Generation of Immune Modulating Small Metabolites—Metabokines—By Adult Schistosomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites and Mice
2.2. Metabokine Detection in Murine Plasma
2.3. Statistical Analysis
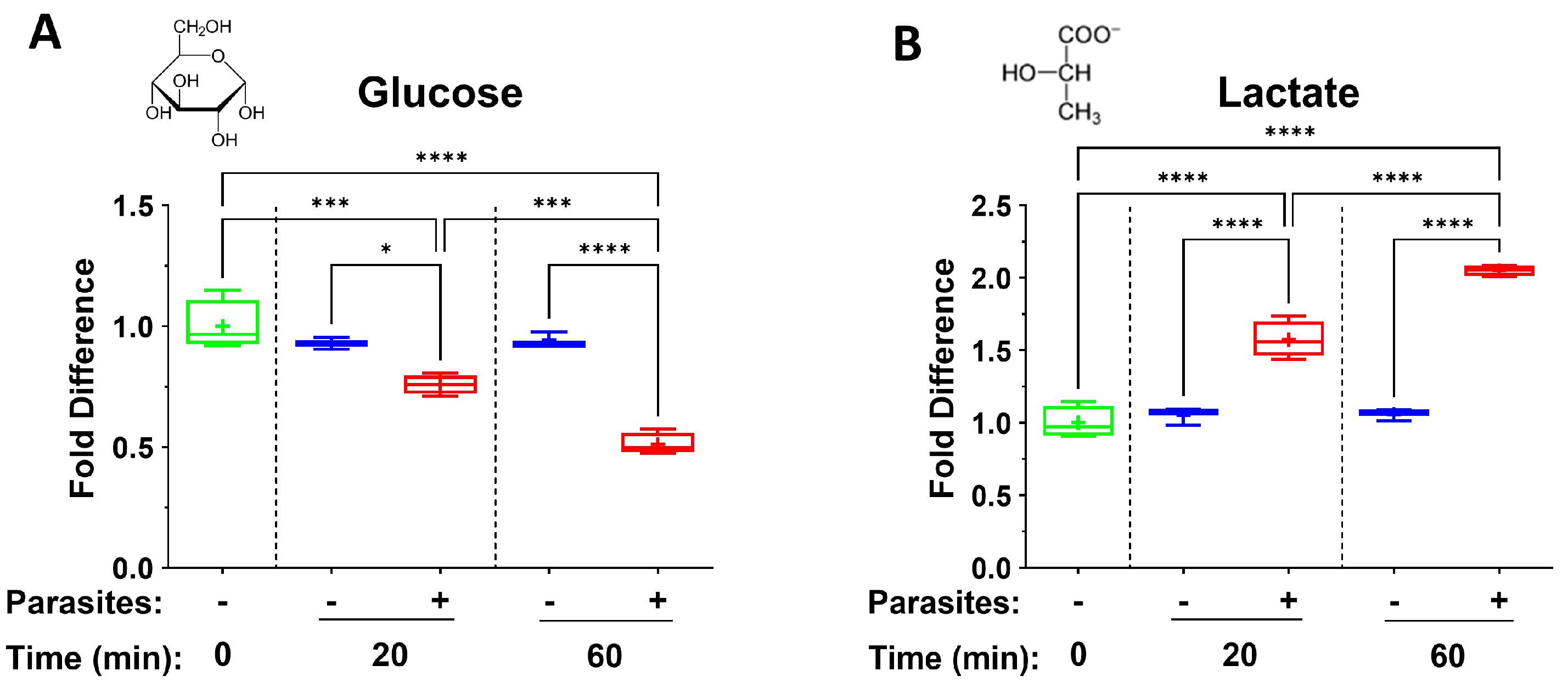
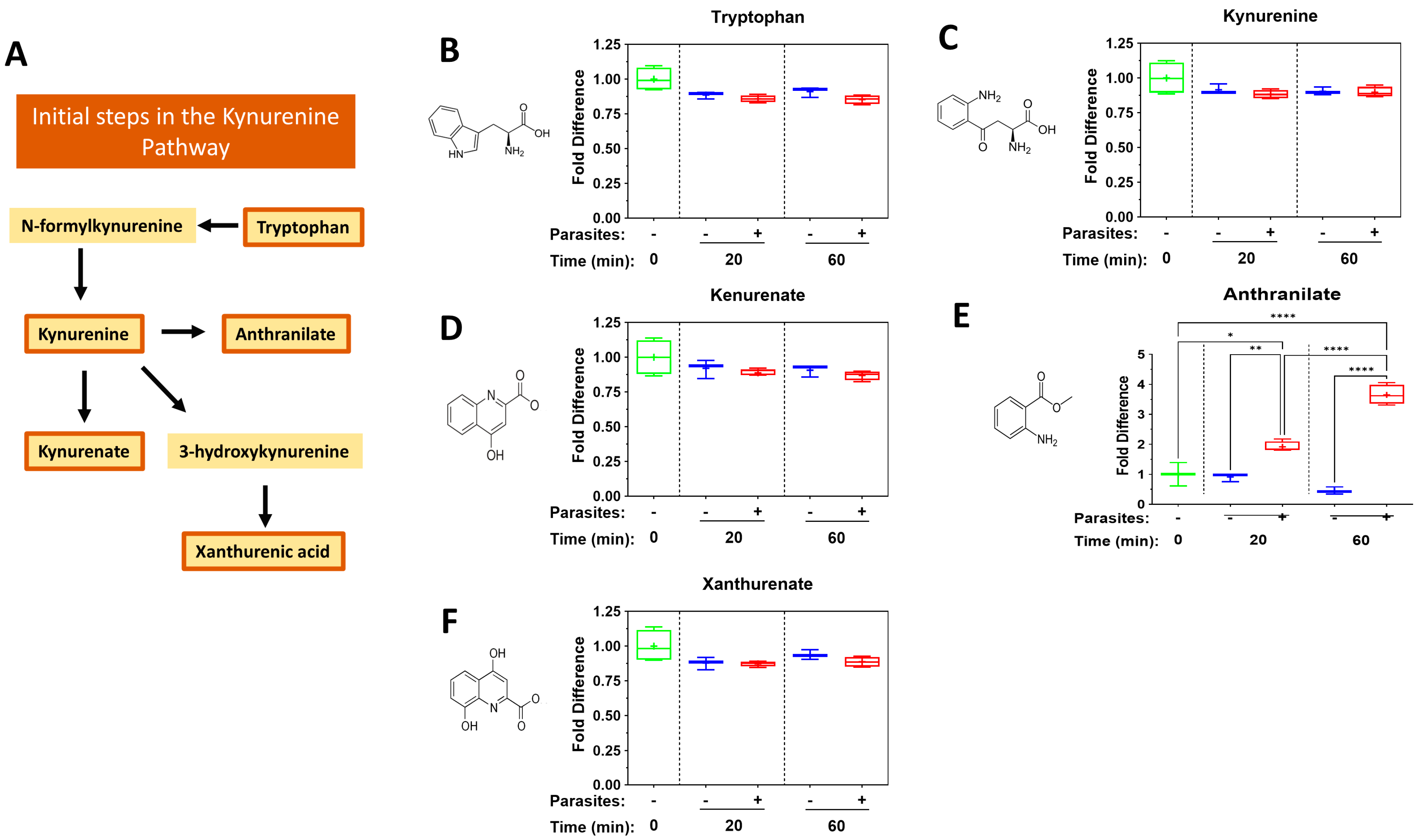
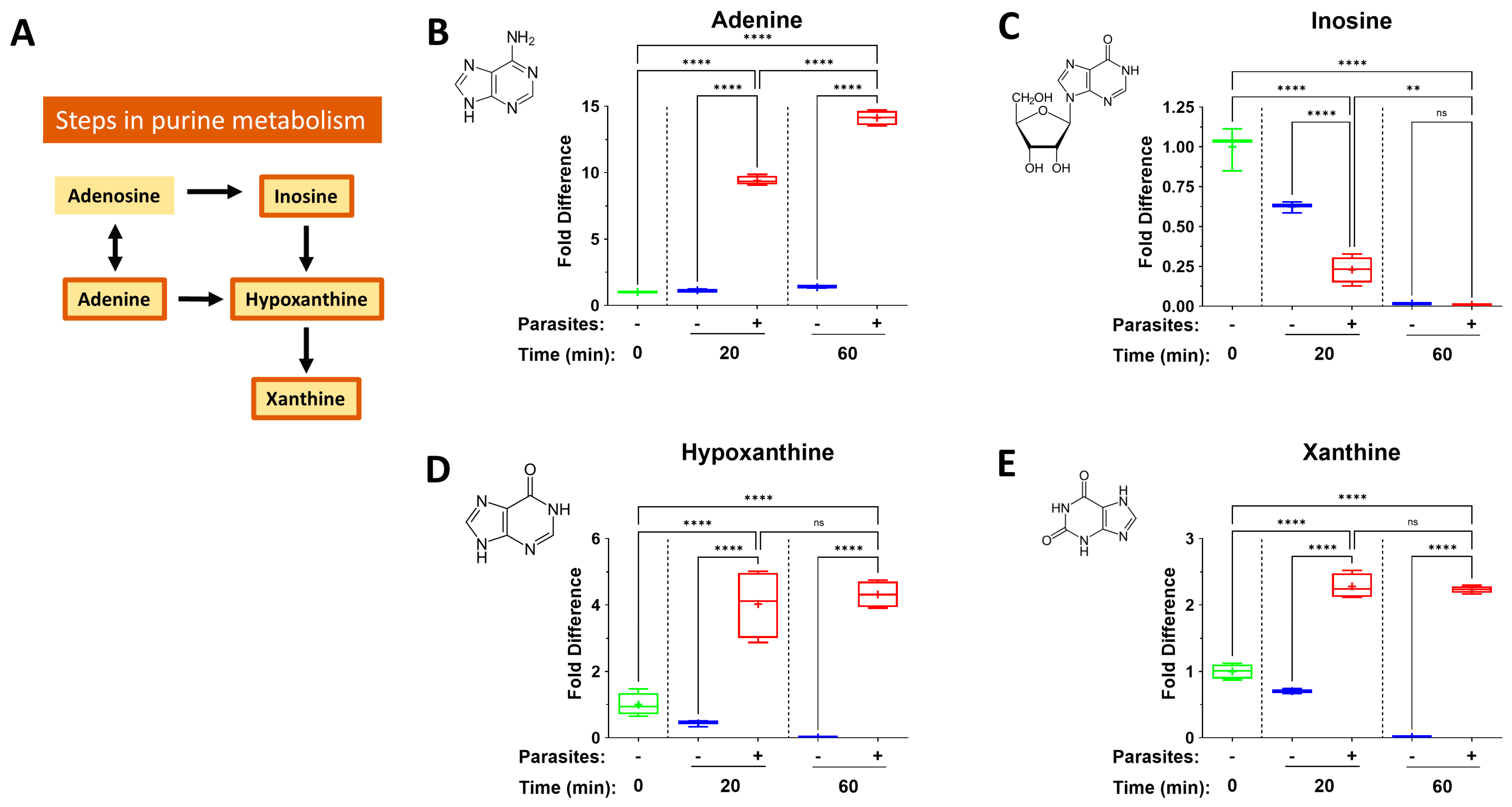
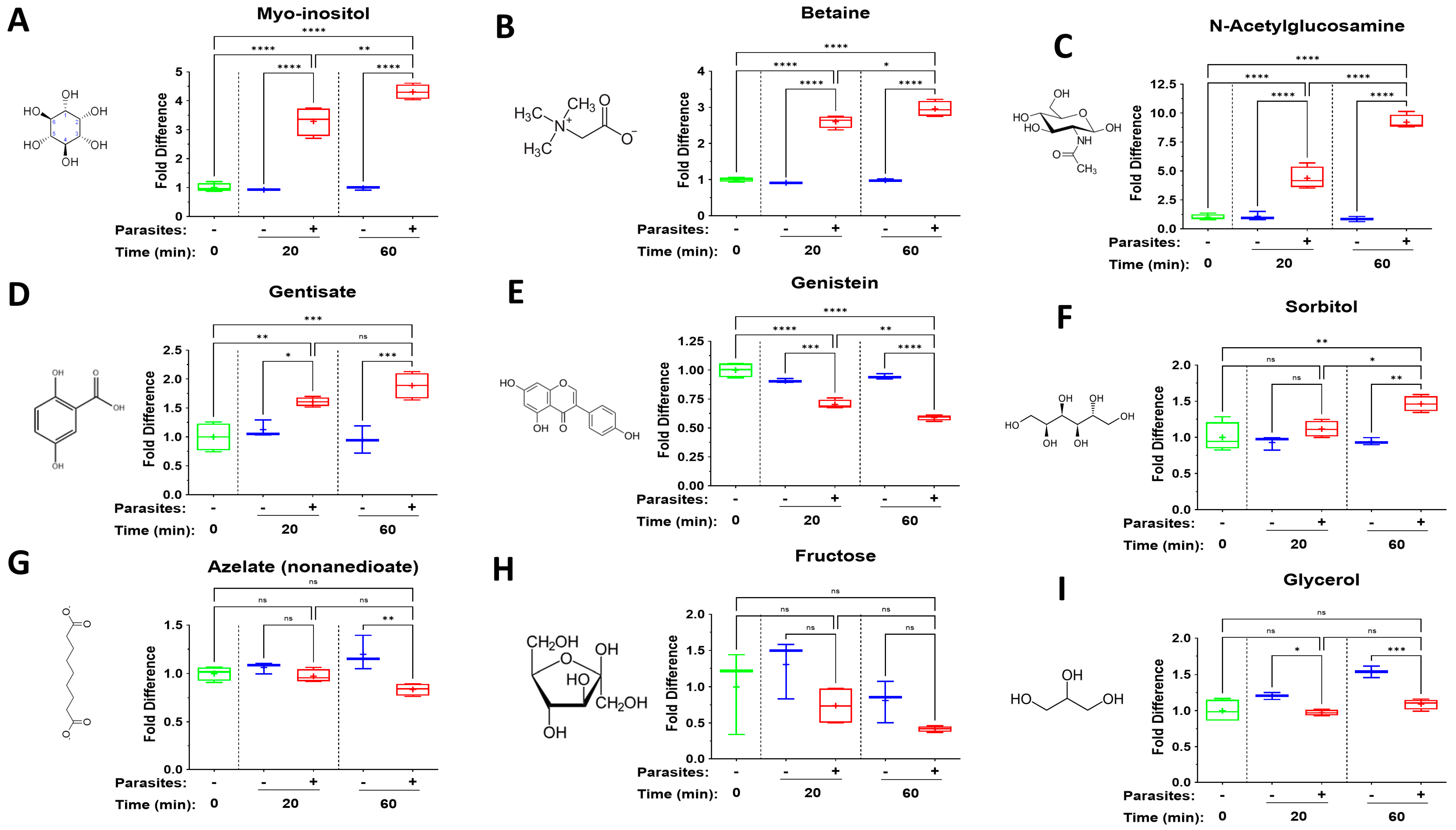
3. Results
4. Discussion
4.1. TCA Cycle Intermediates
4.2. The Kynurenine Pathway
4.3. The Purinergic Halo
4.4. Additional Diverse Metabokines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-HG | 2-hydroxyglutarate |
| ADP | Adenosine Diphosphate |
| ATP | Adenosine Triphosphate |
| GlcNAc | N-acetylglucosamine |
| GPR | G-protein-coupled receptor |
| MCT | Monocarboxylate transporter |
| NAD | Nicotinamide adenine dinucleotide |
| SUCNR1 | succinate receptor 1 |
| TCA | Tricarboxylic acid |
| Treg | T regulatory cell |
References
- Skelly, P. Fighting killer worms. Sci. Am. 2008, 298, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Da’dara, A.A.; Skelly, P.J. Schistosome immunomodulators. PLoS Pathog. 2021, 17, e1010064. [Google Scholar] [CrossRef]
- Skelly, P.J.; Nation, C.S.; Da’Dara, A.A. Schistosoma mansoni and the purinergic halo. Trends Parasitol. 2022, 38, 1080–1088. [Google Scholar] [CrossRef]
- Skelly, P.J.; Da’Dara, A.A. Schistosome secretomes. Acta Trop. 2022, 236, 106676. [Google Scholar] [CrossRef]
- Zasłona, Z.; O’neill, L.A. Cytokine-like Roles for Metabolites in Immunity. Mol. Cell 2020, 78, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Morrot, A.; Da Fonseca, L.M.; Salustiano, E.J.; Gentile, L.B.; Conde, L.; Filardy, A.A.; Franklim, T.N.; da Costa, K.M.; Freire-De-Lima, C.G.; Freire-De-Lima, L. Metabolic Symbiosis and Immunomodulation: How Tumor Cell-Derived Lactate May Disturb Innate and Adaptive Immune Responses. Front. Oncol. 2018, 8, 81. [Google Scholar] [CrossRef]
- Weiss, H.J.; Angiari, S. Metabolite Transporters as Regulators of Immunity. Metabolites 2020, 10, 418. [Google Scholar] [CrossRef]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2017, 114, 580–585. [Google Scholar] [CrossRef]
- Mills, E.; O’neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, M.J.; McDonough, K.L.; Pituch, J.J.; Christopherson, P.L.; Cornell, T.T.; Selewski, D.T.; Shanley, T.P.; Blatt, N.B. Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses. Clin. Exp. Immunol. 2015, 180, 520–530. [Google Scholar] [CrossRef] [PubMed]
