A High-Throughput Inhibitor Screen Targeting CLAG3 Export and Membrane Insertion on Human Erythrocytes Infected with Malaria Parasites
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Cultivation of Plasmodium falciparum Strains
2.2. Miniaturized Luminescence Measurements Using Split NanoLuc
2.3. Growth Inhibition Studies
2.4. Immunofluorescence Assays
3. Results
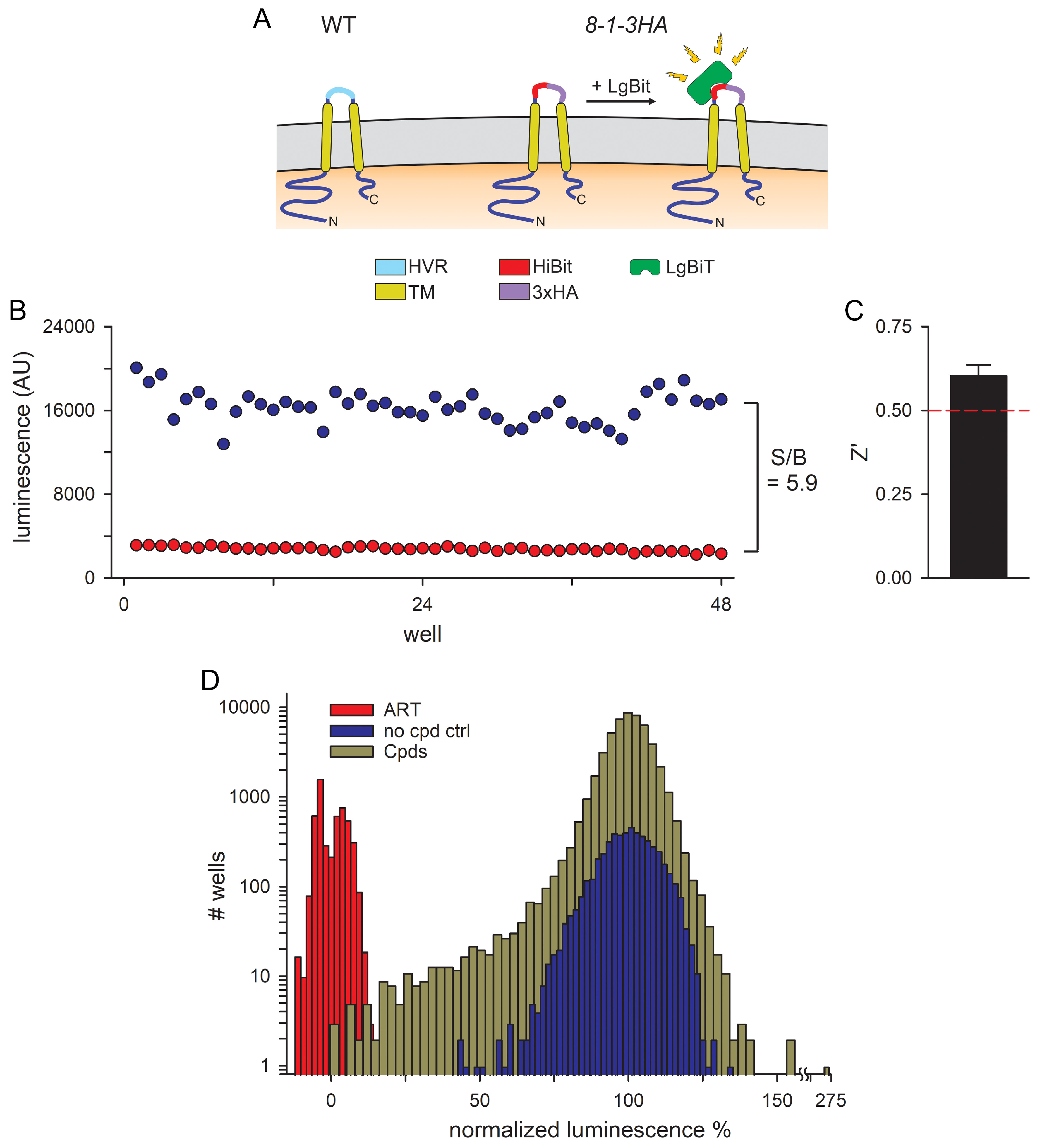
3.1. An Optimized 384-Well Microplate Assay for CLAG3 Exposure on Infected Cells and a High-Throughput Screen for Inhibitors
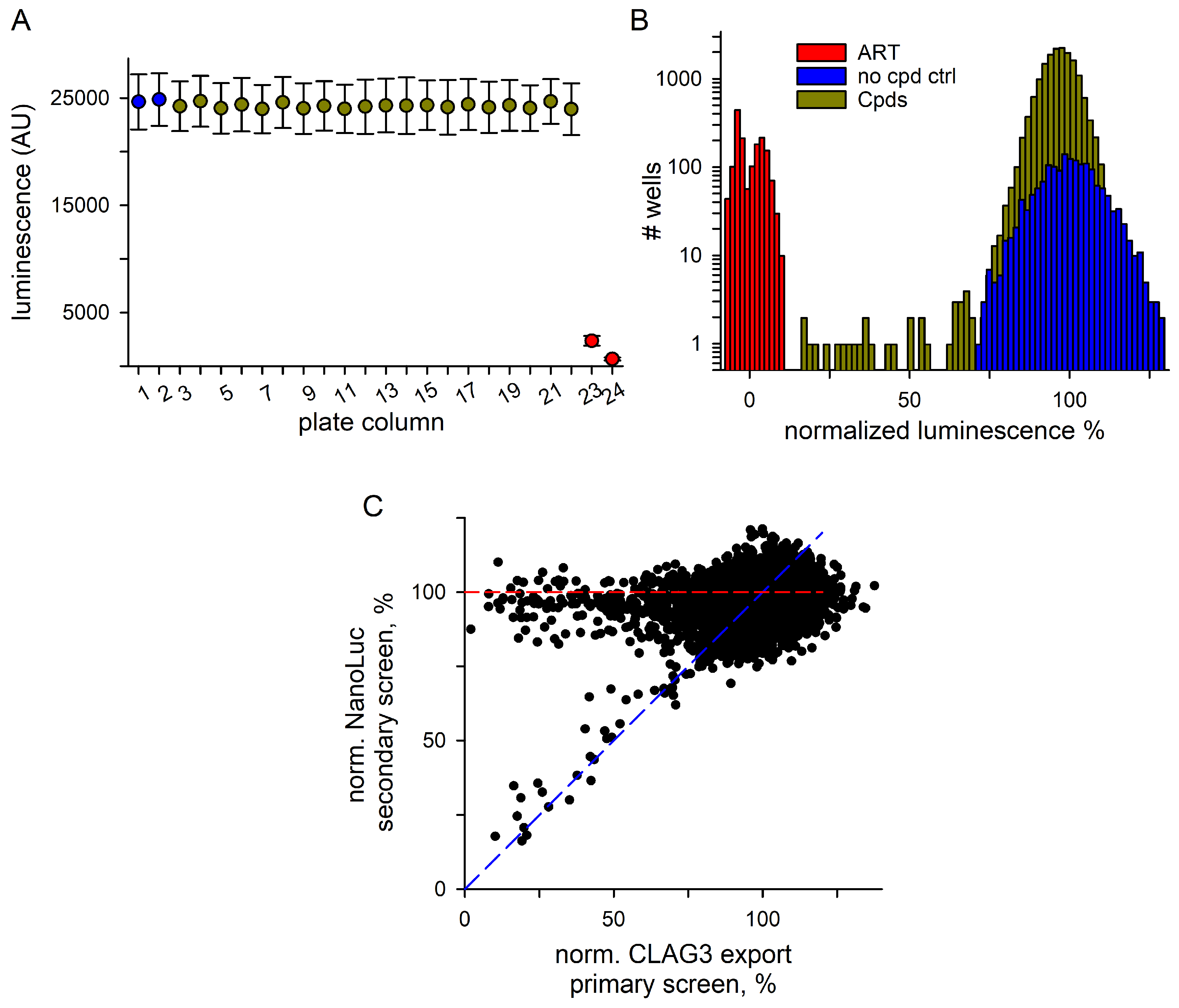
3.2. Hits Filtered for False Positives That Inhibit NanoLuc Signal Development
3.3. Secondary Studies
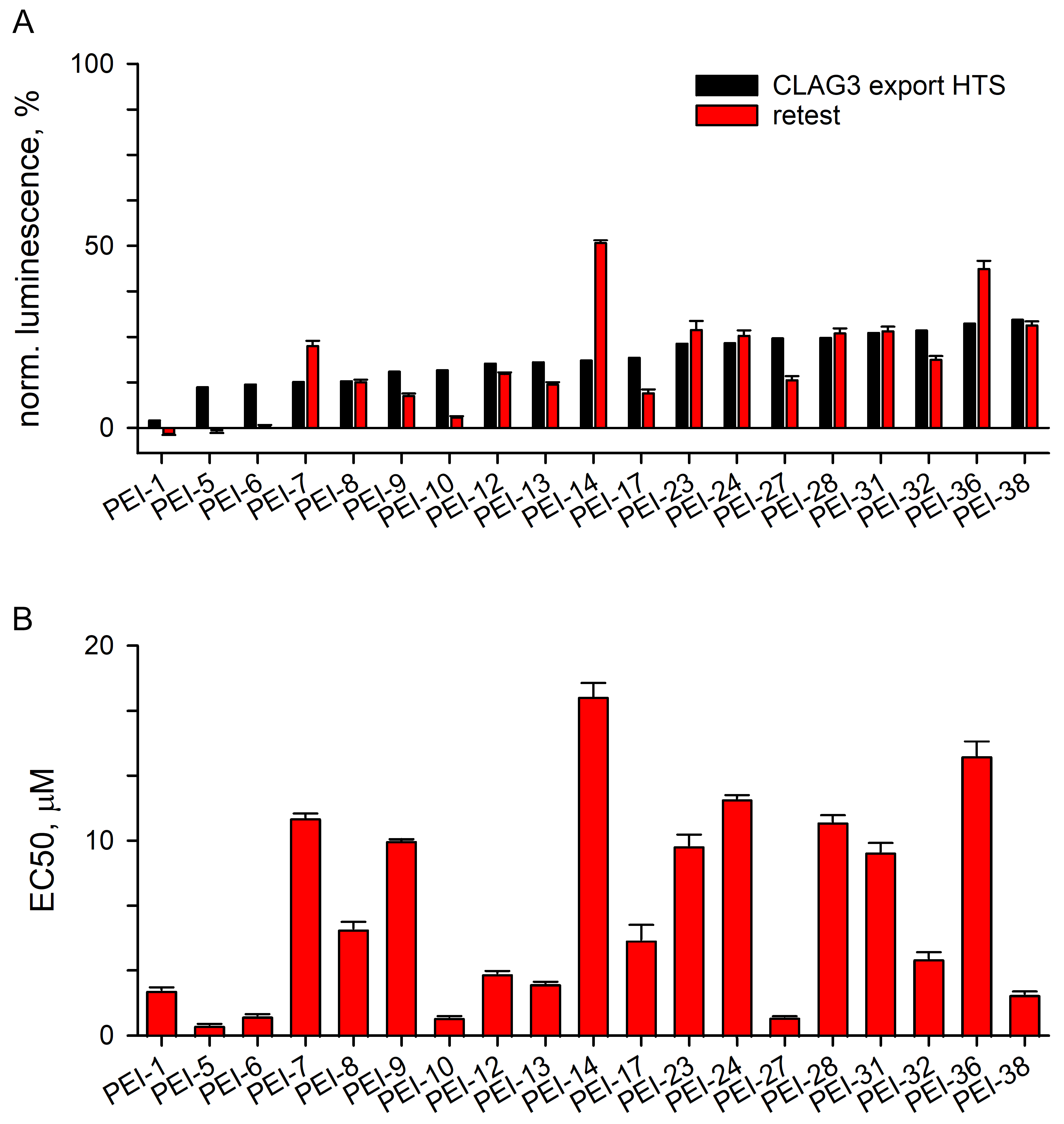
3.3.1. Excellent Retest Rate for Hits Obtained in Resupply
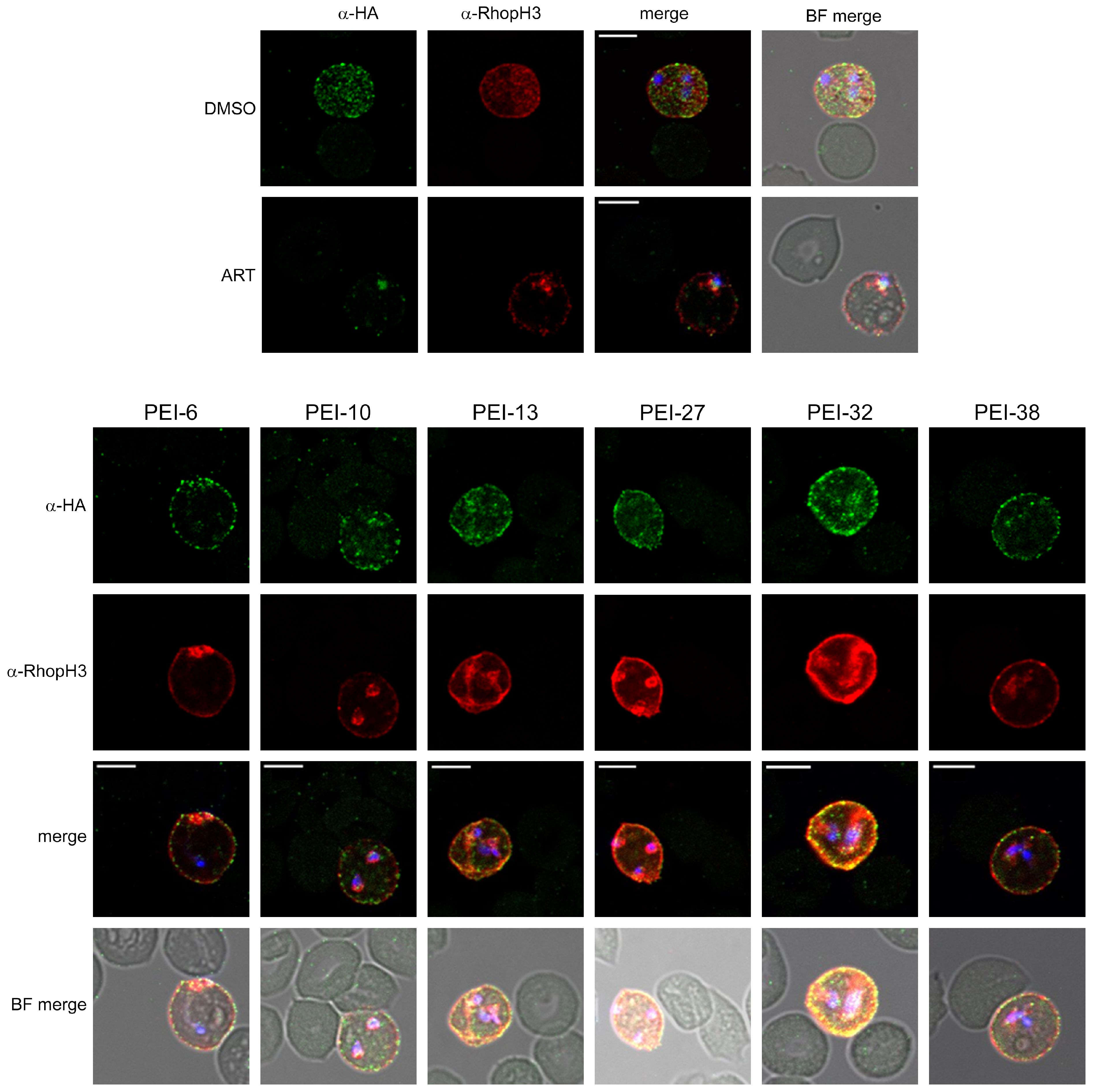
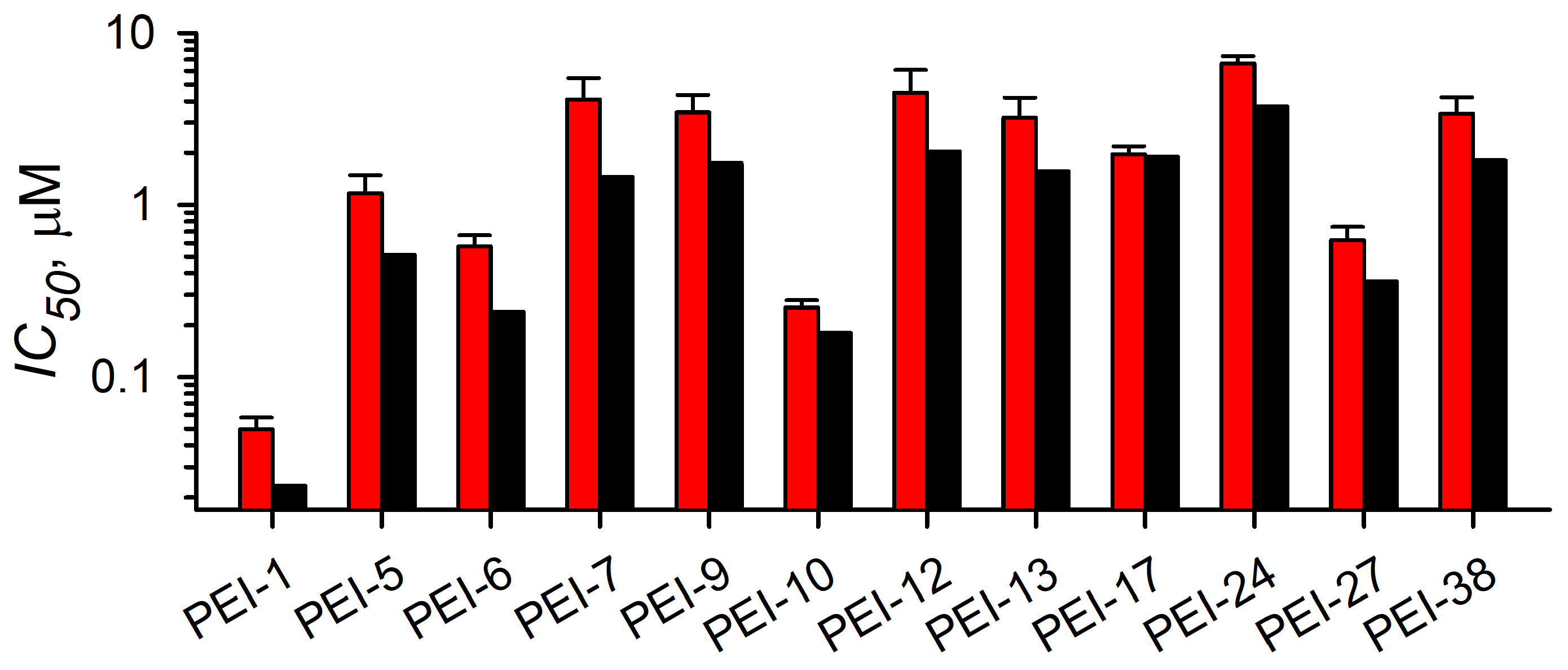
3.3.2. Localization and Growth Inhibition Studies Suggest Off-Target Effects
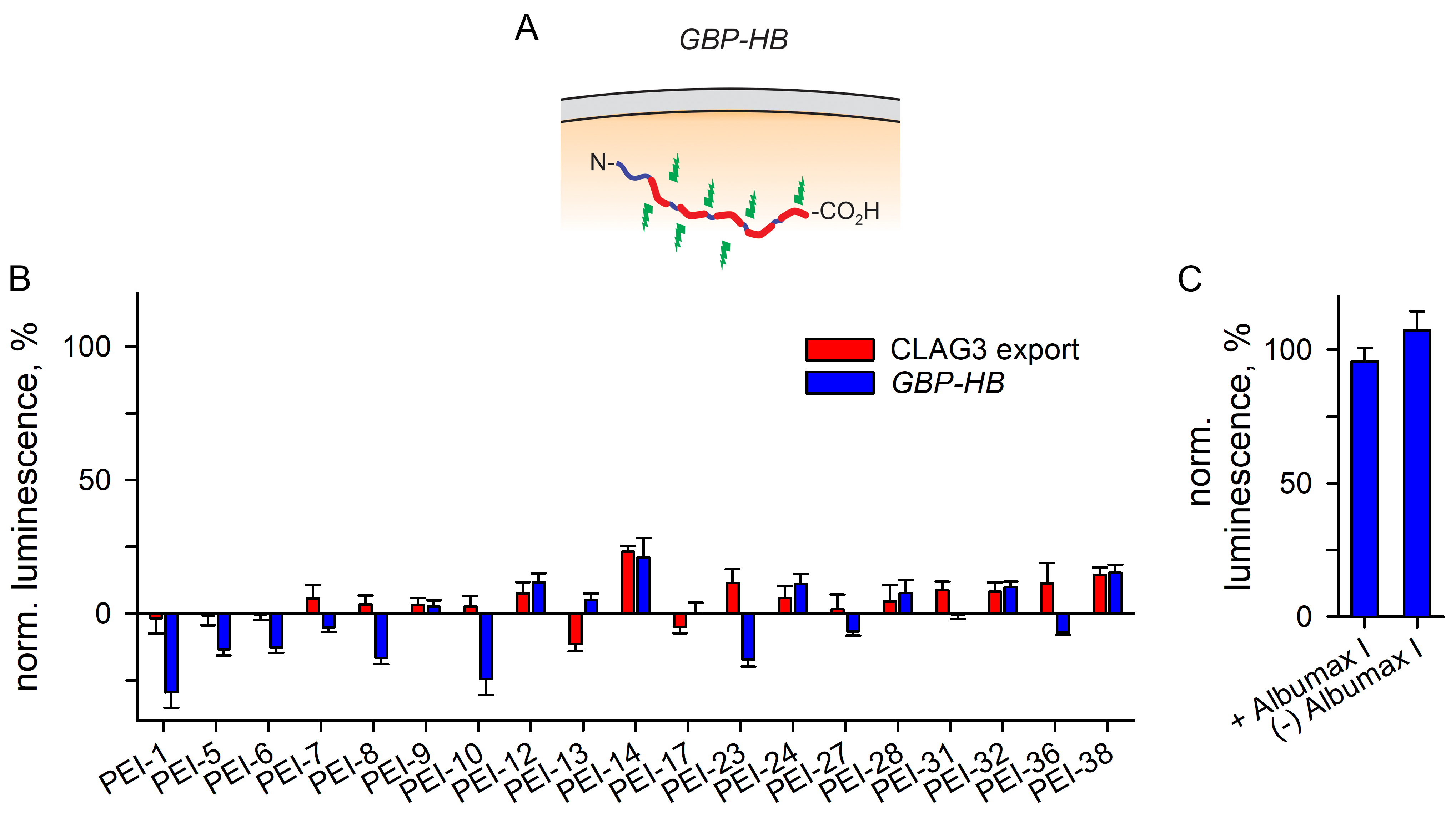
3.3.3. A Separate Reporter Line for Excluding Nonspecific Toxicity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | artemisinin |
| EC50 | 50% effective concentrations for action against target activity |
| GIA | growth inhibition assay |
| HVR | hypervariable domain |
| IC50 | 50% inhibitory concentration in growth inhibition assay |
| IFA | indirect immunofluorescence assay |
| PSAC | plasmodial surface anion channel |
| RISE | Reporter of Insertion and Surface Exposure |
References
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, R.M.; Chilito, K.C.F.; Tokponnon, F.; Messenger, L.A. Malaria vector control in sub-Saharan Africa: Complex trade-offs to combat the growing threat of insecticide resistance. Lancet Planet. Health 2024, 8, e804–e812. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, V.; Hamel, M.J.; Smith, P.G. Malaria vaccines for children: And now there are two. Lancet 2024, 403, 504–505. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, T.K.; Gabriela, M.; Crabb, B.S.; de Koning-Ward, T.F.; Gilson, P.R. Defining the essential exportome of the malaria parasite. Trends Parasitol. 2021, 37, 664–675. [Google Scholar] [CrossRef]
