Neglected Avian Blood Parasites (Onchocercidae and Trypanosomatidae) in Migratory Passerines of the Temperate Zone, Eastern Baltic Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Collection, Blood Fixation and Staining
2.2. DNA Extraction, PCR and Sequencing
2.3. Statistical Methods
3. Results
3.1. Prevalence of Parasites in Different Species of Birds
3.2. The Prevalence of Parasites in Spring and Autumn
3.3. The Prevalence of Parasites in Long-Distance and Short-Distance Migrants
3.4. The Prevalence of Parasites in Birds with Different Diets
3.5. The Prevalence of Parasites in Open-Nesting Birds and Birds Nesting in Nest Boxes
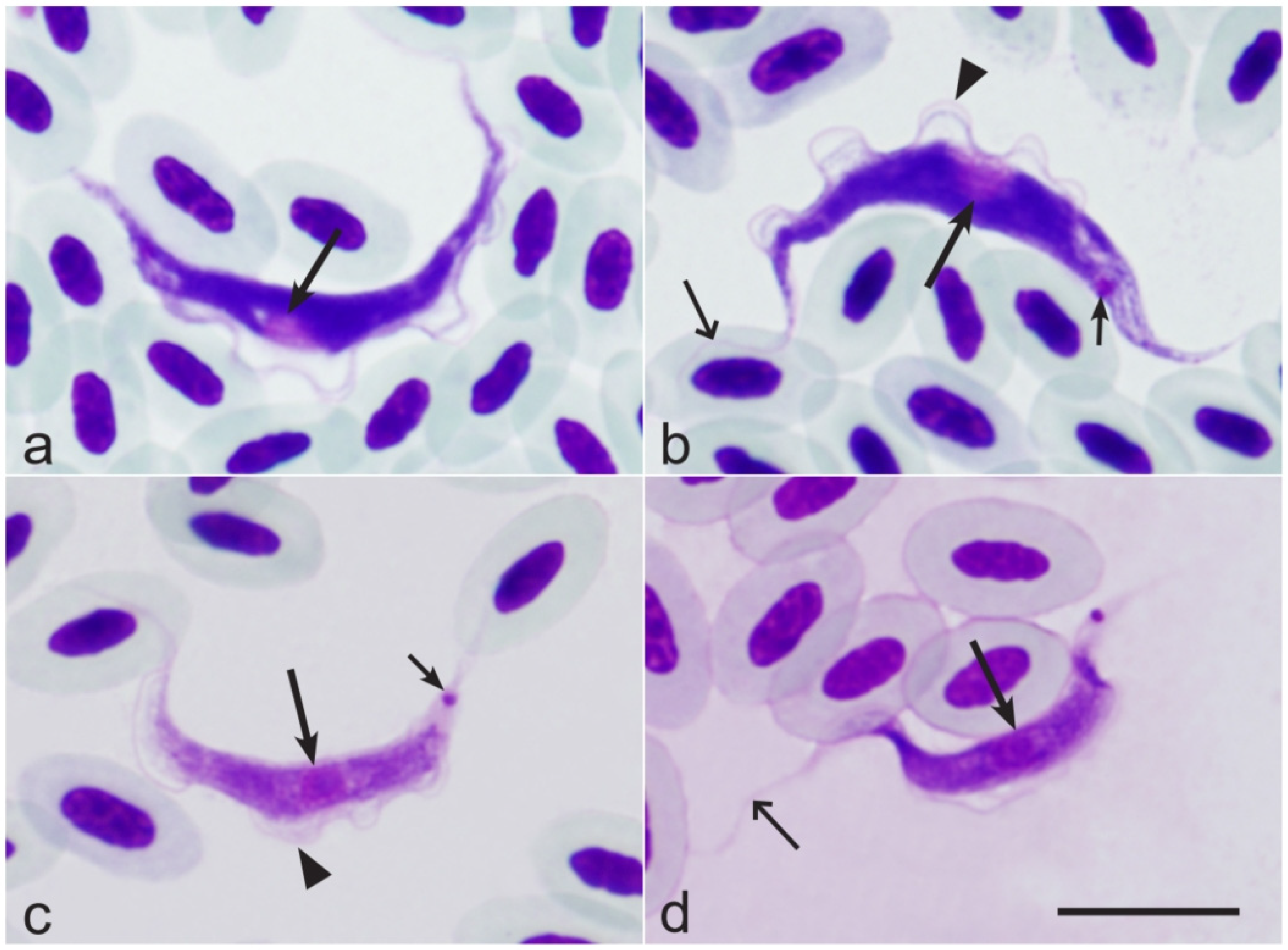
3.6. Composition of Trypanosoma Parasites
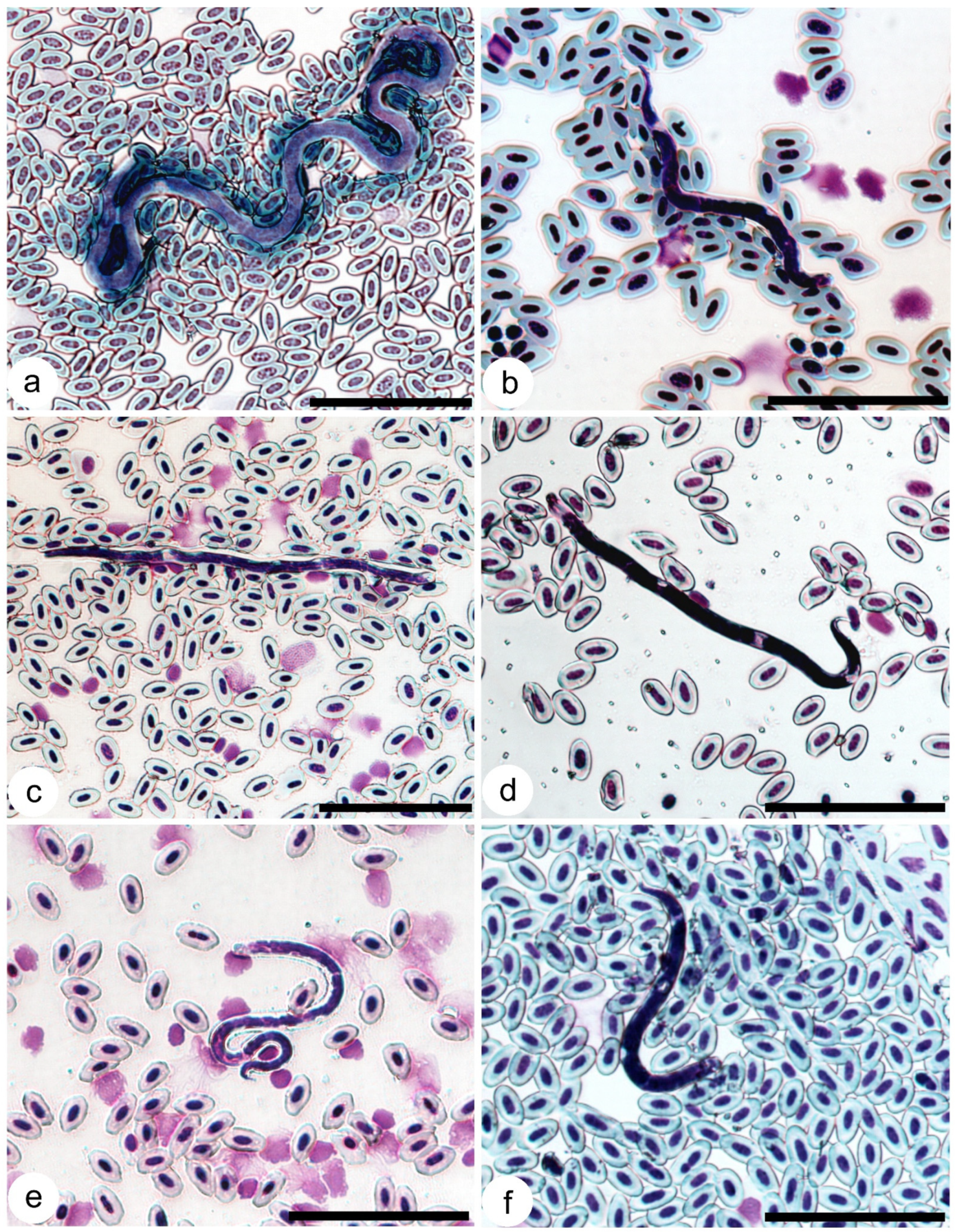
3.7. Composition of Onchocercidae Parasites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cox1 | Mitochondrial cytochrome c oxidase I |
| µm | Micrometres |
| µL | Microlitres |
| bp | Base pair |
| DNA | Deoxyribonucleic acid |
References
- Wobeser, G.A. Parasitism: Costs and Effects. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, B.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 3–12. [Google Scholar]
- Anderson, R.C.; Bain, O. Spirurida: Diplotriaenoidea, Aproctoidea and Filarioidea. In Keys to the Nematode Parasites of Vertebrates: Archival Volume, 1st ed.; Anderson, R.C., Chabaud, A.G., Willmott, S., Eds.; CABI: Wallingford, UK, 2009; pp. 391–448. [Google Scholar] [CrossRef]
- Bain, O.; Mutafchiev, Y.; Junker, K. 7.21 Order Spirurida. In Nematoda; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany; Boston, Ma, USA, 2013; Volume 2, pp. 661–732. [Google Scholar] [CrossRef]
- Bartlett, C.M. Filarioid Nematodes. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, B.D., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 439–462. [Google Scholar]
- Bishop, M.A.; Bennett, G.F. Host-parasite Catalogue of the Avian Haematozoa, Supplement 1; And, Bibliography of the Avian Blood-inhabiting Haematozoa, Supplement 2. Department of Biology, Memorial University of Newfoundland, 1992. Available online: https://books.google.lt/books?id=RG7wAAAAMAAJ (accessed on 10 January 2025).