- E Longbrake, E.; Mao-Draayer, Y.; Cascione, M.; Zielinski, T.; Bame, E.; Brassat, D.; Chen, C.; Kapadia, S.; Mendoza, J.P.; Miller, C.; et al. Dimethyl fumarate treatment shifts the immune environment toward an anti-inflammatory cell profile while maintaining protective humoral immunity. Mult. Scler. 2021, 27, 883–894. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Tucker, M.S.; Karunaratne, L.B.; Lewis, F.A.; Freitas, T.C.; Liang, Y. Schistosomiasis. Curr. Protoc. Immunol. 2013, 103, 19.1.1–19.1.58. [Google Scholar] [CrossRef] [PubMed]
- Elzoheiry, M.; Da’dara, A.A.; Bhardwaj, R.; Wang, Q.; Azab, M.S.; El-Kholy, E.-S.I.; El-Beshbishi, S.N.; Skelly, P.J. Intravascular Schistosoma mansoni Cleave the Host Immune and Hemostatic Signaling Molecule Sphingosine-1-Phosphate via Tegumental Alkaline Phosphatase. Front. Immunol. 2018, 9, 1746. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, J.; Zhang, Y.; Li, H.; Chen, Y.; Zhang, H.; Pan, J.; Zhou, Y.; Zhang, S.; Cheng, L. L-Kynurenine regulates immune response in ICIs-associated myocarditis via JAK/STAT pathway. Int. Immunopharmacol. 2025, 156, 114676. [Google Scholar] [CrossRef]
- Krautz-Peterson, G.; Simoes, M.; Faghiri, Z.; Ndegwa, D.; Oliveira, G.; Shoemaker, C.B.; Skelly, P.J. Suppressing glucose transporter gene expression in schistosomes impairs parasite feeding and decreases survival in the mammalian host. PLoS Pathog. 2010, 6, e1000932. [Google Scholar] [CrossRef]
- Skelly, P.J.; Kim, J.W.; Cunningham, J.; Shoemaker, C.B. Cloning, characterization and functional expression of cDNAs encoding glucose transporter proteins from the human parasite, Schistosoma mansoni. J. Biol. Chem. 1994, 269, 4247–4253. [Google Scholar] [CrossRef]
- Bueding, E. Carbohydrate metabolism of Schistosoma mansoni. J. Gen. Physiol. 1950, 33, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Schiller, E.L.; Bueding, E.; Turner, V.M.; Fisher, J. Aerobic and anaerobic carbohydrate metabolism and egg production of Schistosoma mansoni in vitro. J. Parasitol. 1975, 61, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Faghiri, Z.; Camargo, S.M.R.; Huggel, K.; Forster, I.C.; Ndegwa, D.; Verrey, F.; Skelly, P.J. The tegument of the human parasitic worm Schistosoma mansoni as an excretory organ: The surface aquaporin SmAQP is a lactate transporter. PLoS ONE 2010, 5, e10451. [Google Scholar] [CrossRef] [PubMed]
- Iraporda, C.; Romanin, D.E.; Bengoa, A.A.; Errea, A.J.; Cayet, D.; Foligné, B.; Sirard, J.-C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Local Treatment with Lactate Prevents Intestinal Inflammation in the TNBS-Induced Colitis Model. Front. Immunol. 2016, 7, 651. [Google Scholar] [CrossRef]
- Luo, Y.; Li, L.; Chen, X.; Gou, H.; Yan, K.; Xu, Y. Effects of lactate in immunosuppression and inflammation: Progress and prospects. Int. Rev. Immunol. 2022, 41, 19–29. [Google Scholar] [CrossRef]
