Bacteria and Carcinogenesis and the Management of Cancer: A Narrative Review
Abstract
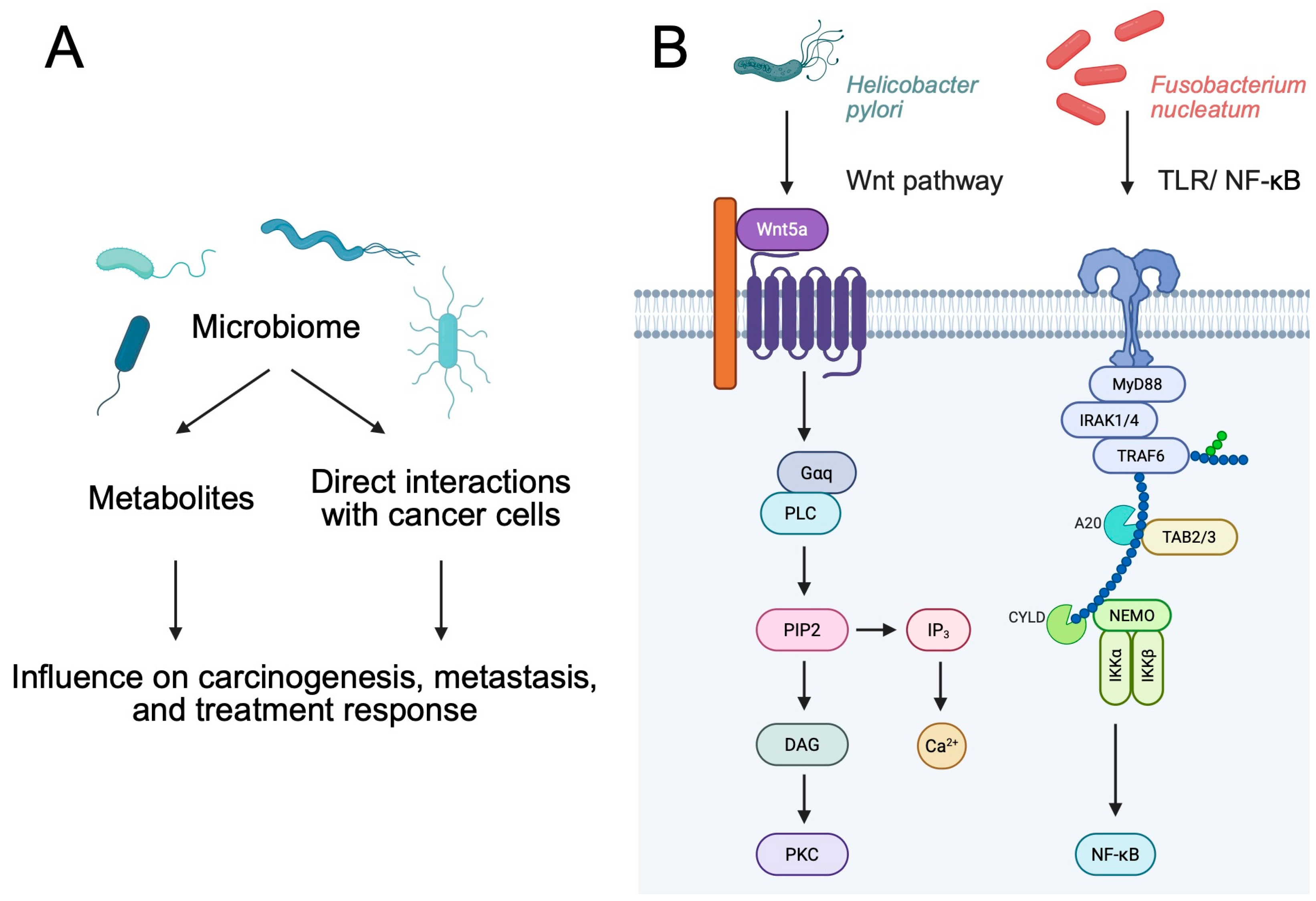
:1. Introduction
2. Immunoregulatory Properties of Bacteria
2.1. Immunoregulatory Properties of Bacterial Metabolites
2.2. Direct Role of Bacteria
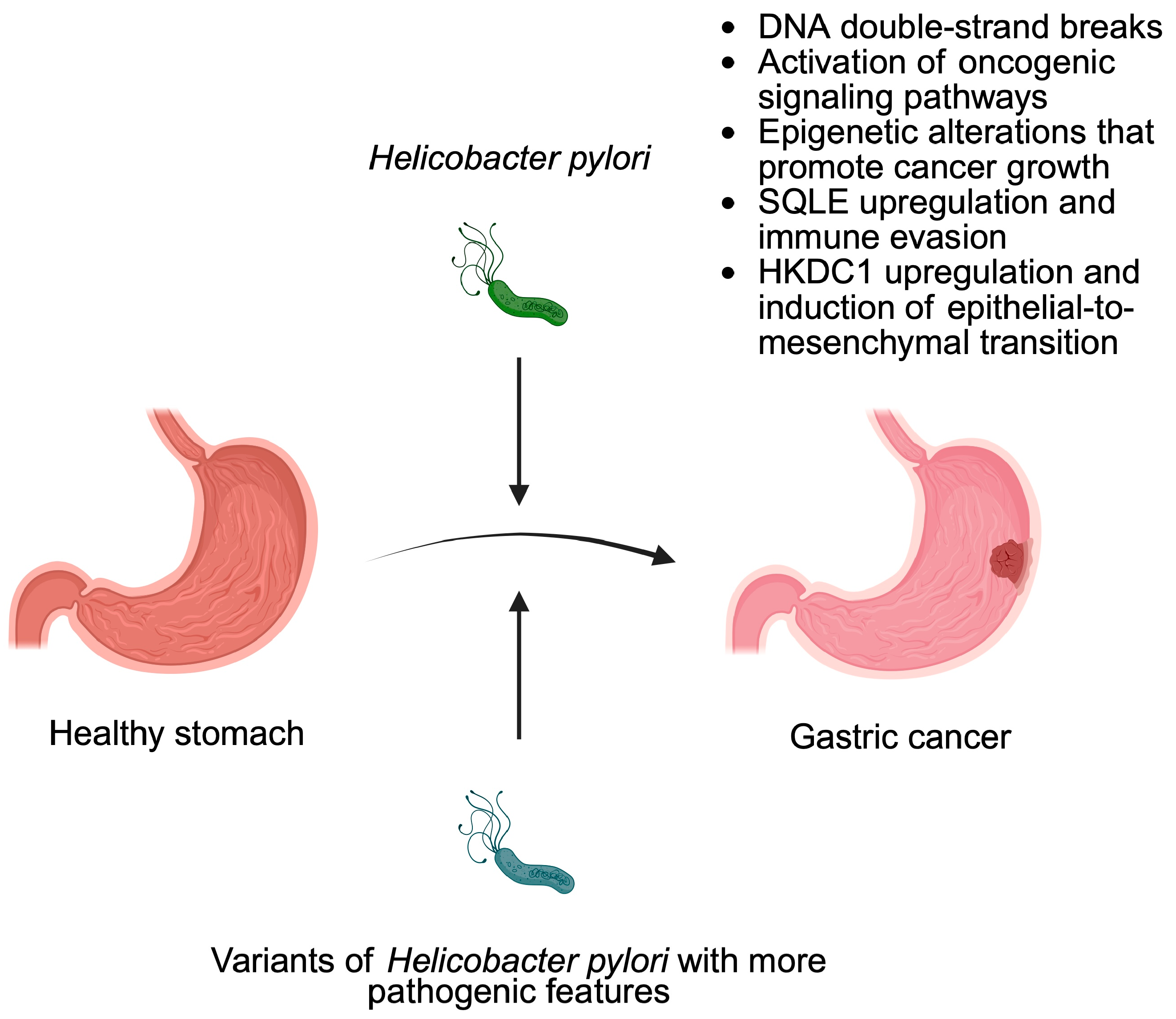
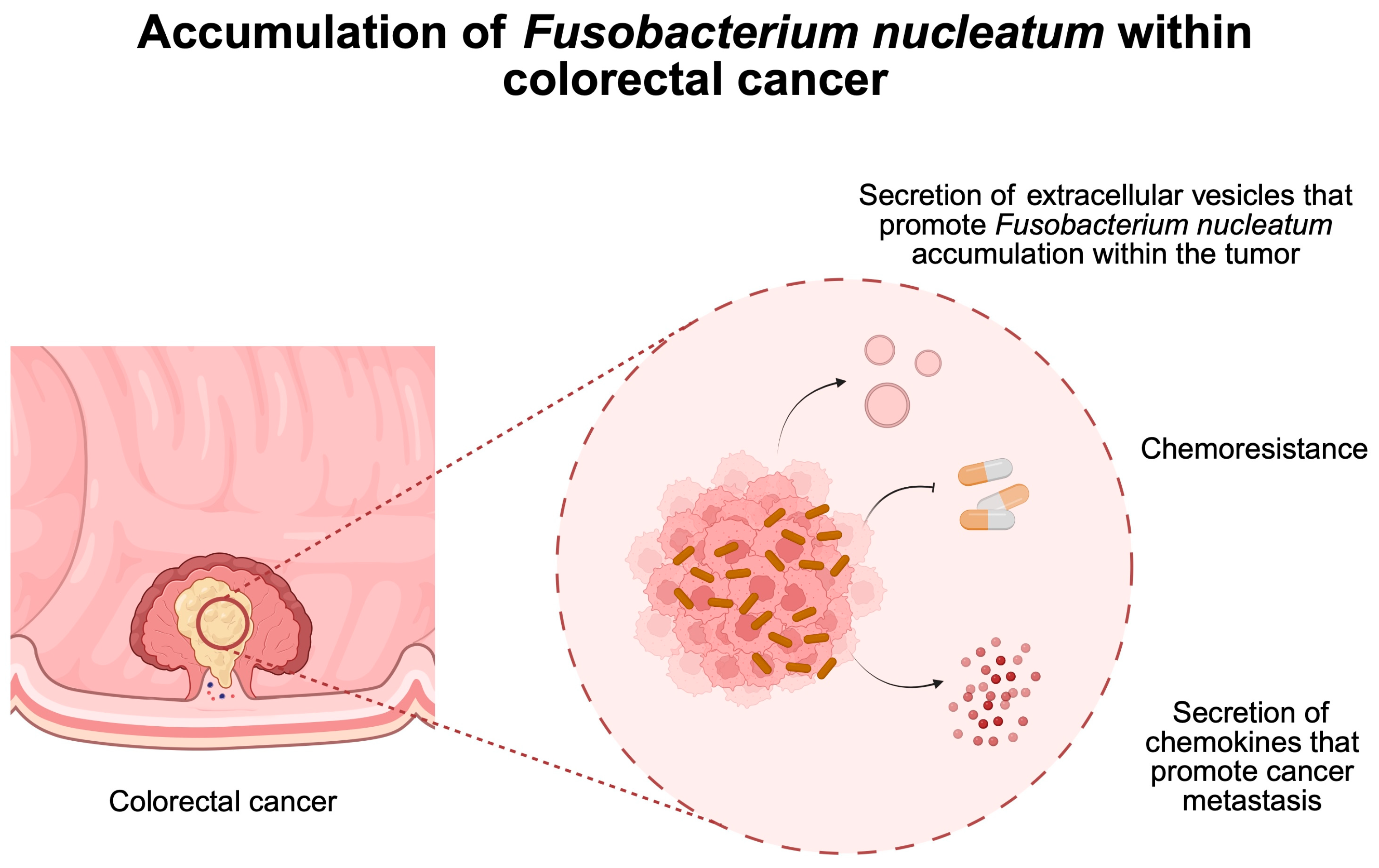
3. Bacteria as Cancer Promoters
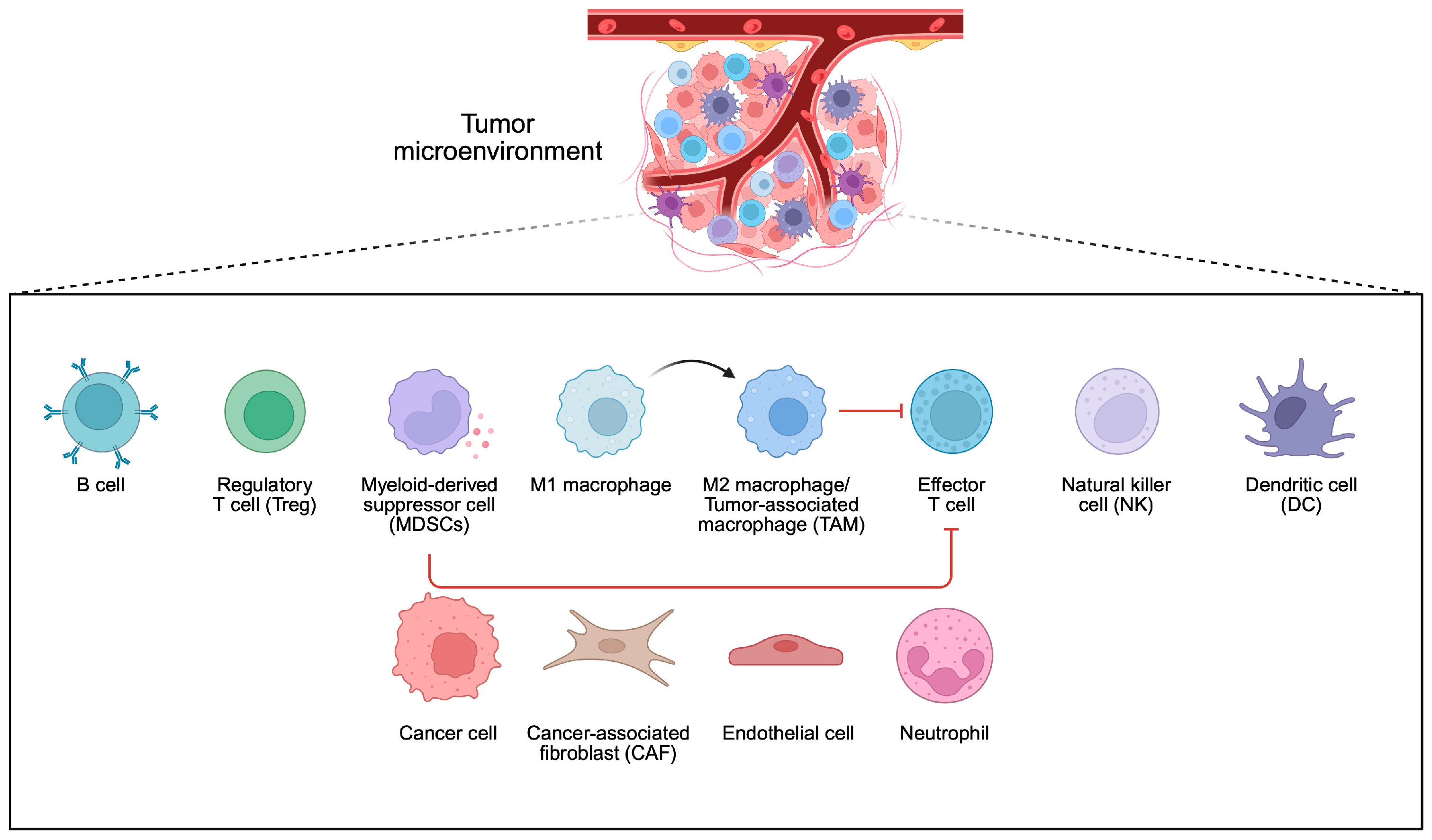
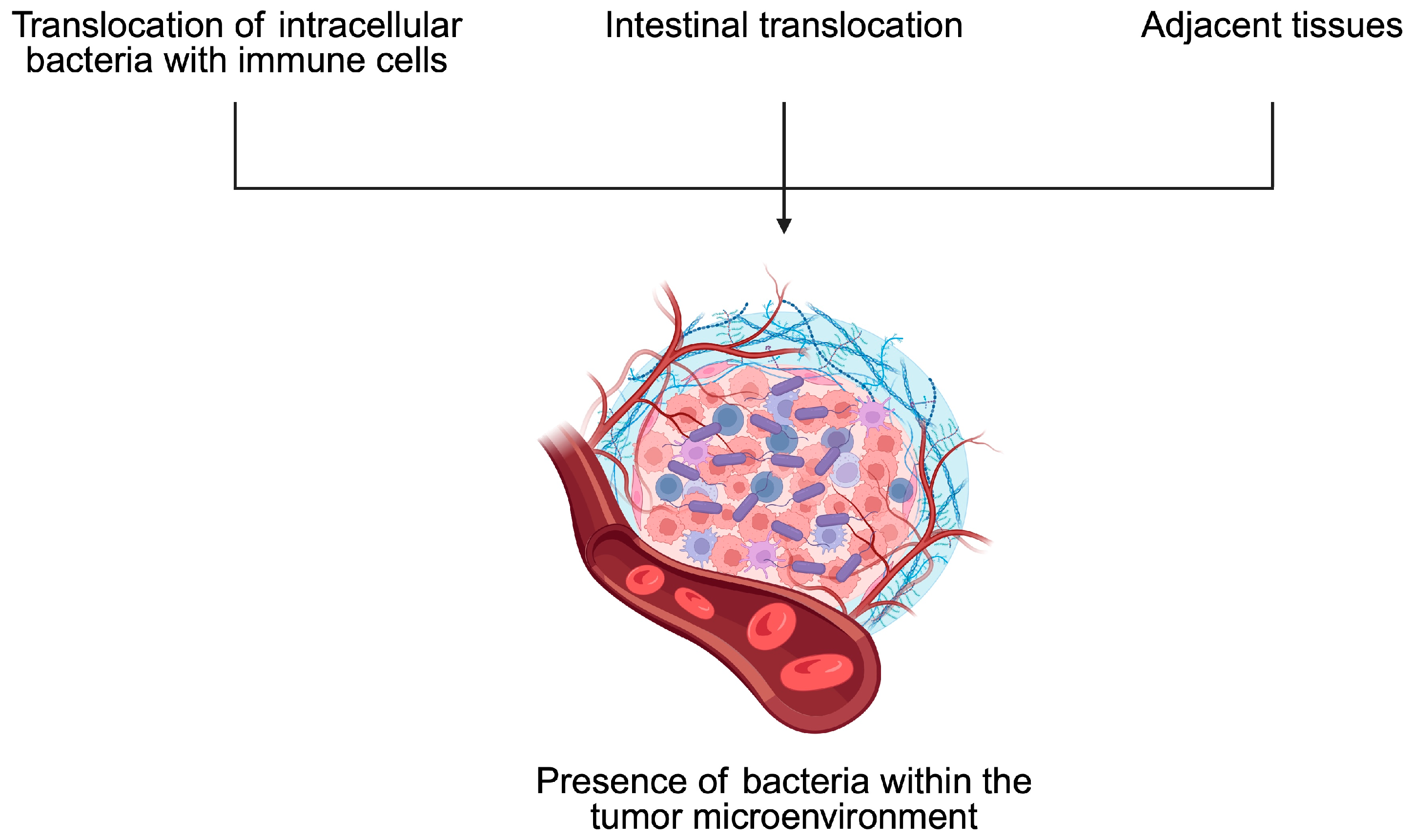
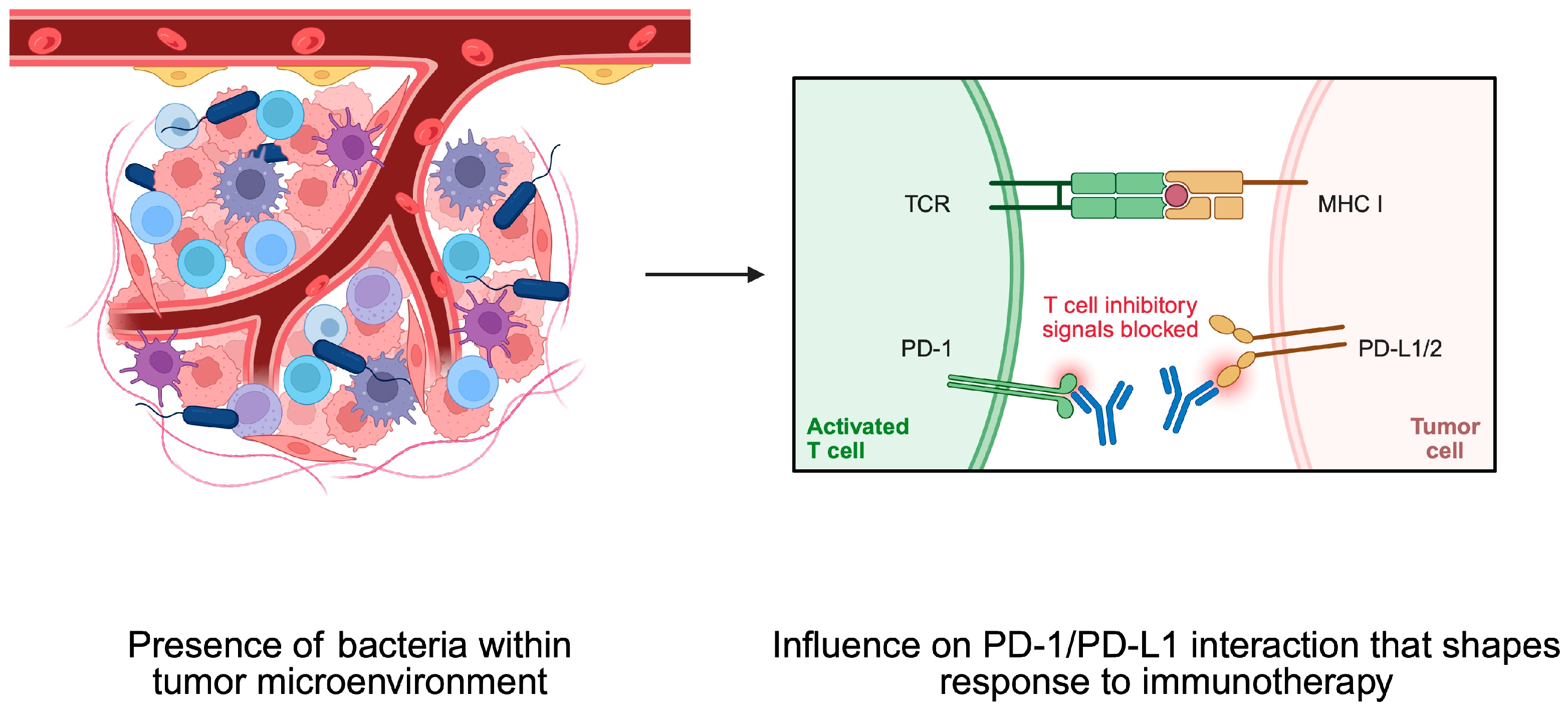
4. Bacteria and Tumor Microenvironment
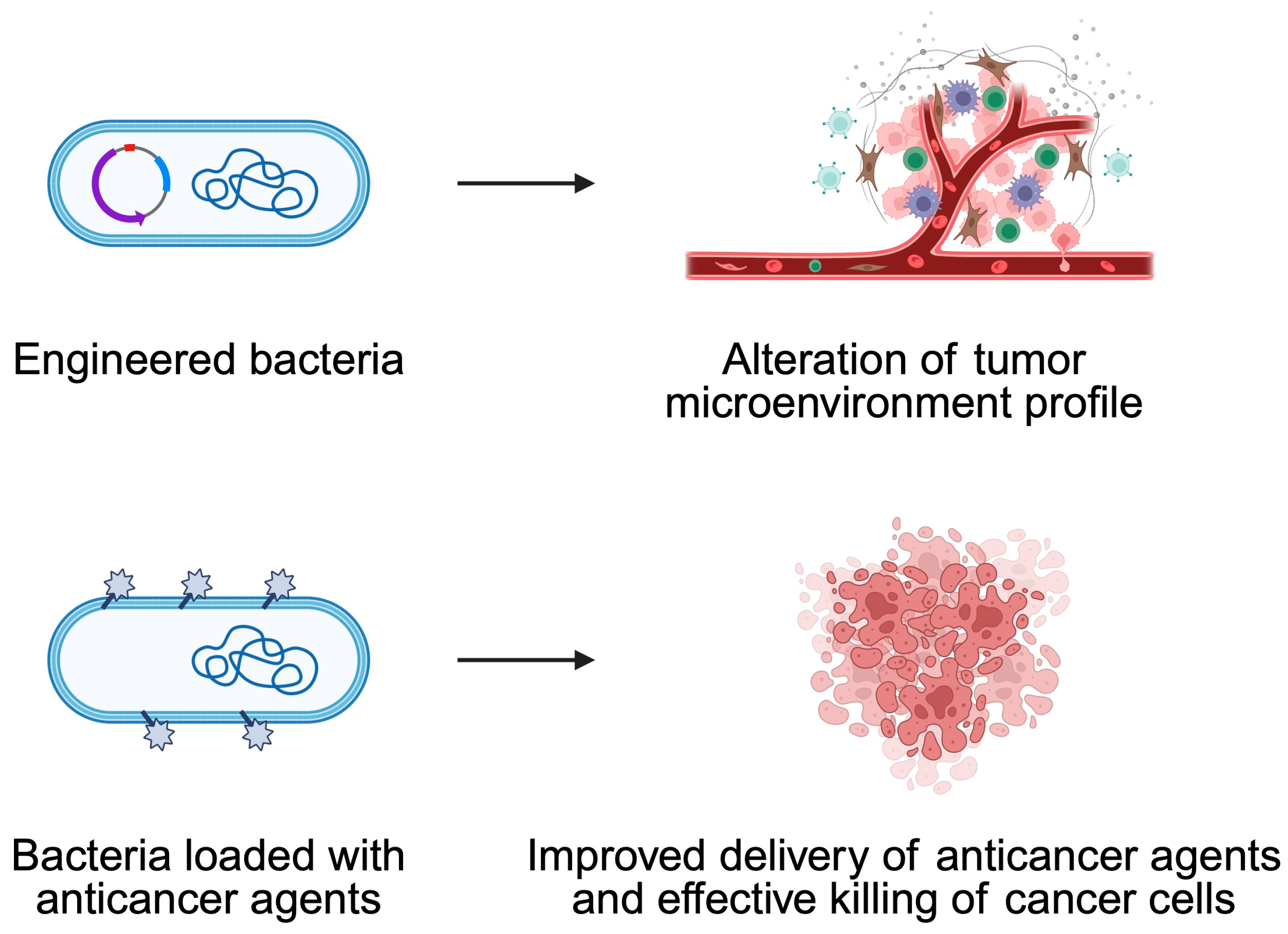
5. Bacteria-Mediated Cancer Therapy
5.1. Engineered Bacteria
5.2. How Can One Increase the Anticancer Effects of Bacteria?
6. The Potential of Probiotics in Cancer Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCFAs | Short-chain fatty acids |
| GPCRs | G-protein-coupled receptors |
| Tregs | Regulatory T cells |
| TGF-β | Transforming growth factor-β |
| DCs | Dendritic cells |
| TME | Tumor microenvironment |
| APCs | Antigen-presenting cells |
| ROS | Reactive oxygen species |
| PI3K | Phosphatidylinositol-3-kinase |
| TLR4 | Toll-like receptor 4 |
| NF-κB | Nuclear factor-kappa B |
| GC | Gastric cancer |
| cagA | Cytotoxic-associated gene |
| vacA | Vacuolating cytotoxin A |
| oipA | Outer inflammatory protein |
| CRC | Colorectal cancer |
| HCC | Hepatocellular carcinoma |
| PD-1 | Programmed cell death protein 1 |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| ICIs | Immune checkpoint inhibitors |
| PFS | Progression-free survival |
| OS | Overall survival |
| TAMs | Tumor-associated macrophages |
| OMVs | Outer membrane vesicles |
| NPs | Nanoparticles |
References
- Chen, H.; Zhu, Y.; Zhang, C.; Hu, L.; Yang, K. Engineered bacteria in tumor immunotherapy. Cancer Lett. 2024, 589, 216817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.X. Microbiota-Associated Metabolites and Related Immunoregulation in Colorectal Cancer. Cancers 2021, 13, 4054. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Miquel, S.; Noordine, M.L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef]
- Zahedifard, Z.; Mahmoodi, S.; Ghasemian, A. Genetically Engineered Bacteria as a Promising Therapeutic Strategy Against Cancer: A Comprehensive Review. Biotechnol. Appl. Biochem. 2025; ahead of print. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Jiang, Z.; Tong, H.; Ma, X.; Liu, Y. Tumor microbiome: Roles in tumor initiation, progression, and therapy. Mol. Biomed. 2025, 6, 9. [Google Scholar] [CrossRef]
- Paterson, Y.; Guirnalda, P.D.; Wood, L.M. Listeria and Salmonella bacterial vectors of tumor-associated antigens for cancer immunotherapy. Semin. Immunol. 2010, 22, 183–189. [Google Scholar] [CrossRef]