- Hviid, L.; Jensen, A.T. PfEMP1—A parasite protein family of key importance in Plasmodium falciparum malaria immunity and pathogenesis. Adv. Parasitol. 2015, 88, 51–84. [Google Scholar] [CrossRef]
- Tan, J.; Pieper, K.; Piccoli, L.; Abdi, A.; Perez, M.F.; Geiger, R.; Tully, C.M.; Jarrossay, D.; Maina Ndungu, F.; Wambua, J.; et al. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature 2016, 529, 105–109. [Google Scholar] [CrossRef]
- Saito, F.; Hirayasu, K.; Satoh, T.; Wang, C.W.; Lusingu, J.; Arimori, T.; Shida, K.; Palacpac, N.M.Q.; Itagaki, S.; Iwanaga, S.; et al. Immune evasion of Plasmodium falciparum by RIFIN via inhibitory receptors. Nature 2017, 552, 101–105. [Google Scholar] [CrossRef]
- Hadjimichael, E.; Deitsch, K.W. Variable surface antigen expression, virulence, and persistent infection by Plasmodium falciparum malaria parasites. Microbiol. Mol. Biol. Rev. 2025, 89, e0011423. [Google Scholar] [CrossRef]
- Kaneko, O.; Yim Lim, B.Y.; Iriko, H.; Ling, I.T.; Otsuki, H.; Grainger, M.; Tsuboi, T.; Adams, J.H.; Mattei, D.; Holder, A.A.; et al. Apical expression of three RhopH1/Clag proteins as components of the Plasmodium falciparum RhopH complex. Mol. Biochem. Parasitol. 2005, 143, 20–28. [Google Scholar] [CrossRef]
- Gupta, A.; Thiruvengadam, G.; Desai, S.A. The conserved clag multigene family of malaria parasites: Essential roles in host-pathogen interaction. Drug Resist. Updat. 2015, 18, 47–54. [Google Scholar] [CrossRef]
- Otto, T.D.; Bohme, U.; Sanders, M.; Reid, A.; Bruske, E.I.; Duffy, C.W.; Bull, P.C.; Pearson, R.D.; Abdi, A.; Dimonte, S.; et al. Long read assemblies of geographically dispersed Plasmodium falciparum isolates reveal highly structured subtelomeres. Wellcome Open Res. 2018, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.A.; Bezrukov, S.M.; Zimmerberg, J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 2000, 406, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, A.; Cohn, J.V.; Wagner, M.A.; Cabrera, J.S.; Rajapandi, T.; Desai, S.A. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood 2004, 104, 4279–4286. [Google Scholar] [CrossRef]