- Manoharan, I.; Prasad, P.D.; Thangaraju, M.; Manicassamy, S. Lactate-Dependent Regulation of Immune Responses by Dendritic Cells and Macrophages. Front. Immunol. 2021, 12, 691134. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z.; Zhang, H.; Liang, X.; Zhang, X.; Wen, Z.; Luo, P.; Zhang, J.; Liu, Z.; Zhang, M.; et al. Tumor-secreted lactate contributes to an immunosuppressive microenvironment and affects CD8 T-cell infiltration in glioblastoma. Front. Immunol. 2023, 14, 894853. [Google Scholar] [CrossRef]
- Comito, G.; Iscaro, A.; Bacci, M.; Morandi, A.; Ippolito, L.; Parri, M.; Montagnani, I.; Raspollini, M.R.; Serni, S.; Simeoni, L.; et al. Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene 2019, 38, 3681–3695. [Google Scholar] [CrossRef]
- Abebayehu, D.; Spence, A.J.; Caslin, H.; Taruselli, M.; Haque, T.T.; Kiwanuka, K.N.; Kolawole, E.M.; Chumanevich, A.P.; Sell, S.A.; Oskeritzian, C.A.; et al. Lactic acid suppresses IgE-mediated mast cell function in vitro and in vivo. Cell Immunol. 2019, 341, 103918. [Google Scholar] [CrossRef]
- Farsani, S.S.M.; Verma, V. Lactate mediated metabolic crosstalk between cancer and immune cells and its therapeutic implications. Front. Oncol. 2023, 13, 1175532. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Kueth, G.; Shao, E.; Wang, X.; Ha, T.; Williams, D.L.; Li, C.; Fan, M.; Yang, K. Lactate’s impact on immune cells in sepsis: Unraveling the complex interplay. Front. Immunol. 2024, 15, 1483400. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Selva, D.; Fairfax, K. Schistosome and intestinal helminth modulation of macrophage immunometabolism. Immunology 2021, 162, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C.; et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Vadevoo, S.M.P.; Gunassekaran, G.R.; Lee, C.; Lee, N.; Lee, J.; Chae, S.; Park, J.-Y.; Koo, J.; Lee, B. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc. Natl. Acad. Sci. USA 2021, 118, e2102434118. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Adalid-Peralta, L.; Fragoso, G.; Fleury, A.; Sciutto, E. Mechanisms underlying the induction of regulatory T cells and its relevance in the adaptive immune response in parasitic infections. Int. J. Biol. Sci. 2011, 7, 1412–1426. [Google Scholar] [CrossRef]
- Skelly, P.J.; Stein, L.D.; Shoemaker, C.B. Expression of Schistosoma mansoni genes involved in anaerobic and oxidative glucose metabolism during the cercaria to adult transformation. Mol. Biochem. Parasitol. 1993, 60, 93–104. [Google Scholar] [CrossRef]
- Milanović, M.; Bekić, M.; Đokić, J.; Vučević, D.; Čolić, M.; Tomić, S. Exogenous alpha-ketoglutarate Modulates Redox Metabolism and Functions of Human Dendritic Cells, Altering Their Capacity to Polarise T Cell Response. Int. J. Biol. Sci. 2024, 20, 1064–1087. [Google Scholar] [CrossRef]