- Singh, M.; Quispe-Tintaya, W.; Chandra, D.; Jahangir, A.; Venkataswamy, M.M.; Ng, T.W.; Sharma-Kharkwal, S.; Carreño, L.J.; Porcelli, S.A.; Gravekamp, C. Direct incorporation of the NKT-cell activator α-galactosylceramide into a recombinant Listeria monocytogenes improves breast cancer vaccine efficacy. Br. J. Cancer 2014, 111, 1945–1954. [Google Scholar] [CrossRef]
- Quispe-Tintaya, W.; Chandra, D.; Jahangir, A.; Harris, M.; Casadevall, A.; Dadachova, E.; Gravekamp, C. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 8668–8673. [Google Scholar] [CrossRef]
- Lu, J.; Tong, Q. From pathogenesis to treatment: The impact of bacteria on cancer. Front. Microbiol. 2024, 15, 1462749. [Google Scholar] [CrossRef]
- Devaraja, K.; Aggarwal, S. Dysbiosis of Oral Microbiome: A Key Player in Oral Carcinogenesis? A Critical Review. Biomedicines 2025, 13, 448. [Google Scholar] [CrossRef]
- Prasad, S.K.; Bhat, S.; Shashank, D.; CR, A.; Rachtanapun, P.; Devegowda, D.; Santhekadur, P.K.; Sommano, S.R. Bacteria-Mediated Oncogenesis and the Underlying Molecular Intricacies: What We Know So Far. Front. Oncol. 2022, 12, 836004. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Murray, B.; Choi, S. Biofilm and Cancer: Interactions and Future Directions for Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 12836. [Google Scholar] [CrossRef]
- El Tekle, G.; Garrett, W.S. Bacteria in cancer initiation, promotion and progression. Nat. Rev. Cancer 2023, 23, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.H.; Parsonnet, J. Role of bacteria in oncogenesis. Clin. Microbiol. Rev. 2010, 23, 837–857. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic bacteria in colorectal cancer: Mechanisms and treatments. Cancer Biol. Med. 2021, 19, 147–162. [Google Scholar] [CrossRef]
- Li, Q. Bacterial infection and microbiota in carcinogenesis and tumor development. Front. Cell. Infect. Microbiol. 2023, 13, 1294082. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Fiorentini, C.; Carlini, F.; Germinario, E.A.P.; Maroccia, Z.; Travaglione, S.; Fabbri, A. Gut Microbiota and Colon Cancer: A Role for Bacterial Protein Toxins? Int. J. Mol. Sci. 2020, 21, 6201. [Google Scholar] [CrossRef]
- Laliani, G.; Ghasemian Sorboni, S.; Lari, R.; Yaghoubi, A.; Soleimanpour, S.; Khazaei, M.; Hasanian, S.M.; Avan, A. Bacteria and cancer: Different sides of the same coin. Life Sci. 2020, 246, 117398. [Google Scholar] [CrossRef]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef]
- Khatun, S.; Appidi, T.; Rengan, A.K. The role played by bacterial infections in the onset and metastasis of cancer. Curr. Res. Microb. Sci. 2021, 2, 100078. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y. Intratumor microbiome in cancer progression: Current developments, challenges and future trends. Biomark. Res. 2022, 10, 37. [Google Scholar] [CrossRef]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef]