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; CABI Pub: Wallingford, UK; New York, NY, USA, 2000. [Google Scholar]
- Atkinson, C.T. Avian malaria and the extinction of Hawaiian forest birds. In Wildlife Disease and Health in Conservation; Jessup, D.A., Radcliffe, R.W., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2023; pp. 326–347. ISBN 9781421446745. [Google Scholar]
- Gulliver, E.; Hunter, S.; Howe, L.; Castillo-Alcala, F. The Pathology of Fatal Avian Malaria Due to Plasmodium elongatum (GRW6) and Plasmodium matutinum (LINN1) Infection in New Zealand Kiwi (Apteryx spp.). Animals 2022, 12, 3376. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Zídková, L.; Cepicka, I.; Szabová, J.; Svobodová, M. Biodiversity of avian trypanosomes. Infect. Genet. Evol. 2012, 12, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, A.; Palinauskas, V.; Platonova, E.; Kobylkov, D.; Vakoliuk, I.; Valkiūnas, G. The Strategy to Survive Primary Malaria Infection: An Experimental Study on Behavioural Changes in Parasitized Birds. PLoS ONE 2016, 11, e0159216. [Google Scholar] [CrossRef]
- Bain, O.; Petit, G.; Kozek, W.J.; Chabaud, A.-G. Sur les Filaires Splendidofilariinae du genre Aproctella. Ann. Parasitol. Hum. Comp. 1981, 56, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, C.M.; Greener, E.C. A revision of Pelecitus Rail lief & Henry, 1910 (Filarioidea, Dirofilariinae) and evidence for the “capture” by mammals of filarioids from birds. Bull. Mus. Natl. Hist. Nat. Paris 1986, 4, 47–99. [Google Scholar]
- Binkienė, R.; Chagas, C.R.F.; Bernotienė, R.; Valkiūnas, G. Molecular and morphological characterization of three new species of avian Onchocercidae (Nematoda) with emphasis on circulating microfilariae. Parasit. Vectors 2021, 14, 137. [Google Scholar] [CrossRef]
- Chabaud, A.G. Chandlerella inversa n. sp., filaire parasite de Nectariniidés au Gabon. Bull. Mus. Natl. Hist. Nat. 1979, 1, 295–297. [Google Scholar] [CrossRef]
