Ex Vivo Drug Susceptibility of Plasmodium malariae Isolates to Antimalarial Drugs in Gabon
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Ex Vivo Susceptibility Test
2.3. Real-Time qPCR Assay
2.4. Statistical Analysis
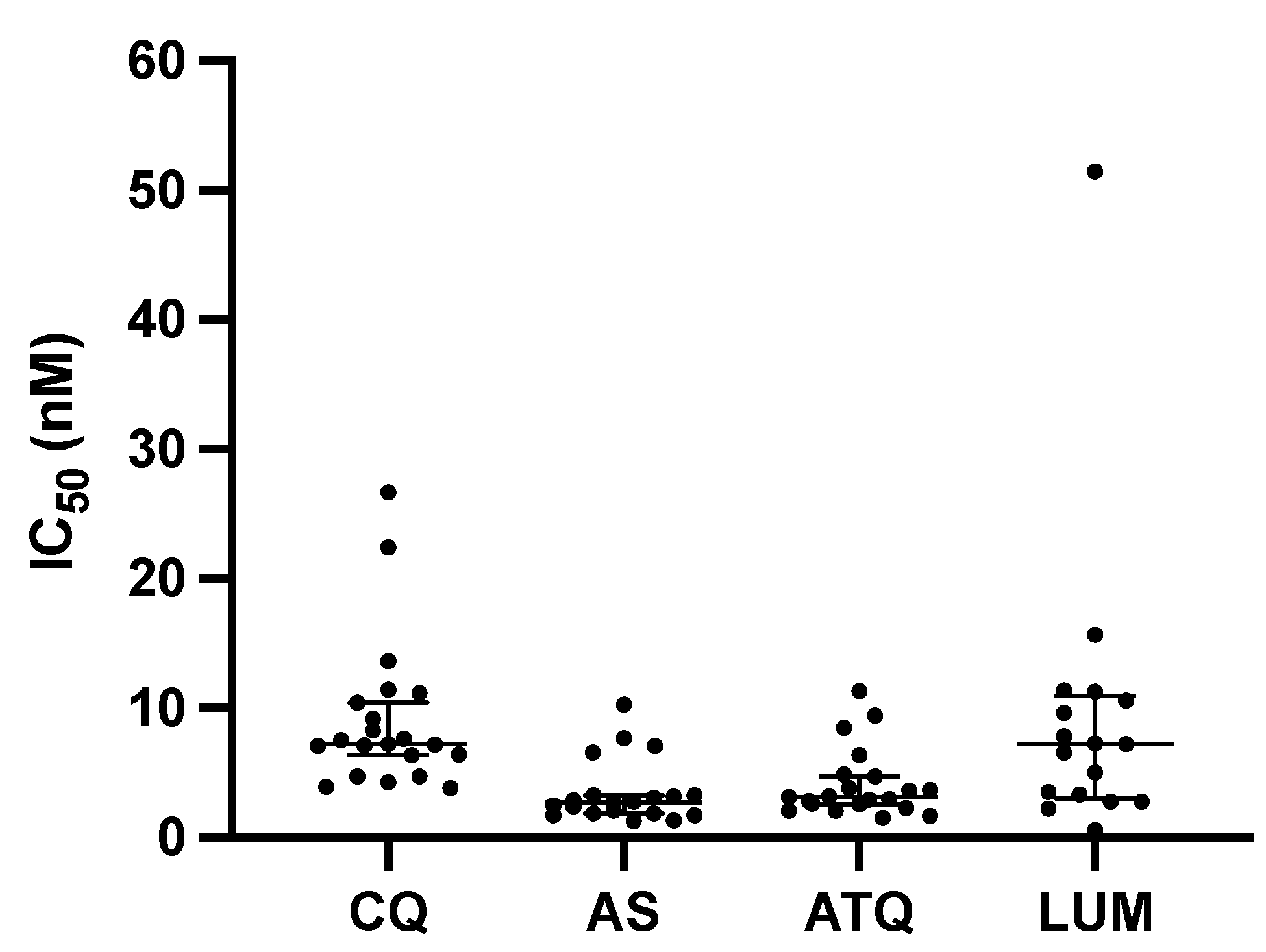
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase Chain Reaction |
| WHO | World Health Organization |
| ACT | Artemisinin-based combination therapy |
| AS | Artesunate |
| ATQ | Atovaquone |
| LUM | Lumefantrine |
| CQ | Chloroquine |
| DNA | Deoxyribonucleic Acid |
| RNA | Ribonucleic Acid |
| IC50 | Half-maximal inhibitory concentration |
| ATPG | Atovaquone-proguanil |
| BMM | Blood Medium Mixture |
References
- Oriero, E.C.; Amenga-Etego, L.; Ishengoma, D.S.; Amambua-Ngwa, A. Plasmodium malariae, current knowledge and future research opportunities on a neglected malaria parasite species. Crit. Rev. Microbiol. 2021, 47, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.J. Persistent Parasitism: The Adaptive Biology of Malariae and Ovale Malaria. Trends Parasitol. 2016, 32, 808–819. [Google Scholar] [CrossRef]
- Collins, W.E.; Jeffery, G.M. Plasmodium malariae: Parasite and disease. Clin. Microbiol. Rev. 2007, 20, 579–592. [Google Scholar] [CrossRef]
- Langford, S.; Douglas, N.M.; Lampah, D.A.; Simpson, J.A.; Kenangalem, E.; Sugiarto, P.; Anstey, N.M.; Poespoprodjo, J.R.; Price, R.N. Plasmodium malariae Infection Associated with a High Burden of Anemia: A Hospital-Based Surveillance Study. PLoS Negl. Trop. Dis. 2015, 9, e0004195. [Google Scholar] [CrossRef]
- Yman, V.; Wandell, G.; Mutemi, D.D.; Miglar, A.; Asghar, M.; Hammar, U.; Karlsson, M.; Lind, I.; Nordfjell, C.; Rooth, I.; et al. Persistent transmission of Plasmodium malariae and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in eastern Tanzania. PLoS Negl. Trop. Dis. 2019, 13, e0007414. [Google Scholar] [CrossRef]