- Upadhayay, A.; Pal, D.; Kumar, A. Salmonella typhi induced oncogenesis in gallbladder cancer: Co-relation and progression. Adv. Cancer Biol. Metastasis 2022, 4, 100032. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Doocey, C.M.; Finn, K.; Murphy, C.; Guinane, C.M. The impact of the human microbiome in tumorigenesis, cancer progression, and biotherapeutic development. BMC Microbiol. 2022, 22, 53. [Google Scholar] [CrossRef]
- Herrero, R.; Park, J.Y.; Forman, D. The fight against gastric cancer—The IARC Working Group report. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1107–1114. [Google Scholar] [CrossRef]
- Chang, W.L.; Yeh, Y.C.; Sheu, B.S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 68. [Google Scholar] [CrossRef]
- Khosravi, Y.; Dieye, Y.; Poh, B.H.; Ng, C.G.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Culturable bacterial microbiota of the stomach of Helicobacter pylori positive and negative gastric disease patients. Sci. World J. 2014, 2014, 610421. [Google Scholar] [CrossRef]
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653. [Google Scholar] [CrossRef] [PubMed]
- Sharafutdinov, I.; Tegtmeyer, N.; Linz, B.; Rohde, M.; Vieth, M.; Tay, A.C.; Lamichhane, B.; Tuan, V.P.; Fauzia, K.A.; Sticht, H.; et al. A single-nucleotide polymorphism in Helicobacter pylori promotes gastric cancer development. Cell Host Microbe 2023, 31, 1345–1358 e1346. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.T.; Johnston, E.; Krishna, U.; Yamaoka, Y.; Israel, D.A.; Nagy, T.A.; Wroblewski, L.E.; Piazuelo, M.B.; Correa, P.; Peek, R.M. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008, 68, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, N.; Ji, X.; Yang, S.; Zhao, Z.; Li, P. Helicobacter pylori CagA promotes gastric cancer immune escape by upregulating SQLE. Cell Death Dis. 2025, 16, 17. [Google Scholar] [CrossRef]
- Oldani, A.; Cormont, M.; Hofman, V.; Chiozzi, V.; Oregioni, O.; Canonici, A.; Sciullo, A.; Sommi, P.; Fabbri, A.; Ricci, V.; et al. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 2009, 5, e1000603. [Google Scholar] [CrossRef]
- Tombola, F.; Morbiato, L.; Del Giudice, G.; Rappuoli, R.; Zoratti, M.; Papini, E. The Helicobacter pylori VacA toxin is a urea permease that promotes urea diffusion across epithelia. J. Clin. Investig. 2001, 108, 929–937. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kwon, D.H.; Graham, D.Y. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 2000, 97, 7533–7538. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, W.; Wang, H.; Zhang, C.; Li, J.; Chen, W.; Xu, X.; Wang, L.; Ma, M.; Zhang, S.; et al. Helicobacter pylori promotes gastric cancer progression by activating the TGF-beta/Smad2/EMT pathway through HKDC1. Cell. Mol. Life Sci. 2024, 81, 453. [Google Scholar] [CrossRef]