- Chabaud, A.G.; Bain, O. Trois nouvelles filaires parasites d’Oiseaux de forêts équatoriales humides africaines. Bull. Mus. natn. Hist. Nat. Paris 1990, 12, 9–18. [Google Scholar] [CrossRef]
- Chabaud, A.G.; Golvan, Y. Nouvelle Filaire parasite des Grives en France. Ann. Parasitol. Hum. Comp. 1956, 31, 405–413. [Google Scholar] [CrossRef][Green Version]
- Chagas, C.R.F.; Binkienė, R.; Valkiūnas, G. Description and molecular characterization of two species of avian blood parasites, with remarks on circadian rhythms of avian haematozoa infections. Animals 2021, 11, 3490. [Google Scholar] [CrossRef]
- Garrido-Bautista, J.; Harl, J.; Fuehrer, H.-P.; Comas, M.; Smith, S.; Penn, D.J.; Moreno-Rueda, G. Prevalence, molecular characterization, and ecological associations of filarioid helminths in a wild population of blue tits (Cyanistes caeruleus). Diversity 2023, 15, 609. [Google Scholar] [CrossRef]
- Sonin, M.D. Filariata of Animals and Man and Diseases Caused by Them. Part two: Diplotriaenoidea. In Essentials of Nematodology; Osnovy Nematodologii; Israel Program for Scientific Translations: Jerusalem, Israel, 1975; Volume 21. [Google Scholar]
- Sonin, M.D. Filariata of Animals and Man and Diseases Caused by Them; Akademia Nauk SSSR: Moscow, Russia, 1966; Volume 17. [Google Scholar]
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- Binkienė, R.; Vanstreels, R.E.T.; Duc, M.; Bernotienė, R. Description and circadian rhythms of Chandlerella sinensis Li, 1933 (Nematoda; Onchocercidae), with remarks of microfilariae effects on the host health. Parasitology 2024, 151, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A.; Sehgal, R.N.M. Deforestation does not affect the prevalence of a common trypanosome in African birds. Acta Trop. 2016, 162, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cigler, P.; Moré, G.; Bize, P.; Meier, C.M.; Frey, C.F.; Basso, W.; Keller, S. Trypanosomiasis: An emerging disease in Alpine swift (Tachymarptis melba) nestlings in Switzerland? Int. J. Parasitol. Parasites Wildl 2024, 23, 100895. [Google Scholar] [CrossRef]