- Niño, C.H.; Cubides, J.R.; Camargo-Ayala, P.A.; Rodríguez-Celis, C.A.; Quiñones, T.; Cortés-Castillo, M.T.; Sánchez-Suárez, L.; Sánchez, R.; Patarroyo, M.E.; Patarroyo, M.A. Plasmodium malariae in the Colombian Amazon region: You don’t diagnose what you don’t suspect. Malar. J. 2016, 15, 576. [Google Scholar] [CrossRef]
- Mbama Ntabi, J.D.; Lissom, A.; Djontu, J.C.; Diafouka-Kietela, S.; Vouvoungui, C.; Boumpoutou, R.K.; Mayela, J.; Nguiffo-Nguete, D.; Nkemngo, F.N.; Ndo, C.; et al. Prevalence of non-Plasmodium falciparum species in southern districts of Brazzaville in The Republic of the Congo. Parasit Vectors 2022, 15, 209. [Google Scholar] [CrossRef]
- Woldearegai, T.G.; Lalremruata, A.; Nguyen, T.T.; Gmeiner, M.; Veletzky, L.; Tazemda-Kuitsouc, G.B.; Matsiegui, P.B.; Mordmüller, B.; Held, J. Characterization of Plasmodium infections among inhabitants of rural areas in Gabon. Sci. Rep. 2019, 9, 9784. [Google Scholar] [CrossRef]
- Camargo-Ayala, P.A.; Cubides, J.R.; Niño, C.H.; Camargo, M.; Rodríguez-Celis, C.A.; Quiñones, T.; Sánchez-Suárez, L.; Patarroyo, M.E.; Patarroyo, M.A. High Plasmodium malariae Prevalence in an Endemic Area of the Colombian Amazon Region. PLoS ONE 2016, 11, e0159968. [Google Scholar] [CrossRef]
- Abdulraheem, M.A.; Ernest, M.; Ugwuanyi, I.; Abkallo, H.M.; Nishikawa, S.; Adeleke, M.; Orimadegun, A.E.; Culleton, R. High prevalence of Plasmodium malariae and Plasmodium ovale in co-infections with Plasmodium falciparum in asymptomatic malaria parasite carriers in southwestern Nigeria. Int. J. Parasitol. 2022, 52, 23–33. [Google Scholar] [CrossRef]
- Borrmann, S.; Szlezák, N.; Binder, R.K.; Missinou, M.A.; Lell, B.; Kremsner, P.G. Evidence for the efficacy of artesunate in asymptomatic Plasmodium malariae infections. J. Antimicrob. Chemother. 