- Nguitragool, W.; Bokhari, A.A.; Pillai, A.D.; Rayavara, K.; Sharma, P.; Turpin, B.; Aravind, L.; Desai, S.A. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell 2011, 145, 665–677. [Google Scholar] [CrossRef]
- Kaneko, O. Erythrocyte invasion: Vocabulary and grammar of the Plasmodium rhoptry. Parasitol. Int. 2007, 56, 255–262. [Google Scholar] [CrossRef]
- Trenholme, K.R.; Gardiner, D.L.; Holt, D.C.; Thomas, E.A.; Cowman, A.F.; Kemp, D.J. clag9: A cytoadherence gene in Plasmodium falciparum essential for binding of parasitized erythrocytes to CD36. Proc. Natl. Acad. Sci. USA 2000, 97, 4029–4033. [Google Scholar] [CrossRef]
- Mira-Martinez, S.; Pickford, A.K.; Rovira-Graells, N.; Guetens, P.; Tinto-Font, E.; Cortes, A.; Rosanas-Urgell, A. Identification of antimalarial compounds that require CLAG3 for their uptake by Plasmodium falciparum-infected erythrocytes. Antimicrob. Agents Chemother. 2019, 63, e00052-19. [Google Scholar] [CrossRef]
- Gupta, A.; Balabaskaran-Nina, P.; Nguitragool, W.; Saggu, G.S.; Schureck, M.A.; Desai, S.A. CLAG3 self-associates in malaria parasites and quantitatively determines nutrient uptake channels at the host membrane. mBio 2018, 9, e02293-17. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Rayavara, K.; Ito, D.; Basore, K.; Desai, S.A. A CLAG3 mutation in an amphipathic transmembrane domain alters malaria parasite nutrient channels and confers leupeptin resistance. Infect. Immun. 2015, 83, 2566–2574. [Google Scholar] [CrossRef]
- Ito, D.; Schureck, M.A.; Desai, S.A. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. eLife 2017, 6, e23485. [Google Scholar] [CrossRef]
- Gupta, A.; Bokhari, A.A.B.; Pillai, A.D.; Crater, A.K.; Gezelle, J.; Saggu, G.; Nasamu, A.S.; Ganesan, S.M.; Niles, J.C.; Desai, S.A. Complex nutrient channel phenotypes despite Mendelian inheritance in a Plasmodium falciparum genetic cross. PLoS Pathog. 2020, 16, e1008363. [Google Scholar] [CrossRef] [PubMed]