- Molyneux, D.H. Trypanosoma everetti sp. Nov. A trypanosome from the black-rumped waxbill Estrilda t. Troglodytes Lichtenstein. Ann. Trop. Med. Parasitol. 1973, 67, 219–222. [Google Scholar] [CrossRef]
- Bernotienė, R.; Iezhova, T.A.; Bukauskaitė, D.; Chagas, C.R.F.; Kazak, M.; Valkiūnas, G. Development of Trypanosoma everetti in Culicoides biting midges. Acta Trop. 2020, 210, 105555. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, M.; Dolnik, O.V.; Čepička, I.; Rádrová, J. Biting midges (Ceratopogonidae) as vectors of avian trypanosomes. Parasit. Vectors 2017, 10, 224. [Google Scholar] [CrossRef]
- Bennett, G.F.; Earlé, R.A.; Squires-Parsons, D. Trypanosomes of some sub-Saharan birds. Onderstepoort J. Vet. Res. 1994, 61, 263–271. [Google Scholar]
- Votypka, J.; Svobodova, M. Trypanosoma avium: Experimental transmission from black flies to canaries. Parasitol. Res. 2004, 92, 147–151. [Google Scholar] [CrossRef]
- Genchi, C.; Rinaldi, L.; Mortarino, M.; Genchi, M.; Cringoli, G. Climate and Dirofilaria infection in Europe. Vet. Parasitol. 2009, 163, 286–292. [Google Scholar] [CrossRef]
- Koprivnikar, J.; Leung, T.L.F. Flying with diverse passengers: Greater richness of parasitic nematodes in migratory birds. Oikos 2015, 124, 399–405. [Google Scholar] [CrossRef]
- Bukauskaitė, D.; Bernotienė, R.; Iezhova, T.A.; Valkiūnas, G. Mechanisms of mortality in Culicoides biting midges due to Haemoproteus infection. Parasitology 2016, 143, 1748–1754. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Kazlauskienė, R.; Bernotienė, R.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.A. Haemoproteus infections (Haemosporida, Haemoproteidae) kill bird-biting mosquitoes. Parasitol. Res. 2014, 113, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Logminas, V. Lietuvos Fauna: Paukščiai 2; Mokslas: Vilnius, Lithuania, 1991; Volume 2. [Google Scholar]
- Spina, F.; Baillie, S.R.; Fiedler, W.; Thorup, K. The Eurasian African Bird Migration Atlas. 2025. Available online: https://migrationatlas.org (accessed on 15 March 2025).