- Liu, G.M.; Lu, J.J.; Sun, W.X.; Jia, G.; Zhao, H.; Chen, X.L.; Tian, G.; Cai, J.Y.; Zhang, R.N.; Wang, J. Dietary alpha-ketoglutarate enhances intestinal immunity by Th17/Treg immune response in piglets after lipopolysaccharide challenge. J. Anim. Sci. 2023, 101, skad213. [Google Scholar] [CrossRef]
- Najjar, E.; Staun-Ram, E.; Volkowich, A.; Miller, A. Dimethyl fumarate promotes B cell-mediated anti-inflammatory cytokine profile in B and T cells, and inhibits immune cell migration in patients with MS. J. Neuroimmunol. 2020, 343, 577230. [Google Scholar] [CrossRef]
- Gill, A.J.; Kolson, D.L. Dimethyl fumarate modulation of immune and antioxidant responses: Application to HIV therapy. Crit. Rev. Immunol. 2013, 33, 307–359. [Google Scholar] [CrossRef] [PubMed]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Vicario, N.; Costa, A.S.; Kwok, C.K.; Leonardi, T.; Booty, L.M.; Bicci, I.; Balzarotti, B.; Volpe, G.; et al. Macrophage-Derived Extracellular Succinate Licenses Neural Stem Cells to Suppress Chronic Neuroinflammation. Cell Stem Cell 2018, 22, 355–368.e13. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, G.; He, Y.; Chen, J.; Yan, J.; Zhang, Q.; Li, L.; Cai, X. Cellular succinate metabolism and signaling in inflammation: Implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef] [PubMed]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Gowda, G.N.; Fontana, M.F.; et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49, 33–41.e7. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Ma, X.; Zhao, J.-C.; Wang, X.-Q.; Gao, C.-Q. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes 2023, 15, 2190300. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Helminths in alternative therapeutics of inflammatory bowel disease. Intest. Res. 2025, 23, 8–22. [Google Scholar] [CrossRef]
- Mickael, C.S.; Graham, B.B. The Role of Type 2 Inflammation in Schistosoma-Induced Pulmonary Hypertension. Front. Immunol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Shen, T.; Lin, R.; Hu, C.; Yu, D.; Ren, C.; Li, T.; Zhu, M.; Wan, Z.; Su, T.; Wu, Y.; et al. Succinate-induced macrophage polarization and RBP4 secretion promote vascular sprouting in ocular neovascularization. J. Neuroinflamm. 2023, 20, 308. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, R. Succinate metabolism: A promising therapeutic target for inflammation, ischemia/reperfusion injury and cancer. Front. Cell Dev. Biol. 2023, 11, 1266973. [Google Scholar] [CrossRef]