2002, 50, 751–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Betson, M.; Clifford, S.; Stanton, M.; Kabatereine, N.B.; Stothard, J.R. Emergence of Nonfalciparum Plasmodium Infection Despite Regular Artemisinin Combination Therapy in an 18-Month Longitudinal Study of Ugandan Children and Their Mothers. J. Infect. Dis. 2018, 217, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Tournoy, T.K.; Rosanas-Urgell, A.; Van Esbroeck, M.; Bottieau, E.; Huits, R. Plasmodium malariae after successful treatment of P. falciparum malaria with artemether-lumefantrine. Int. J. Infect. Dis. 2022, 119, 56–58. [Google Scholar] [CrossRef]
- Rosenthal, P.J.; Asua, V.; Bailey, J.A.; Conrad, M.D.; Ishengoma, D.S.; Kamya, M.R.; Rasmussen, C.; Tadesse, F.G.; Uwimana, A.; Fidock, D.A. The emergence of artemisinin partial resistance in Africa: How do we respond? Lancet Infect Dis. 2024, 24, e591–e600. [Google Scholar] [CrossRef]
- Maguire, J.D.; Sumawinata, I.W.; Masbar, S.; Laksana, B.; Prodjodipuro, P.; Susanti, I.; Sismadi, P.; Mahmud, N.; Bangs, M.J.; Baird, J.K. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 2002, 360, 58–60. [Google Scholar] [CrossRef]
- Smith, A.; Denholm, J.; Shortt, J.; Spelman, D. Plasmodium species co-infection as a cause of treatment failure. Travel Med. Infect. Dis. 2011, 9, 306–309. [Google Scholar]
- Dinko, B.; Oguike, M.C.; Larbi, J.A.; Bousema, T.; Sutherland, C.J. Persistent detection of Plasmodium falciparum, P. malariae, P. ovale curtisi and P. ovale wallikeri after ACT treatment of asymptomatic Ghanaian school-children. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 45–50. [Google Scholar] [CrossRef]
- Joanny, F.; Löhr, S.J.; Engleitner, T.; Lell, B.; Mordmüller, B. Limit of blank and limit of detection of Plasmodium falciparum thick blood smear microscopy in a routine setting in Central Africa. Malar. J. 2014, 14, 234. [Google Scholar]
- World Health Organization. In vitro Micro-Test (Mark III) for the Assessment of the Response of Plasmodium falciparum to Chloroquine, Mefloquine, Quinine, Amodiaquine, Sulfadoxine/Pyrimethamine and Artemisinin. WHO. CTD/MAL/97.20 Rev 2. Available online: https://iris.who.int/bitstream/handle/10665/67373/a76873.pdf?sequence=1&isAllowed=y (accessed on 16 February 2025).