- Guo, R.; Cui, X.; Li, X.; Zang, W.; Chang, M.; Sun, Z.; Liu, Z.; Sun, Y.; Jia, J.; Li, W. CircMAN1A2 is upregulated by Helicobacter pylori and promotes development of gastric cancer. Cell Death Dis. 2022, 13, 409. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, Z.; Cao, J.; Liu, Y.; Zhu, L.; Mao, B.; Cui, F.; Shao, S. Helicobacter pylori upregulates circPGD and promotes development of gastric cancer. J. Cancer Res. Clin. Oncol. 2024, 150, 104. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Mertens, A.; Klein, I.; Leyh, C.; Krieg, S.; Kandler, J.; Luedde, T.; Roderburg, C.; Kostev, K. Association between Helicobacter pylori and its eradication and the development of cancer. BMJ Open Gastroenterol. 2024, 11, e001377. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chiu, H.M.; Su, W.W.; Chou, K.C.; Chen, S.L.; Yen, A.M.; Fann, J.C.; Chiu, S.Y.; Chuang, S.L.; et al. Screening for Helicobacter pylori to Prevent Gastric Cancer: A Pragmatic Randomized Clinical Trial. JAMA 2024, 332, 1642–1651. [Google Scholar] [CrossRef]
- Della Bella, C.; Soluri, M.F.; Puccio, S.; Benagiano, M.; Grassi, A.; Bitetti, J.; Cianchi, F.; Sblattero, D.; Peano, C.; D’Elios, M.M. The Helicobacter pylori CagY protein drives gastric Th1 and Th17 inflammation and B cell proliferation in gastric MALT lymphoma. Int. J. Mol. Sci. 2021, 22, 9459. [Google Scholar] [CrossRef]
- Vlăduţ, C.; Ciocîrlan, M.; Costache, R.S.; Jinga, M.; Balaban, V.D.; Costache, D.O.; Diculescu, M. Is mucosa-associated lymphoid tissue lymphoma an infectious disease? Role of. Exp. Ther. Med. 2020, 20, 3546–3553. [Google Scholar] [CrossRef]
- Park, J.B.; Koo, J.S. Helicobacter pylori infection in gastric mucosa-associated lymphoid tissue lymphoma. World J. Gastroenterol. 2014, 20, 2751–2759. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, X.; Yin, M.; Gao, J.; Weng, Z.; Xu, C. The relationship between Helicobacter pylori and pancreatic cancer: A meta-analysis. Transl. Cancer Res. 2022, 11, 2810–2822. [Google Scholar] [CrossRef]
- Shah, S.C.; Camargo, M.C.; Lamm, M.; Bustamante, R.; Roumie, C.L.; Wilson, O.; Halvorson, A.E.; Greevy, R.; Liu, L.; Gupta, S.; et al. Impact of Helicobacter pylori Infection and Treatment on Colorectal Cancer in a Large, Nationwide Cohort. J. Clin. Oncol. 2024, 42, 1881–1889. [Google Scholar] [CrossRef]
- Xu, C.; Fan, L.; Lin, Y.; Shen, W.; Qi, Y.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, L.; Leng, X.X.; Xie, Y.L.; Kang, Z.R.; Zhao, L.C.; Song, L.H.; Zhou, C.B.; Fang, J.Y. Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes 2024, 16, 2333790. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, D.; Yin, H.S.; Zhang, H.; Hong, K.Q.; Yuan, J.P.; Yu, B.P. Fusobacterium nucleatum promotes esophageal squamous cell carcinoma progression and chemoresistance by enhancing the secretion of chemotherapy-induced senescence-associated secretory phenotype via activation of DNA damage response pathway. Gut Microbes 2023, 15, 2197836. [Google Scholar] [CrossRef]
- Lei, J.; Xu, F.; Deng, C.; Nie, X.; Zhong, L.; Wu, Z.; Li, J.; Wu, X.; He, S.; Chen, Y. Fusobacterium nucleatum promotes the early occurrence of esophageal cancer through upregulation of IL-32/PRTN3 expression. Cancer Sci. 2023, 114, 2414–2428. [Google Scholar] [CrossRef]