- Hooftman, A.; Angiari, S.; Hester, S.; Corcoran, S.E.; Runtsch, M.C.; Ling, C.; Ruzek, M.C.; Slivka, P.F.; McGettrick, A.F.; Banahan, K.; et al. The Immunomodulatory Metabolite Itaconate Modifies NLRP3 and Inhibits Inflammasome Activation. Cell Metab. 2020, 32, 468–478.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, W.; Kong, W.; Zeng, T. Itaconate: A Potent Macrophage Immunomodulator. Inflammation 2023, 46, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- de Goede, K.E.; Harber, K.J.; Gorki, F.S.; Verberk, S.G.; Groh, L.A.; Keuning, E.D.; Struys, E.A.; van Weeghel, M.; Haschemi, A.; de Winther, M.P.; et al. d-2-Hydroxyglutarate is an anti-inflammatory immunometabolite that accumulates in macrophages after TLR4 activation. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166427. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhao, J.; Ding, Q.; Wei, J. Oncometabolite 2-hydroxyglutarate regulates anti-tumor immunity. Heliyon 2024, 10, e24454. [Google Scholar] [CrossRef]
- Notarangelo, G.; Spinelli, J.B.; Perez, E.M.; Baker, G.J.; Kurmi, K.; Elia, I.; Stopka, S.A.; Baquer, G.; Lin, J.-R.; Golby, A.J.; et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8(+) T cell function. Science 2022, 377, 1519–1529. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Goto, N.; Kambara, K.; Fujigaki, S.; Fujigaki, H.; Takemura, M.; Nabeshima, T.; Tomita, A.; Saito, K. Usefulness of the 3-hydroxykynurenine/kynurenic acid ratio as a diagnostic biomarker for diffuse larger B-cell lymphoma. Ann. Clin. Biochem. 2025, 62, 109–117. [Google Scholar] [CrossRef]
- Schlichtner, S.; Yasinska, I.M.; Klenova, E.; Abooali, M.; Lall, G.S.; Berger, S.M.; Ruggiero, S.; Cholewa, D.; Milošević, M.; Gibbs, B.F.; et al. L-Kynurenine participates in cancer immune evasion by downregulating hypoxic signaling in T lymphocytes. Oncoimmunology 2023, 12, 2244330. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Yang, S.-P.; Weaver, D.F. Anti-Inflammatory Anthranilate Analogue Enhances Autophagy through mTOR and Promotes ER-Turnover through TEX264 during Alzheimer-Associated Neuroinflammation. ACS Chem. Neurosci. 2022, 13, 406–422. [Google Scholar] [CrossRef]
- Lin, Z.; Hsieh, P.; Hwang, T.; Chen, C.; Sung, C.T.; Fang, J. Topical application of anthranilate derivatives ameliorates psoriatic inflammation in a mouse model by inhibiting keratinocyte-derived chemokine expression and neutrophil infiltration. FASEB J. 2018, 32, 6783–6795. [Google Scholar] [CrossRef]
- Da’dara, A.A.; Bhardwaj, R.; Ali, Y.B.; Skelly, P.J. Schistosome tegumental ecto-apyrase (SmATPDase1) degrades exogenous pro-inflammatory and pro-thrombotic nucleotides. PeerJ 2014, 2, e316. [Google Scholar] [CrossRef] [PubMed]