- Schureck, M.A.; Darling, J.E.; Merk, A.; Shao, J.; Daggupati, G.; Srinivasan, P.; Olinares, P.D.B.; Rout, M.P.; Chait, B.T.; Wollenberg, K.; et al. Malaria parasites use a soluble RhopH complex for erythrocyte invasion and an integral form for nutrient uptake. eLife 2021, 10, e65282. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.; Chisholm, S.A.; Bullen, H.E.; Srivastava, A.; Sanders, P.R.; Jonsdottir, T.K.; Weiss, G.E.; Ghosh, S.; Crabb, B.S.; Creek, D.J.; et al. Plasmodium falciparum parasites deploy RhopH2 into the host erythrocyte to obtain nutrients, grow and replicate. eLife 2017, 6, e23217. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Jih, J.; Lai, M.; Li, X.; Goldberg, D.E.; Beck, J.R.; Zhou, Z.H. Native structure of the RhopH complex, a key determinant of malaria parasite nutrient acquisition. Proc. Natl. Acad. Sci. USA 2021, 118, e2100514118. [Google Scholar] [CrossRef] [PubMed]
- Lisk, G.; Desai, S.A. The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot. Cell 2005, 4, 2153–2159. [Google Scholar] [CrossRef]
- Ling, I.T.; Kaneko, O.; Narum, D.L.; Tsuboi, T.; Howell, S.; Taylor, H.M.; Scott-Finnigan, T.J.; Torii, M.; Holder, A.A. Characterisation of the rhoph2 gene of Plasmodium falciparum and Plasmodium yoelii. Mol. Biochem. Parasitol. 2003, 127, 47–57. [Google Scholar] [CrossRef]
- Iriko, H.; Kaneko, O.; Otsuki, H.; Tsuboi, T.; Su, X.Z.; Tanabe, K.; Torii, M. Diversity and evolution of the rhoph1/clag multigene family of Plasmodium falciparum. Mol. Biochem. Parasitol. 2008, 158, 11–21. [Google Scholar] [CrossRef]
- Cortes, A.; Carret, C.; Kaneko, O.; Yim Lim, B.Y.; Ivens, A.; Holder, A.A. Epigenetic silencing of Plasmodium falciparum genes linked to erythrocyte invasion. PLoS. Pathog. 2007, 3, e107. [Google Scholar] [CrossRef]
- Vincensini, L.; Fall, G.; Berry, L.; Blisnick, T.; Braun, B.C. The RhopH complex is transferred to the host cell cytoplasm following red blood cell invasion by Plasmodium falciparum. Mol. Biochem. Parasitol. 2008, 160, 81–89. [Google Scholar] [CrossRef]