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting Programmed. Cancers 2019, 11, 1592. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef]
- Dadgar-Zankbar, L.; Elahi, Z.; Shariati, A.; Khaledi, A.; Razavi, S.; Khoshbayan, A. Exploring the role of Fusobacterium nucleatum in colorectal cancer: Implications for tumor proliferation and chemoresistance. Cell Commun. Signal. 2024, 22, 547. [Google Scholar] [CrossRef]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal. 2023, 21, 201. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Kang, W.Y.; Jia, Z.L.; Tang, D.; Zhang, Z.W.; Gao, H.; He, K.L.; Feng, Q. Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-κB Signaling Pathways in Human Gingival Fibroblasts. Oxidative Med. Cell. Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Miguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Gong, T.; Luo, W.; Hu, B.; Gao, J.; Li, Y.; Liu, R.; Xie, N.; Yang, W.; Xu, X.; et al. Fusobacterium nucleatum extracellular vesicles are enriched in colorectal cancer and facilitate bacterial adhesion. Sci. Adv. 2024, 10, eado0016. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, Z.; Wang, Z.; Xu, F.; Yang, J.; Lin, B.; Chen, Y.; Wenren, H.; Wu, L.; Guo, X.; et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/beta-catenin/GPX4 axis in colorectal cancer. Free. Radic. Biol. Med. 2024, 220, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Ternes, D.; Tsenkova, M.; Pozdeev, V.I.; Meyers, M.; Koncina, E.; Atatri, S.; Schmitz, M.; Karta, J.; Schmoetten, M.; Heinken, A.; et al. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat. Metab. 2022, 4, 458–475. [Google Scholar] [CrossRef]
- Zou, Q.; Wu, Y.; Zhang, S.; Li, S.; Li, S.; Su, Y.; Zhang, L.; Li, Q.; Zou, H.; Zhang, X.; et al. Escherichia coli and HPV16 coinfection may contribute to the development of cervical cancer. Virulence 2024, 15, 2319962. [Google Scholar] [CrossRef]
- Miyasaka, T.; Yamada, T.; Uehara, K.; Sonoda, H.; Matsuda, A.; Shinji, S.; Ohta, R.; Kuriyama, S.; Yokoyama, Y.; Takahashi, G.; et al. Pks-positive Escherichia coli in tumor tissue and surrounding normal mucosal tissue of colorectal cancer patients. Cancer Sci. 2024, 115, 1184–1195. [Google Scholar] [CrossRef]
- Kang, F.P.; Chen, Z.W.; Liao, C.Y.; Wu, Y.D.; Li, G.; Xie, C.K.; Lin, H.Y.; Huang, L.; Tian, Y.F.; Wang, Z.W.; et al. Escherichia coli-Induced cGLIS3-Mediated Stress Granules Activate the NF-kappaB Pathway to Promote Intrahepatic Cholangiocarcinoma Progression. Adv. Sci. 2024, 11, e2306174. [Google Scholar] [CrossRef]
- Cuevas-Ramos, G.; Petit, C.R.; Marcq, I.; Boury, M.; Oswald, E.; Nougayrède, J.P. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl. Acad. Sci. USA 2010, 107, 11537–11542. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Chen, B.; Ramazzotti, D.; Heide, T.; Spiteri, I.; Fernandez-Mateos, J.; James, C.; Magnani, L.; Graham, T.A.; Sottoriva, A. Contribution of pks(+) E. coli mutations to colorectal carcinogenesis. Nat. Commun. 2023, 14, 7827. [Google Scholar] [CrossRef] [PubMed]