- Elzoheiry, M.; Da’Dara, A.A.; Delaforcade, A.M.; El-Beshbishi, S.N.; Skelly, P.J. The Essential Ectoenzyme SmNPP5 from the Human Intravascular Parasite Schistosoma mansoni is an ADPase and a Potent Inhibitor of Platelet Aggregation. Thromb. Haemost. 2018, 118, 979–989. [Google Scholar] [CrossRef]
- Nation, C.S.; Da’dara, A.A.; Skelly, P.J. The essential schistosome tegumental ectoenzyme SmNPP5 can block NAD-induced T cell apoptosis. Virulence 2020, 11, 568–579. [Google Scholar] [CrossRef]
- Nation, C.S.; Da’Dara, A.A.; Skelly, P.J. NAD-catabolizing ectoenzymes of Schistosoma mansoni. Biochem. J. 2022, 479, 1165–1180. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Skelly, P.J. Characterization of schistosome tegumental alkaline phosphatase (SmAP). PLoS Negl. Trop. Dis. 2011, 5, e1011. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Hodge, J.W. Targeting Immunosuppressive Adenosine Signaling: A Review of Potential Immunotherapy Combination Strategies. Int. J. Mol. Sci. 2023, 24, 8871. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef]
- Fukuda, T.; Kuroda, T.; Kono, M.; Hyoguchi, M.; Tajiri, S.; Tanaka, M.; Mine, Y.; Matsui, T. Adenine attenuates the Ca(2+) contraction-signaling pathway via adenine receptor-mediated signaling in rat vascular smooth muscle cells. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 999–1007. [Google Scholar] [CrossRef]
- Harber, K.J.; Nguyen, T.-A.; Schomakers, B.V.; Heister, D.A.F.; de Vries, H.E.; van Weeghel, M.; Bossche, J.V.D.; de Winther, M.P.J. Adenine is an anti-inflammatory metabolite found to be more abundant in M-CSF over GM-CSF-differentiated human macrophages. Immunol. Lett. 2024, 265, 23–30. [Google Scholar] [CrossRef]
- Senft, A.W.; Senft, D.G.; Miech, R.P. Pathways of nucleotide metabolism in Schistosoma mansoni. II. Disposition of adenosine by whole worms. Biochem. Pharmacol. 1973, 22, 437–447. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Hu, Z.; Chen, S.; Wang, Q.; Zeng, Y.; Yin, H.; Zhao, J.; Zhan, Y.; Gao, C.; et al. Microbiota-Derived Inosine Suppresses Systemic Autoimmunity via Restriction of B Cell Differentiation and Migration. Adv. Sci. 2025, e2409837. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Sitkovsky, M.V.; Szabó, C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Komiyama, A. New clinical applications of xanthine derivatives: Modulatory actions on leukocyte survival and function. Int. J. Hematol. 2001, 73, 87–92. [Google Scholar] [CrossRef]
- Baldassarre, M.P.A.; Di Tomo, P.; Centorame, G.; Pandolfi, A.; Di Pietro, N.; Consoli, A.; Formoso, G. Myoinositol Reduces Inflammation and Oxidative Stress in Human Endothelial Cells Exposed In Vivo to Chronic Hyperglycemia. Nutrients 2021, 13, 2210. [Google Scholar] [CrossRef]