- Pinilla, Y.T.; Boussougou-Sambe, S.T.; Gräßle, S.; Ngossanga, B.; Doumba-Ndalembouly, A.G.; Weierich, A.; Bingoulou, G.; Malinga, E.G.; Nguiffo-Nguete, D.; Ntoumi, F.; et al. Experimental Transmission of Plasmodium malariae to Anopheles gambiae. J. Infect. Dis. 2021, 223, 522–526. [Google Scholar] [CrossRef]
- Groger, M.; Veletzky, L.; Lalremruata, A.; Cattaneo, C.; Mischlinger, J.; Zoleko-Manego, R.; Endamne, L.; Klicpera, A.; Kim, J.; Nguyen, T. Prospective Clinical Trial Assessing Species-Specific Efficacy of Artemether-Lumefantrine for the Treatment of Plasmodium malariae, Plasmodium ovale, and Mixed Plasmodium Malaria in Gabon. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Dembele, L.; Aniweh, Y.; Diallo, N.; Sogore, F.; Sangare, C.P.O.; Haidara, A.S.; Traore, A.; Diakité, S.A.S.; Diakite, M.; Campo, B.; et al. Plasmodium malariae and Plasmodium falciparum comparative susceptibility to antimalarial drugs in Mali. J. Antimicrob. Chemother. 2021, 76, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Siswantoro, H.; Russell, B.; Ratcliff, A.; Prasetyorini, B.; Chalfein, F.; Marfurt, J.; Kenangalem, E.; Wuwung, M.; Piera, K.A.; Ebsworth, E.P.; et al. In vivo and in vitro efficacy of chloroquine against Plasmodium malariae and P. ovale in Papua, Indonesia. Antimicrob. Agents Chemother. 2011, 55, 197–202. [Google Scholar] [CrossRef] [PubMed]
- van Schalkwyk, D.A.; Moon, R.W.; Duffey, M.; Leroy, D.; Sutherland, C.J. Ex Vivo susceptibility to new antimalarial agents differs among human-infecting Plasmodium species. Int. J. Parasitol. Drugs Drug Resist. 2021, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mombo-Ngoma, G.; Kleine, C.; Basra, A.; Würbel, H.; Diop, D.A.; Capan, M.; Adegnika, A.A.; Kurth, F.; Mordmüller, B.; Joanny, F.; et al. Prospective evaluation of artemether-lumefantrine for the treatment of non-falciparum and mixed-species malaria in Gabon. Malar. J. 2012, 11, 120. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mohring, F.; Manko, E.; van Schalkwyk, D.A.; Phelan, J.E.; Nolder, D.; Borrmann, S.; Adegnika, A.A.; Di Santi, S.M.; Alam, M.S.; et al. Genome sequencing of Plasmodium malariae identifies continental segregation and mutations associated with reduced pyrimethamine susceptibility. Nat Commun. 2024, 15, 10779. [Google Scholar] [CrossRef]
- Khositnithikul, R.; Tan-Ariya, P.; Mungthin, M. In vitro atovaquone/proguanil susceptibility and characterization of the cytochrome b gene of Plasmodium falciparum from different endemic regions of Thailand. Malar. J. 2008, 7, 23. [Google Scholar] [CrossRef]
- van Schalkwyk, D.A.; Pratt, S.; Nolder, D.; Stewart, L.B.; Liddy, H.; Muwanguzi-Karugaba, J.; Beshir, K.B.; Britten, D.; Victory, E.; Rogers, C.; et al. Treatment Failure in a UK Malaria Patient Harboring Genetically Variant Plasmodium falciparum From Uganda With Reduced In Vitro Susceptibility to Artemisinin and Lumefantrine. Clin. Infect. Dis. 2024, 78, 445–452. [Google Scholar] [CrossRef]
- Lingani, M.; Bonkian, L.N.; Yerbanga, I.; Kazienga, A.; Valéa, I.; Sorgho, H.; Ouédraogo, J.B.; Mens, P.F.; Schallig, H.D.F.H.; Ravinetto, R.; et al. In vivo/Ex Vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine as first-line treatment for uncomplicated falciparum malaria in children: An open label randomized controlled trial in Burkina Faso. Malar. J. 2020, 19, 8. [Google Scholar] [CrossRef]