- Raisch, J.; Buc, E.; Bonnet, M.; Sauvanet, P.; Vazeille, E.; de Vallée, A.; Déchelotte, P.; Darcha, C.; Pezet, D.; Bonnet, R.; et al. Colon cancer-associated B2 Escherichia coli colonize gut mucosa and promote cell proliferation. World J. Gastroenterol. 2014, 20, 6560–6572. [Google Scholar] [CrossRef]
- Wei, Z.; Cao, S.; Liu, S.; Yao, Z.; Sun, T.; Li, Y.; Li, J.; Zhang, D.; Zhou, Y. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients’ survival? A pilot study on relevant mechanism. Oncotarget 2016, 7, 46158–46172. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Minot, S.S.; Li, N.; Srinivasan, H.; Ayers, J.L.; Yu, M.; Koester, S.T.; Stangis, M.M.; Dominitz, J.A.; Halberg, R.B.; Grady, W.M.; et al. Colorectal cancer-associated bacteria are broadly distributed in global microbiomes and drivers of precancerous change. Sci. Rep. 2024, 14, 23646. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Cai, Y.; Wong, C.C.; Wang, J.; Wang, N.; Lau, H.C.; Jiao, Y.; Zhou, X.; et al. Gut-liver translocation of pathogen Klebsiella pneumoniae promotes hepatocellular carcinoma in mice. Nat. Microbiol. 2025, 10, 169–184. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Badve, S.S.; Gokmen-Polar, Y. Targeting the Tumor-Tumor Microenvironment Crosstalk. Expert Opin. Ther. Targets 2023, 27, 447–457. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhang, R.; Gong, H.; Jiang, X.; Xia, S. Targeting the tumor immune microenvironment: GPCRs as key regulators in triple-negative breast cancer. Int. Immunopharmacol. 2025, 147, 113930. [Google Scholar] [CrossRef]
- Li, S.; Xia, H.; Wang, Z.; Zhang, X.; Song, T.; Li, J.; Xu, L.; Zhang, N.; Fan, S.; Li, Q.; et al. Intratumoral microbial heterogeneity affected tumor immune microenvironment and determined clinical outcome of HBV-related HCC. Hepatology 2023, 78, 1079–1091. [Google Scholar] [CrossRef]
- Chen, F.; Guo, S.; Li, Y.; Lu, Y.; Liu, L.; Chen, S.; An, J.; Zhang, G. Fusobacterium nucleatum-driven CX3CR1(+) PD-L1(+) phagocytes route to tumor tissues and reshape tumor microenvironment. Gut Microbes 2025, 17, 2442037. [Google Scholar] [CrossRef] [PubMed]
- Galeano Nino, J.L.; Wu, H.; LaCourse, K.D.; Kempchinsky, A.G.; Baryiames, A.; Barber, B.; Futran, N.; Houlton, J.; Sather, C.; Sicinska, E.; et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 2022, 611, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Chen, Y.; Xie, Y.; Wang, X.; Hu, Y.; Sun, Y.; Cao, Y.; Zhang, L.; Wang, Y.; Wang, Z.; et al. Helicobacter pylori and immunotherapy for gastrointestinal cancer. Innovation 2024, 5, 100561. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer. Version 2.2025—April 4. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434 (accessed on 15 April 2025).