- Oganezovi, N.; Lagani, V.; Kikvidze, M.; Gamkrelidze, G.; Tsverava, L.; Lepsveridze, E.; Kelly, K.M.; Solomonia, R. Long-term effects of myo-inositol on traumatic brain injury: Epigenomic and transcriptomic studies. IBRO Neurosci. Rep. 2024, 16, 291–299. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Hua, X.; Su, Z.; Deng, R.; Lin, J.; Li, D.-Q.; Pflugfelder, S.C. Effects of L-carnitine, erythritol and betaine on pro-inflammatory markers in primary human corneal epithelial cells exposed to hyperosmotic stress. Curr. Eye Res. 2015, 40, 657–667. [Google Scholar] [CrossRef]
- Grigorian, A.; Araujo, L.; Naidu, N.N.; Place, D.J.; Choudhury, B.; Demetriou, M. N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem. 2011, 286, 40133–40141. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; De Berardis, B.; Bigioni, I.; Mariano, A.; Superti, F.; D’abusco, A.S. In Vitro Antiviral and Anti-Inflammatory Activities of N-Acetylglucosamine: Development of an Alternative and Safe Approach to Fight Viral Respiratory Infections. Int. J. Mol. Sci. 2023, 24, 5129. [Google Scholar] [CrossRef]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741. [Google Scholar] [CrossRef]
- Guo, X. Antibacterial and anti-inflammatory effects of genistein in Staphylococcus aureus induced osteomyelitis in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23298. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef] [PubMed]
- Freyschmidt, E.J.; Alonso, A.; Hartmann, G.; Gissmann, L. Activation of dendritic cells and induction of T cell responses by HPV 16 L1/E7 chimeric virus-like particles are enhanced by CpG ODN or sorbitol. Antivir. Ther. 2004, 9, 479–489. [Google Scholar] [CrossRef]
- Mastrofrancesco, A.; Ottaviani, M.; Aspite, N.; Cardinali, G.; Izzo, E.; Graupe, K.; Zouboulis, C.C.; Camera, E.; Picardo, M. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARgamma activation. Exp. Dermatol. 2010, 19, 813–820. [Google Scholar] [CrossRef]
- Jones, N.; Blagih, J.; Zani, F.; Rees, A.; Hill, D.G.; Jenkins, B.J.; Bull, C.J.; Moreira, D.; Bantan, A.I.M.; Cronin, J.G.; et al. Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation. Nat. Commun. 2021, 12, 1209. [Google Scholar] [CrossRef]
- Widyarman, A.S.; Drestia, A.M.; Bachtiar, E.W.; Bachtiar, B.M. The Anti-inflammatory Effects of Glycerol-supplemented Probiotic Lactobacillus reuteri on Infected Epithelial cells In vitro. Contemp. Clin. Dent. 2018, 9, 298–303. [Google Scholar] [CrossRef]
- Szél, E.; Polyánka, H.; Szabó, K.; Hartmann, P.; Degovics, D.; Balázs, B.; Németh, I.; Korponyai, C.; Csányi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef]