- Beck, J.R.; Muralidharan, V.; Oksman, A.; Goldberg, D.E. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 2014, 511, 592–595. [Google Scholar] [CrossRef]
- Gehde, N.; Hinrichs, C.; Montilla, I.; Charpian, S.; Lingelbach, K.; Przyborski, J.M. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol. Microbiol. 2009, 71, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.M.; Kalanon, M.; de Koning-Ward, T.F. Uncoupling the threading and unfoldase actions of Plasmodium HSP101 reveals differences in export between soluble and insoluble proteins. mBio 2019, 10, e01106-19. [Google Scholar] [CrossRef] [PubMed]
- Mundwiler-Pachlatko, E.; Beck, H.P. Maurer’s clefts, the enigma of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 19987–19994. [Google Scholar] [CrossRef]
- Shao, J.; Arora, G.; Manzella-Lapeira, J.; Brzostowski, J.A.; Desai, S.A. Kinetic tracking of Plasmodium falciparum antigens on infected erythrocytes with a novel reporter of protein insertion and surface exposure. mBio 2022, 13, e0040422. [Google Scholar] [CrossRef]
- Pillai, A.D.; Nguitragool, W.; Lyko, B.; Dolinta, K.; Butler, M.M.; Nguyen, S.T.; Peet, N.P.; Bowlin, T.L.; Desai, S.A. Solute restriction reveals an essential role for clag3-associated channels in malaria parasite nutrient acquisition. Mol. Pharmacol. 2012, 82, 1104–1114. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Shun, T.Y.; Lazo, J.S.; Sharlow, E.R.; Johnston, P.A. Identifying actives from HTS data sets: Practical approaches for the selection of an appropriate HTS data-processing method and quality control review. J. Biomol. Screen. 2011, 16, 1–14. [Google Scholar] [CrossRef]
- Nemergut, M.; Pluskal, D.; Horackova, J.; Sustrova, T.; Tulis, J.; Barta, T.; Baatallah, R.; Gagnot, G.; Novakova, V.; Majerova, M.; et al. Illuminating the mechanism and allosteric behavior of NanoLuc luciferase. Nat. Commun. 2023, 14, 7864. [Google Scholar] [CrossRef] [PubMed]