- Rha, S.Y.; Oh, D.Y.; Yanez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, C.C.; Ding, Y.; Gao, M.; Wen, J.; Lau, H.C.; Cheung, A.H.; Huang, D.; Huang, H.; Yu, J. Peptostreptococcus anaerobius mediates anti-PD1 therapy resistance and exacerbates colorectal cancer via myeloid-derived suppressor cells in mice. Nat. Microbiol. 2024, 9, 1467–1482. [Google Scholar] [CrossRef]
- Benej, M.; Hoyd, R.; Kreamer, M.; Wheeler, C.E.; Grencewicz, D.J.; Choueiry, F.; Chan, C.H.F.; Zakharia, Y.; Ma, Q.; Dodd, R.D.; et al. The Tumor Microbiome Reacts to Hypoxia and Can Influence Response to Radiation Treatment in Colorectal Cancer. Cancer Res. Commun. 2024, 4, 1690–1701. [Google Scholar] [CrossRef]
- Yan, S.; Gan, Y.; Xu, H.; Piao, H. Bacterial carrier-mediated drug delivery systems: A promising strategy in cancer therapy. Front. Bioeng. Biotechnol. 2024, 12, 1526612. [Google Scholar] [CrossRef]
- Jin, K.; Huang, Y.; Che, H.; Wu, Y. Engineered Bacteria for Disease Diagnosis and Treatment Using Synthetic Biology. Microb. Biotechnol. 2025, 18, e70080. [Google Scholar] [CrossRef]
- He, X.; Guo, J.; Bai, Y.; Sun, H.; Yang, J. Salmonella-based therapeutic strategies: Improving tumor microenvironment and bringing new hope for cancer immunotherapy. Med. Oncol. 2024, 42, 27. [Google Scholar] [CrossRef]
- Zhu, D.; Pan, W.; Li, H.; Hua, J.; Zhang, C.; Zhao, K. Innovative Applications of Bacteria and Their Derivatives in Targeted Tumor Therapy. ACS Nano 2025, 19, 5077–5109. [Google Scholar] [CrossRef]
- Senevirathne, A.; Lloren, K.K.S.; Aganja, R.P.; Kwon, J.; Lee, J.H. Transforming bacterial pathogens into wonder tools in cancer immunotherapy. Mol. Ther. 2025, 33, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Michán, C.; Prados, J.; Ramos, J.L. Bacteria as Precision Tools for Cancer Therapy. Microb. Biotechnol. 2025, 18, e70090. [Google Scholar] [CrossRef]
- Sapkota, H.; Dasgupta, S.; Roy, B.; Pathan, E.K. Human Commensal Bacteria: Next-generation Pro- and Post-biotics for Anticancer Therapy. Front. Biosci. Elite Ed. 2025, 17, 26809. [Google Scholar] [CrossRef]
- Kuhl, G.C.; Tangney, M. Bacterial-Mediated In Situ Engineering of Tumour-Associated Macrophages for Cancer Immunotherapy. Cancers 2025, 17, 723. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, C.; Li, S.; Liang, Y.; Lee, L.T.; Aggarwal, N.; Wun, K.S.; Liu, J.; Nadarajan, S.P.; Weng, C.; et al. Prodrug-conjugated tumor-seeking commensals for targeted cancer therapy. Nat. Commun. 2024, 15, 4343. [Google Scholar] [CrossRef]
- Mahdizade Ari, M.; Dadgar, L.; Elahi, Z.; Ghanavati, R.; Taheri, B. Genetically Engineered Microorganisms and Their Impact on Human Health. Int. J. Clin. Pract. 2024, 2024, 6638269. [Google Scholar] [CrossRef]
- Savage, T.M.; Vincent, R.L.; Rae, S.S.; Huang, L.H.; Ahn, A.; Pu, K.; Li, F.; de Los Santos-Alexis, K.; Coker, C.; Danino, T.; et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity. Sci. Adv. 2023, 9, eadc9436. [Google Scholar] [CrossRef]
- Nguyen, D.H.; You, S.H.; Ngo, H.T.; Van Nguyen, K.; Tran, K.V.; Chu, T.H.; Kim, S.Y.; Ha, S.J.; Hong, Y.; Min, J.J. Reprogramming the tumor immune microenvironment using engineered dual-drug loaded Salmonella. Nat. Commun. 2024, 15, 6680. [Google Scholar] [CrossRef]
- Thomas, S.C.; Madaan, T.; Kamble, N.S.; Siddiqui, N.A.; Pauletti, G.M.; Kotagiri, N. Engineered Bacteria Enhance Immunotherapy and Targeted Therapy through Stromal Remodeling of Tumors. Adv. Healthc. Mater. 2022, 11, e2101487. [Google Scholar] [CrossRef]
- Mi, Z.; Feng, Z.C.; Li, C.; Yang, X.; Ma, M.T.; Rong, P.F. Salmonella-Mediated Cancer Therapy: An Innovative Therapeutic Strategy. J. Cancer 2019, 10, 4765–4776. [Google Scholar] [CrossRef]
- Piñero-Lambea, C.; Ruano-Gallego, D.; Fernández, L. Engineered bacteria as therapeutic agents. Curr. Opin. Biotechnol. 2015, 35, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.; Chong, A.; Hong, Y.; Min, J.J. Bioengineering of bacteria for cancer immunotherapy. Nat. Commun. 2023, 14, 3553. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Qin, Y.; You, S.H.; Min, J.J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Raman, V.; Van Dessel, N.; Hall, C.L.; Wetherby, V.E.; Whitney, S.A.; Kolewe, E.L.; Bloom, S.M.K.; Sharma, A.; Hardy, J.A.; Bollen, M.; et al. Intracellular delivery of protein drugs with an autonomously lysing bacterial system reduces tumor growth and metastases. Nat. Commun. 2021, 12, 6116. [Google Scholar] [CrossRef]
- Pan, H.Z.; Li, L.Y.; Pang, G.J.; Han, C.L.; Liu, B.N.; Zhang, Y.Y.; Shen, Y.; Sun, T.; Liu, J.; Chang, J.; et al. Engineered NIR light-responsive bacteria as anti-tumor agent for targeted and precise cancer therapy. Chem. Eng. J. 2021, 426, 130842. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, X.; Fang, M.; Pang, G.; Xing, Y.; Zhang, X.; Li, L.; Chen, Q.; Wang, Y.; Chang, J.; et al. Upconversion Optogenetic Engineered Bacteria System for Time-Resolved Imaging Diagnosis and Light-Controlled Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 46351–46361. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Z.; Zhang, M.; Lin, S.; Liu, J. Spatiotemporally Controllable Distribution of Combination Therapeutics in Solid Tumors by Dually Modified Bacteria. Adv. Mater. 2022, 34, e2106669. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Arpaia, N.; Danino, T. Engineering bacteria as interactive cancer therapies. Science 2022, 378, 858–864. [Google Scholar] [CrossRef]
- Shong, J.; Collins, C.H. Quorum sensing-modulated AND-gate promoters control gene expression in response to a combination of endogenous and exogenous signals. ACS Synth. Biol. 2014, 3, 238–246. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef]
- Zalatan, J.G.; Petrini, L.; Geiger, R. Engineering bacteria for cancer immunotherapy. Curr. Opin. Biotechnol. 2024, 85, 103061. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.; Harimoto, T.; Kepecs, B.; Gray, K.; Coker, C.; Hou, N.; Pu, K.; Azad, T.; Nolasco, A.; Pavlicova, M.; et al. Enhancing the tropism of bacteria via genetically programmed biosensors. Nat. Biomed. Eng. 2022, 6, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Shi, H.; Zhang, Y.; Fan, Y.; Wang, L.; Xiang, L.; Liu, Y.; Zhao, L.; Fu, S. Bacteria-driven hypoxia targeting delivery of chemotherapeutic drug proving outcome of breast cancer. J. Nanobiotechnol. 2022, 20, 178. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef]