- Kokova, D.; Mayboroda, O.A. Twenty Years on: Metabolomics in Helminth Research. Trends Parasitol. 2019, 35, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, A.S.; Mundhe, S.D.; Awobode, H.O.; Onile, O.S.; Agunloye, A.M.; Isokpehi, R.D.; Shouche, Y.S.; Santhakumari, B.; Anumudu, C.I. Metabolite profiling for biomarkers in Schistosoma haematobium infection and associated bladder pathologies. PLoS Negl. Trop. Dis. 2018, 12, e0006452. [Google Scholar] [CrossRef]
- Reddy, A.; Winther, S.; Tran, N.; Xiao, H.; Jakob, J.; Garrity, R.; Smith, A.; Ordonez, M.; Laznik-Bogoslavski, D.; Rothstein, J.D.; et al. Monocarboxylate transporters facilitate succinate uptake into brown adipocytes. Nat. Metab. 2024, 6, 567–577. [Google Scholar] [CrossRef]
- Engstrom, M.; Schott, U.; Nordstrom, C.-H.; Romner, B.; Reinstrup, P. Increased lactate levels impair the coagulation system—A potential contributing factor to progressive hemorrhage after traumatic brain injury. J. Neurosurg. Anesth. 2006, 18, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, K.; Sugita, C.; Yamashita, A.; Moriguchi-Goto, S.; Furukoji, E.; Sakae, T.; Gi, T.; Hirai, T.; Asada, Y. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: Potential markers for early deep vein thrombosis. Thromb. Res. 2019, 177, 136–144. [Google Scholar] [CrossRef] [PubMed]

| Metabokine | Mean Fold Change at 20 min (+Parasites) | Mean Fold Change at 60 min (+Parasites) |
|---|---|---|
| Adenine | 9.4 | 14.1 |
| N-Acetylglucosamine | 4.4 | 9.2 |
| Hypoxanthine | 4.1 | 4.3 |
| Myo-inositol | 3.3 | 4.3 |
| Anthranilate | 1.9 | 3.7 |
| Betaine | 2.6 | 3.0 |
| 2-Hydroxyglutarate | 1.5 | 2.6 |
| Xanthine | 2.3 | 2.2 |
| Succinate | 1.5 | 2.1 |
| Lactate | 1.6 | 2.1 |
| Gentisate | 1.6 | 1.9 |
| Aconitate | 1.3 | 1.8 |
| Sorbitol | 1.1 | 1.5 |
| Genistein | 0.7 | 0.6 |
| Glucose | 0.8 | 0.5 |
| Inosine | 0.2 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skelly, P.J.; Da’dara, A.A. Generation of Immune Modulating Small Metabolites—Metabokines—By Adult Schistosomes. Pathogens 2025, 14, 526. https://doi.org/10.3390/pathogens14060526
Skelly PJ, Da’dara AA. Generation of Immune Modulating Small Metabolites—Metabokines—By Adult Schistosomes. Pathogens. 2025; 14(6):526. https://doi.org/10.3390/pathogens14060526
Chicago/Turabian StyleSkelly, Patrick J., and Akram A. Da’dara. 2025. "Generation of Immune Modulating Small Metabolites—Metabokines—By Adult Schistosomes" Pathogens 14, no. 6: 526. https://doi.org/10.3390/pathogens14060526
APA StyleSkelly, P. J., & Da’dara, A. A. (2025). Generation of Immune Modulating Small Metabolites—Metabokines—By Adult Schistosomes. Pathogens, 14(6), 526. https://doi.org/10.3390/pathogens14060526