- Willett, P. Similarity-based virtual screening using 2D fingerprints. Drug Discov. Today 2006, 11, 1046–1053. [Google Scholar] [CrossRef]
- Butler, M.M.; Waidyarachchi, S.L.; Shao, J.; Nguyen, S.T.; Ding, X.; Cardinale, S.C.; Morin, L.R.; Kwasny, S.M.; Ito, M.; Gezelle, J.; et al. Optimized pyridazinone nutrient channel inhibitors are potent and specific antimalarial leads. Mol. Pharmacol. 2022, 102, 172–182. [Google Scholar] [CrossRef]
- Kutner, S.; Breuer, W.V.; Ginsburg, H.; Aley, S.B.; Cabantchik, Z.I. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: Association with parasite development. J. Cell. Physiol. 1985, 125, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Chavez, Z.; Desai, S.A. Plasmodium falciparum CLAG paralogs all traffic to the host membrane but knockouts have distinct phenotypes. Microorganisms 2024, 12, 1172. [Google Scholar] [CrossRef] [PubMed]
- Lanne, A.; Usselmann, L.E.J.; Llowarch, P.; Michaelides, I.N.; Fillmore, M.; Holdgate, G.A. A perspective on the changing landscape of HTS. Drug Discov. Today 2023, 28, 103670. [Google Scholar] [CrossRef] [PubMed]
- Nada, H.; Choi, Y.; Kim, S.; Jeong, K.S.; Meanwell, N.A.; Lee, K. New insights into protein-protein interaction modulators in drug discovery and therapeutic advance. Signal Transduct. Target. Ther. 2024, 9, 341. [Google Scholar] [CrossRef]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-Moran, O.; Galietta, L.J.; Verkman, A.S. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef]
- Dave, K.; Dobra, R.; Scott, S.; Saunders, C.; Matthews, J.; Simmonds, N.J.; Davies, J.C. Entering the era of highly effective modulator therapies. Pediatr. Pulmonol. 2021, 56 (Suppl. S1), S79–S89. [Google Scholar] [CrossRef]
- Pauwels, E.; Schulein, R.; Vermeire, K. Inhibitors of the Sec61 complex and novel high throughput screening strategies to target the protein translocation pathway. Int. J. Mol. Sci. 2021, 22, 12007. [Google Scholar] [CrossRef]