| Isolate No. | Parasite Count, No. Parasites/µL | Plasmodium Species Microscopy | Plasmodium Species qPCR |
|---|---|---|---|
| 12 | 153 | P.m | P.m |
| 27 | 2610 | P.m-P.f | P.m-P.f |
| 148 | 8620 | P.m-P.f | P.m-P.f |
| 149 | 1013 | P.m-P.f | P.m-P.f |
| 167 | 677 | P.m | P.m |
| 268 | 831 | P.m-P.f | P.m-P.f |
| 269 | 503 | P.m-P.f | P.m-P.f |
| 325 | 1237 | P.m | P.m |
| 326 | 773 | P.m | P.m |
| 392 | 2960 | P.m | P.m |
| 589 | 677 | P.m | P.m-P.f |
| 627 | 2175 | P.m | P.m-P.f |
| 678 | 1856 | P.m-P.f | P.m-P.f |
| 742 | 2436 | P.m | P.m |
| 1037 | 803 | P.m-P.f | P.m-P.f |
| 1063 | 387 | P.m | P.m |
| 1077 | ND | P.m-P.f | ND |
| 1179 | 462 | P.m | P.m-P.f |
| 1194 | 406 | P.m | P.m |
| 1320 | 715 | P.m | P.m |
| 1351 | 358 | P.m | P.m-P.f |
| Isolate No. | CQ (nM) | AS (nM) | ATQ (nM) | LUM (nM) | Incubation Time (h) |
|---|---|---|---|---|---|
| 12 | 7.14 | 1.73 | 11.33 | 2.76 | 36 |
| 27 | 3.93 | 2.09 | 3.60 | 2.20 | 36 |
| 148 | 9.20 | 7.68 | 6.37 | 6.60 | 36 |
| 149 | 22.45 | 2.90 | 3.81 | 3.52 | 36 |
| 167 | 6.39 | 7.10 | 3.69 | 7.24 | 36 |
| 268 | 7.20 | 6.55 | 4.88 | 7.85 | 36 |
| 269 | 3.83 | 2.36 | 3.17 | 9.63 | 36 |
| 325 | 7.51 | 2.76 | 2.58 | 10.59 | 36 |
| 326 | 7.64 | 1.70 | 2.83 | 5.05 | 36 |
| 392 | 4.29 | 2.48 | 2.65 | 11.37 | 36 |
| 589 | 26.67 | 3.09 | 8.46 | 15.68 | 36 |
| 627 | 7.18 | 2.69 | 2.95 | 51.48 | 36 |
| 678 | 10.41 | 10.26 | 9.45 | 11.26 | 36 |
| 742 | 7.10 | NA | 4.72 | 7.28 | 36 |
| 1037 | 4.74 | 1.29 | 1.52 | 3.32 | 36 |
| 1063 | 8.29 | 1.90 | 2.93 | 2.77 | 36 |
| 1077 | 11.17 | 3.30 | 2.09 | NA | 45 |
| 1179 | 6.40 | 1.85 | 2.29 | NA | 13 |
| 1194 | 11.44 | 3.30 | 2.09 | NA | 24 |
| 1320 | 4.74 | 1.32 | 1.68 | 0.59 | 36 |
| 1351 | 13.60 | 3.17 | 3.11 | NA | 48 |
| NF54 | 5.81 | 3.03 | 4.44 | 3.57 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinilla, Y.T.; Hoffmann, A.; Viehweg, M.; Saison, N.; Sambe, S.T.B.; Ndalembouly, A.G.D.; Ngossanga, B.; Awamu, F.; Adegnika, A.A.; Borrmann, S. Ex Vivo Drug Susceptibility of Plasmodium malariae Isolates to Antimalarial Drugs in Gabon. Pathogens 2025, 14, 453. https://doi.org/10.3390/pathogens14050453
Pinilla YT, Hoffmann A, Viehweg M, Saison N, Sambe STB, Ndalembouly AGD, Ngossanga B, Awamu F, Adegnika AA, Borrmann S. Ex Vivo Drug Susceptibility of Plasmodium malariae Isolates to Antimalarial Drugs in Gabon. Pathogens. 2025; 14(5):453. https://doi.org/10.3390/pathogens14050453
Chicago/Turabian StylePinilla, Yudi T., Anton Hoffmann, Maxim Viehweg, Nathanaël Saison, Stravensky Terence Boussougou Sambe, Ange Gatien Doumba Ndalembouly, Barclaye Ngossanga, Florence Awamu, Ayola Akim Adegnika, and Steffen Borrmann. 2025. "Ex Vivo Drug Susceptibility of Plasmodium malariae Isolates to Antimalarial Drugs in Gabon" Pathogens 14, no. 5: 453. https://doi.org/10.3390/pathogens14050453
APA StylePinilla, Y. T., Hoffmann, A., Viehweg, M., Saison, N., Sambe, S. T. B., Ndalembouly, A. G. D., Ngossanga, B., Awamu, F., Adegnika, A. A., & Borrmann, S. (2025). Ex Vivo Drug Susceptibility of Plasmodium malariae Isolates to Antimalarial Drugs in Gabon. Pathogens, 14(5), 453. https://doi.org/10.3390/pathogens14050453







