu, W.; Dunn, J.J.; Bosler, E.M.; Luft, B.J. Genetic Diversity of ospC in a Local Population of Borrelia burgdorferi sensu stricto. Genetics 1999, 151, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Pearson, P.; Skaltsis, O.; Luo, C.-Y.; Xu, G.; Oppler, Z.; Brisson, D.; Rich, S.M. A Borrelia burgdorferi outer surface protein C (OspC) genotyping method using Luminex technology. PLoS ONE 2022, 17, e0269266. [Google Scholar] [CrossRef] [PubMed]
- Staden, R.; Beal, K.F.; Bonfield, J.K. The Staden Package. In Bioinformatics Methods and Protocols; Humana Press: Totowa, NJ, USA, 1998; pp. 115–130. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Milutinović, M.; Masuzawa, T.; Tomanović, S.; Radulović, Ž.; Fukui, T.; Okamoto, Y. Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Francisella tularensis and their co-infections in host-seeking Ixodes ricinus ticks collected in Serbia. Exp. Appl. Acarol. 2008, 45, 171–183. [Google Scholar] [CrossRef]
- Bogunović, D.; Stević, N.; Sidi-Boumedine, K.; Mišić, D.; Tomanović, S.; Kulišić, Z.; Magaš, V.; Radojičić, S. Molecular Evidence of Q Fever Agent Coxiella Burnetii in Ixodid Ticks Collected from Stray Dogs in Belgrade (Serbia). Acta Vet. Brno. 2018, 68, 257–268. [Google Scholar] [CrossRef]
- Banović, P.; Čapo, I.; Ogorelica, D.; Vranješ, N.; Simin, V.; Lalošević, D. Mysterious path of Borrelia spielmanii: Spreading without morphological alteration of collagen type I and IV. Futur. Microbiol. 2019, 14, 1469–1475. [Google Scholar] [CrossRef]
- Simin, V.; Lalosevic, D.; Mijatovic, D.; Tomanovic, S.; Miljevic, M.; Cabrilo, B.; Bogdan, I.; Banovic, P. Borellia burgdorferi infection in removed ticks and anti-borrelia antibodies in infested patients admitted to the Pasteur institute, Novi Sad. Vet. Glas. 2020, 74, 164–177. [Google Scholar] [CrossRef]
- Banovic, P. Early-Stage Diagnosis and Risk Factors for Tick Infestation and Development of Lyme Borreliosis in Residents of South Bačka District. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2022. [Google Scholar]
- Anderson, M.L. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology 2007, 68, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Kalmár, Z.; Dumitrache, M.O.; D’amico, G.; Matei, I.A.; Ionică, A.M.; Gherman, C.M.; Lupșe, M.; Mihalca, A.D. Multiple Tick-Borne Pathogens in Ixodes ricinus Ticks Collected from Humans in Romania. Pathogens 2020, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Maiwald, M.; Oehme, R.; March, O.; Petney, T.N.; Kimmig, P.; Naser, K.; Zappe, H.A.; Hassler, D.; Doeberitz, M.V.K. Transmission risk of Borrelia burgdorferi sensu lato from Ixodes ricinus ticks to humans in southwest Germany. Epidemiol. Infect. 1998, 121, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lasić, L.; Ušanović, L.; Ćakić, S.; Hanjalić, J.; Stroil, B.K. First molecular detection of Borrelia burgdorferi in Ixodes ricinu ticks collected from humans in the Sarajevo Canton. Syst. Appl. Acarol. 2020, 25, 169–172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).