- Saffari, A.; Brechmann, B.; Boger, C.; Saber, W.A.; Jumo, H.; Whye, D.; Wood, D.; Wahlster, L.; Alecu, J.E.; Ziegler, M.; et al. High-content screening identifies a small molecule that restores AP-4-dependent protein trafficking in neuronal models of AP-4-associated hereditary spastic paraplegia. Nat. Commun. 2024, 15, 584. [Google Scholar] [CrossRef]
- Gechijian, L.N.; Muncipinto, G.; Rettenmaier, T.J.; Labenski, M.T.; Rusu, V.; Rosskamp, L.; Conway, L.; van Kalken, D.; Gross, L.; Iantosca, G.; et al. Novel corrector for variants of SLC6A8: A therapeutic opportunity for creatine transporter deficiency. ACS Chem. Biol. 2024, 19, 2372–2382. [Google Scholar] [CrossRef]
- de Koning-Ward, T.F.; Gilson, P.R.; Boddey, J.A.; Rug, M.; Smith, B.J.; Papenfuss, A.T.; Sanders, P.R.; Lundie, R.J.; Maier, A.G.; Cowman, A.F.; et al. A newly discovered protein export machine in malaria parasites. Nature 2009, 459, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Cowman, A.F. Moving in and renovating: Exporting proteins from Plasmodium into host erythrocytes. Nat. Rev. Microbiol. 2010, 8, 617–621. [Google Scholar] [CrossRef]
- Ginsburg, H.; Kutner, S.; Krugliak, M.; Cabantchik, Z.I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 1985, 14, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Dharia, N.V.; Bopp, S.E.; Gluzman, I.; Winzeler, E.A.; Goldberg, D.E. Validation of isoleucine utilization targets in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2011, 108, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.A.; Solomon, T.; Desai, S.A. Two distinct mechanisms of transport through the plasmodial surface anion channel. J. Membr. Biol. 2008, 226, 27–34. [Google Scholar] [CrossRef]
- Roberts, B.F.; Zheng, Y.; Cleaveleand, J.; Lee, S.; Lee, E.; Ayong, L.; Yuan, Y.; Chakrabarti, D. 4-Nitro styrylquinoline is an antimalarial inhibiting multiple stages of Plasmodium falciparum asexual life cycle. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 120–129. [Google Scholar] [CrossRef]
| ID | Structure | CLAG3 Export HTS, % | NanoLuc HTS, % |
|---|---|---|---|
| PEI-1, UCF-501 | 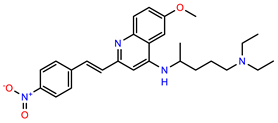 | 2.0 | 87.4 |
| PEI-5 |  | 11.2 | 110.1 |