- Altonsy, M.O.; Andrews, S.C.; Tuohy, K.M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int. J. Food Microbiol. 2010, 137, 190–203. [Google Scholar] [CrossRef]
- Borowicki, A.; Michelmann, A.; Stein, K.; Scharlau, D.; Scheu, K.; Obst, U.; Glei, M. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr. Cancer 2011, 63, 151–160. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Russo, F.; Orlando, A.; Linsalata, M.; Cavallini, A.; Messa, C. Effects of Lactobacillus rhamnosus GG on the cell growth and polyamine metabolism in HGC-27 human gastric cancer cells. Nutr. Cancer 2007, 59, 106–114. [Google Scholar] [CrossRef]
- Sadeghi-Aliabadi, H.; Mohammadi, F.; Fazeli, H.; Mirlohi, M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran. J. Basic Med. Sci. 2014, 17, 815–819. [Google Scholar]
- Łoniewski, I.; Kaźmierczak-Siedlecka, K.; Komorniak, N.; Stachowska, E. Probiotics—When and for whom in the oncological patient population. Nowotw. J. Oncol. 2024, 74, 141–152. [Google Scholar] [CrossRef]
- Thananimit, S.; Pahumunto, N.; Teanpaisan, R. Characterization of Short Chain Fatty Acids Produced by Selected Potential Probiotic Lactobacillus Strains. Biomolecules 2022, 12, 1829. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Du, M.; Li, X.; Gao, J.; Li, Q.; Li, H.; Li, J.; Gao, X.; Cong, H.; Huang, Y.; et al. Upregulation of Lactobacillus spp. in gut microbiota as a novel mechanism for environmental eustress-induced anti-pancreatic cancer effects. Gut Microbes 2025, 17, 2470372. [Google Scholar] [CrossRef] [PubMed]
- Abedin-Do, A.; Taherian-Esfahani, Z.; Ghafouri-Fard, S.; Ghafouri-Fard, S.; Motevaseli, E. Immunomodulatory effects of Lactobacillus strains: Emphasis on their effects on cancer cells. Immunotherapy 2015, 7, 1307–1329. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224–1232. [Google Scholar] [CrossRef]
- Li, N.; Niu, L.; Liu, Y.; Wang, Y.; Su, X.; Xu, C.; Sun, Z.; Guo, H.; Gong, J.; Shen, S. Taking SCFAs produced by Lactobacillus reuteri orally reshapes gut microbiota and elicits antitumor responses. J. Nanobiotechnol. 2024, 22, 241. [Google Scholar] [CrossRef]
- Takada, K.; Takamori, S.; Shimokawa, M.; Pinato, D.J.; Cortellini, A. Prior antibiotics, proton pump inhibitors, and probiotics in patients with extensive stage small cell lung cancer treated with immune checkpoint blockade: A post-hoc analysis of the phase I/III IMpower 133 trial. Int. J. Cancer 2025, 156, 914–919. [Google Scholar] [CrossRef]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8(+) T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Gao, G.; Shen, S.; Zhang, T.; Zhang, J.; Huang, S.; Sun, Z.; Zhang, H. Lacticaseibacillus rhamnosus Probio-M9 enhanced the antitumor response to anti-PD-1 therapy by modulating intestinal metabolites. EBioMedicine 2023, 91, 104533. [Google Scholar] [CrossRef]
| Characteristic | Description | References |
|---|---|---|
| Tumor Targeting | Bacteria such as Escherichia coli, Salmonella, and Listeria monocytogenes naturally migrate toward and colonize the hypoxic and necrotic regions of tumors due to their chemotactic abilities and motility. | [92] |
| Adaptability to the TME | The unique conditions within the tumor microenvironment—such as low oxygen levels, acidity, and abundant nutrients—create a favorable niche for bacterial survival and proliferation, which is not present in healthy tissues. | [92] |
| Immune Activation | Certain bacteria can stimulate both innate and adaptive immune responses, acting as natural adjuvants to enhance antitumor immunity. | [90] |
| Genetic Manipulability | Advances in genetic engineering have enabled the modification of bacterial strains to attenuate virulence, control therapeutic gene expression, and incorporate regulatory circuits, thereby increasing safety and efficacy. | [90,91] |
| Bacterial Strain | Key Properties | References |
|---|---|---|
| Escherichia coli (e.g., BL21, DH5α) | Genetically engineered to produce therapeutic agents; effectively colonizes tumor tissues due to natural tumor-targeting abilities. | [90,92] |
| Salmonella typhimurium | Exhibits robust motility and chemotaxis; engineered into attenuated “suicide” strains that deliver cytotoxic proteins and prodrug-converting enzymes to induce apoptosis. | [90,94] |
| Listeria monocytogenes | Stimulates the immune system; induces direct tumor cell death through mechanisms involving reactive oxygen species (ROS) and mitochondrial disruption. | [94] |
| Probiotic Strains (Lactobacillus plantarum, Enterococcus faecalis, Bifidobacterium spp.) | Exhibit selective anticancer activity; investigated for their potential to inhibit tumor growth and work synergistically with conventional therapies. | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plewa, P.; Kiełbowski, K.; Mentel, O.; Figiel, K.; Bakinowska, E.; Becht, R.; Banach, B.; Pawlik, A. Bacteria and Carcinogenesis and the Management of Cancer: A Narrative Review. Pathogens 2025, 14, 509. https://doi.org/10.3390/pathogens14050509
Plewa P, Kiełbowski K, Mentel O, Figiel K, Bakinowska E, Becht R, Banach B, Pawlik A. Bacteria and Carcinogenesis and the Management of Cancer: A Narrative Review. Pathogens. 2025; 14(5):509. https://doi.org/10.3390/pathogens14050509
Chicago/Turabian StylePlewa, Paulina, Kajetan Kiełbowski, Oliwia Mentel, Karolina Figiel, Estera Bakinowska, Rafał Becht, Bolesław Banach, and Andrzej Pawlik. 2025. "Bacteria and Carcinogenesis and the Management of Cancer: A Narrative Review" Pathogens 14, no. 5: 509. https://doi.org/10.3390/pathogens14050509
APA StylePlewa, P., Kiełbowski, K., Mentel, O., Figiel, K., Bakinowska, E., Becht, R., Banach, B., & Pawlik, A. (2025). Bacteria and Carcinogenesis and the Management of Cancer: A Narrative Review. Pathogens, 14(5), 509. https://doi.org/10.3390/pathogens14050509