- Rubcov, I.A. Blackflies (Simuliidae), 2nd ed.; Fauna of the USSR Diptera; Brill: Leiden, The Netherlands, 1990; Volume 6. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Binkienė, R.; Ilgūnas, M.; Iezhova, T.; Valkiūnas, G. The buffy coat method: A tool for detection of blood parasites without staining procedures. Parasit. Vectors 2020, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Kazak, M.; Bernotienė, R.; Treinys, R.; Bukauskaitė, D. Trypanosomatids in bloodsucking Diptera insects (Ceratopogonidae and Simuliidae) wild-caught at raptor bird nests in temperate forests. Diversity 2023, 15, 692. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Iezhova, T.A.; Marzec, T.; Valkiūnas, G. Trypanosoma naviformis sp. nov. (Kinetoplastidae: Trypanosomatidae) from widespread African songbirds, the Olive sunbird (Cyanomitra olivacea) and Yellow-whiskered greenbul (Andropadus latirostris). Zootaxa 2015, 4034, 342. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Iezhova, T.A.; Carlson, J.S.; Sehgal, R.N.M. Two new Trypanosoma species from African birds, with notes on the taxonomy of avian trypanosomes. J. Parasitol. 2011, 97, 924–930. [Google Scholar] [CrossRef]
- Stunžėnas, V.; Petkevičiūtė, R.; Stanevičiūtė, G. Phylogeny of Sphaerium solidum (Bivalvia) based on karyotype and sequences of 16S and ITS1 rDNA. Open Life Sci. 2011, 6, 105–117. [Google Scholar] [CrossRef]
- Petkevičiūtė, R.; Stunžėnas, V.; Stanevičiūtė, G. Differentiation of European freshwater bucephalids (Digenea: Bucephalidae) based on karyotypes and DNA sequences. Syst. Parasitol. 2014, 87, 199–212. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Jones, H.I.; Smith, T.B. Molecular evidence for host specificity of parasitic nematode microfilariae in some African rainforest birds: Microfilariae in african rainforest birds. Mol. Ecol. 2005, 14, 3977–3988. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575. [Google Scholar] [CrossRef]
- Svensson, L.; Zetterström, D.; Mullarney, K. Collins Bird Guide, 3rd ed.; HarperCollins Publishers: New York, NY, USA, 2022. [Google Scholar]
- Demongin, L. Identification guide to birds in the hand: The 301 species most frequently caught in Western Europe identification, measurements, geographical variation, moult, sex and age; Laurent Demongin: Totnes, UK, 2016. [Google Scholar]
- Votýpka, J.; Szabová, J.; Rádrová, J.; Zídková, L.; Svobodová, M. Trypanosoma culicavium sp. nov., an avian trypanosome transmitted by Culex mosquitoes. Int. J. Syst. Evo.l Microbiol. 2012, 62, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G. Immunity, resistance and tolerance in bird–parasite interactions. Parasite Immunol. 2013, 35, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Erritzøe, J. Host immune defence and migration in birds. Evol. Ecol. 1998, 12, 945–953. [Google Scholar] [CrossRef]