| PEI-6 | 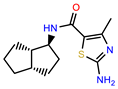 | 11.9 | 94.3 |
| PEI-7 | 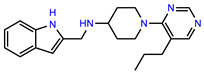 | 12.6 | 97.3 |
| PEI-8 | 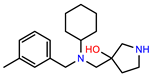 | 12.8 | 98.0 |
| PEI-9 |  | 15.4 | 101.3 |
| PEI-10 | 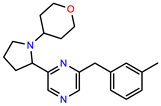 | 15.8 | 97.4 |
| PEI-12 | 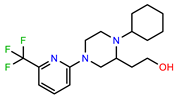 | 17.6 | 103.8 |
| PEI-13 | 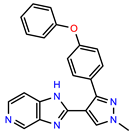 | 18.0 | 84.4 |
| PEI-14 |  | 18.5 | 93.9 |
| PEI-17 | 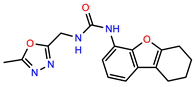 | 19.2 | 96.4 |
| PEI-23 | 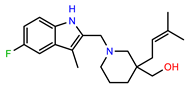 | 23.1 | 98.4 |
| PEI-24 |  | 23.3 | 96.1 |
| PEI-27 | 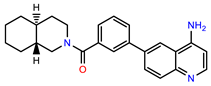 | 24.6 | 96.9 |
| PEI-28 |  | 24.8 | 103.9 |
| PEI-31 | 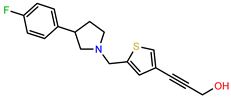 | 26.1 | 106.6 |
| PEI-32 |  | 26.8 | 88.1 |
| PEI-36 | 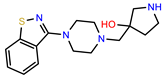 | 28.7 | 97.0 |
| PEI-38 | 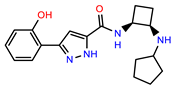 | 29.7 | 94.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Chu, J.; Mohammad, K.; Desai, S.A. A High-Throughput Inhibitor Screen Targeting CLAG3 Export and Membrane Insertion on Human Erythrocytes Infected with Malaria Parasites. Pathogens 2025, 14, 520. https://doi.org/10.3390/pathogens14060520
Shao J, Chu J, Mohammad K, Desai SA. A High-Throughput Inhibitor Screen Targeting CLAG3 Export and Membrane Insertion on Human Erythrocytes Infected with Malaria Parasites. Pathogens. 2025; 14(6):520. https://doi.org/10.3390/pathogens14060520
Chicago/Turabian StyleShao, Jinfeng, Jonathan Chu, Kashif Mohammad, and Sanjay A. Desai. 2025. "A High-Throughput Inhibitor Screen Targeting CLAG3 Export and Membrane Insertion on Human Erythrocytes Infected with Malaria Parasites" Pathogens 14, no. 6: 520. https://doi.org/10.3390/pathogens14060520
APA StyleShao, J., Chu, J., Mohammad, K., & Desai, S. A. (2025). A High-Throughput Inhibitor Screen Targeting CLAG3 Export and Membrane Insertion on Human Erythrocytes Infected with Malaria Parasites. Pathogens, 14(6), 520. https://doi.org/10.3390/pathogens14060520