- Valkiūnas, G. The role of seasonal migrations in the distribution of Haemosporidia of birds in North Palaearctic. Ekologija 1993, 2, 57–67. [Google Scholar]
- Wilkerson, R.C.; Linton, Y.-M.; Strickman, D. Mosquitoes of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2021. [Google Scholar]
- Žiegytė, R.; Platonova, E.; Kinderis, E.; Mukhin, A.; Palinauskas, V.; Bernotienė, R. Culicoides biting midges involved in transmission of haemoproteids. Parasit. Vectors 2021, 14, 27. [Google Scholar] [CrossRef]
- Knott, J. A method for making microfilarial surveys on day blood. Trans. R. Soc. Trop. Med. Hyg. 1939, 33, 191–196. [Google Scholar] [CrossRef]
- Bouteille, B.; Buguet, A. The detection and treatment of human African trypanosomiasis. Res. Rep. Trop. Med. 2012, 3, 35–45. [Google Scholar] [CrossRef]
- Mumba Ngoyi, D.; Ekangu, R.A.; Kodi, M.F.M.; Pyana, P.P.; Balharbi, F.; Decq, M.; Betu, V.K.; Van der Veken, W.; Sese, C.; Menten, J.; et al. Performance of parasitological and molecular techniques for the diagnosis and surveillance of Gambiense sleeping sickness. PLoS Negl. Trop. Dis. 2014, 8, e2954. [Google Scholar] [CrossRef]
- Marcos, R.; Pereira, C.; Santos, M.; Luzzago, C.; Lauzi, S.; Maia, J.P.; Faustino, A.; Puente-Payo, P. Buffy coat smear or Knott’s test: Which to choose for canine microfilaria screening in field studies? Vet. Clin. Pathol. 2016, 45, 201–205. [Google Scholar] [CrossRef]
- Noda, R.; Nagata, S. Struthiofilaria megalocephala gen. et sp.n. (Nematoda: Filarioidea) from the body cavity of an ostrich. Bull. Univ. Osaka Pref. Ser. B 1976, 28, 1–4. [Google Scholar]
- Okulewicz, A. Pasożytnicze nicienie sikor (Paridae) w Polsce, Wiad. Parazytol. 1991, 37, 491–498. [Google Scholar]
- Haas, M.; Baruš, V.; Benedikt, V.; Literák, I. Microfilariae in birds in the Czech Republic, including a note on adult nematodes Eufilaria delicata in a song thrush Turdus philomelos. Parasitol. Res. 2011, 109, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Šupić, J.; Alić, A.Š.; Hasanić, M.; Goletić, Š.; Duscher, G.G.; Hodžić, A.; Alić, A. Eulimdana clava (Nematoda: Filarioidea) infection in domestic pigeons (Columba livia domestica): Molecular characterization and pathological changes. Vet. Parasitol. 2018, 251, 44–49. [Google Scholar] [CrossRef]
- Uni, S.; Udin, A.S.M.; Tan, P.E.; Rodrigues, J.; Martin, C.; Junker, K.; Agatsuma, T.; Low, V.L.; Lim, Y.; Saijuntha, W.; et al. Description and molecular characterisation of Pelecitus copsychi Uni, Mat Udin and Martin n. sp. (Nematoda: Onchocercidae) from the white-rumped shama Copsychus malabaricus (Scopoli) (Passeriformes: Muscicapidae) of Pahang, Malaysia. Curr. Res. Parasitol. Vector. Borne. Dis. 2022, 2, 100078. [Google Scholar] [CrossRef]
- Available online: https://ornitologija.lt/orni/web/phenology/steam (accessed on 15 March 2025).
| Bird Species | Spring | Autumn | ||||
|---|---|---|---|---|---|---|
| N | Mf N (P%) | Try N (P%) | N | Mf N (P%) | Try N (P%) | |
| Acrocephalus schoenobaenus (L.) | 209 | 3 (1.4) | 29 (13.9) | 33 | 0 | 0 |
| Acrocephalus scirpaceus (Hermann, 1804) | 93 | 2 (2.2) | 14 (15.1) | 50 | 0 | 2 (4.0) |
| Cyanistes caeruleus (L.) | 90 | 4 (4.4) | 15 (16.7) | 228 | 12 (5.3) | 39 (17.1) |
| Erithacus rubecula | 174 | 4 (2.3) | 12 6.9) | 261 | 4 (1.5) | 20 (7.7) |
| Fringilla coelebs L. | 138 | 6 (4.3) | 31 (22.5) | 42 | 0 | 8 (19.0) |
| Hirundo rustica (L.) | 142 | 2 (1.4) | 50 (35.2) | 133 | 0 | 0 |
| Parus major L. | 118 | 1 (0.8) | 4 (3.4) | 288 | 6 (2.1) | 12 (4.2) |
| Phoenicurus phoenicurus (L.) | 24 | 0 | 10 (41.7) | 37 | 1 (2.7) | 12 (32.4) |
| Phylloscopus collybita (Vieillot, 1817) | 32 | 1 (3.1) | 5 (15.6) | 54 | 3 (5.6) | 11 (20.4) |
| Phylloscopus trochilus (L.) | 107 | 0 | 28 (26.2) | 17 | 0 | 3 (17.6) |
| Prunella modularis (L.) | 38 | 0 | 10 (26.3) | 42 | 2 (4.8) | 18 (42.9) |
| Regulus regulus Sundevall, 1850 | 19 | 0 | 5 (26.3) | 24 | 0 | 6 (25.0) |
| Spinus spinus (L.) | 9 | 0 | 1 (11.1) | 144 | 0 | 37 (25.7) |
| Sturnus vulgaris L. | 218 | 0 | 5 (2.3) | 49 | 0 | 1 (2.0) |
| Sylvia atricapilla (L.) | 101 | 2 (2.0) | 34 (33.7) | 32 | 0 | 5 (15.6) |
| Sylvia borin (Boddaert, 1783) | 60 | 3 (5.0) | 5 (8.3) | 52 | 0 | 2 (3.8) |
| Troglodytes troglodytes (L.) | 24 | 0 | 3 (12.5) | 86 | 0 | 11 (12.8) |
| Turdus merula L. | 33 | 4 (12.1) | 8 (24.2) | 36 | 3 (8.3) | 4 (11.1) |
| Turdus philomelos Brehm, 1831 | 20 | 5 (25.0) | 0 | 78 | 12 (15.4) | 23 (29.5) |
| Total | 1649 | 37 (2.2) | 269 (16.3) | 1686 | 43 (2.6) | 214 (12.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernotienė, R.; Iezhova, T.; Eigirdas, V.; Jusys, V.; Kazak, M.; Binkienė, R. Neglected Avian Blood Parasites (Onchocercidae and Trypanosomatidae) in Migratory Passerines of the Temperate Zone, Eastern Baltic Region. Pathogens 2025, 14, 452. https://doi.org/10.3390/pathogens14050452
Bernotienė R, Iezhova T, Eigirdas V, Jusys V, Kazak M, Binkienė R. Neglected Avian Blood Parasites (Onchocercidae and Trypanosomatidae) in Migratory Passerines of the Temperate Zone, Eastern Baltic Region. Pathogens. 2025; 14(5):452. https://doi.org/10.3390/pathogens14050452
Chicago/Turabian StyleBernotienė, Rasa, Tatjana Iezhova, Vytautas Eigirdas, Vytautas Jusys, Margarita Kazak, and Rasa Binkienė. 2025. "Neglected Avian Blood Parasites (Onchocercidae and Trypanosomatidae) in Migratory Passerines of the Temperate Zone, Eastern Baltic Region" Pathogens 14, no. 5: 452. https://doi.org/10.3390/pathogens14050452
APA StyleBernotienė, R., Iezhova, T., Eigirdas, V., Jusys, V., Kazak, M., & Binkienė, R. (2025). Neglected Avian Blood Parasites (Onchocercidae and Trypanosomatidae) in Migratory Passerines of the Temperate Zone, Eastern Baltic Region. Pathogens, 14(5), 452. https://doi.org/10.3390/pathogens14050452









