Transmissible Viral Proventriculitis in Broiler Chickens from Bosnia and Herzegovina
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Nucleic Acid Extraction
2.3. CPNV Reverse Transcriptase Quantitative PCR (RT-qPCR)
2.4. Gyrovirus-Specific Multiplex Quantitative Real-Time PCR (qPCR)
2.5. IBDV Reverse Transcriptase Quantitative PCR (RT-qPCR)
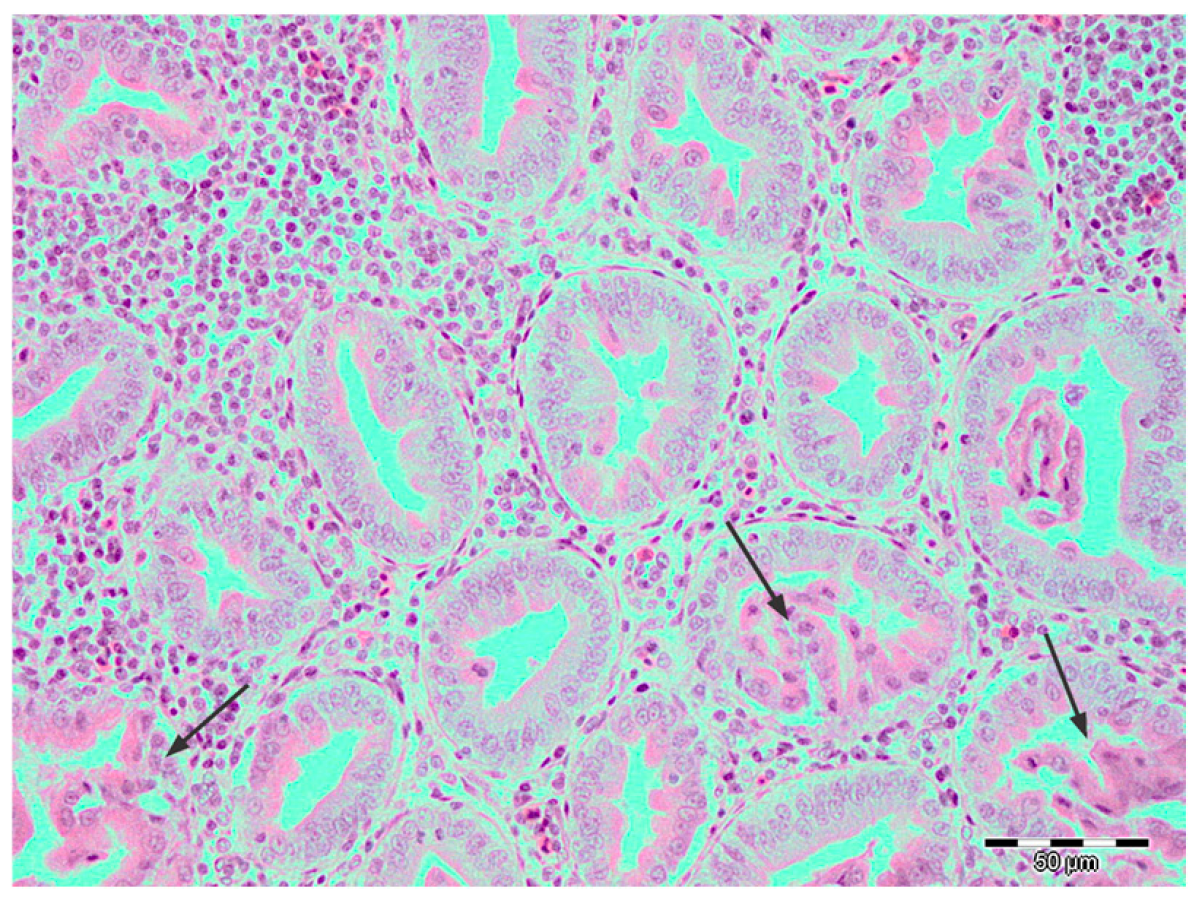
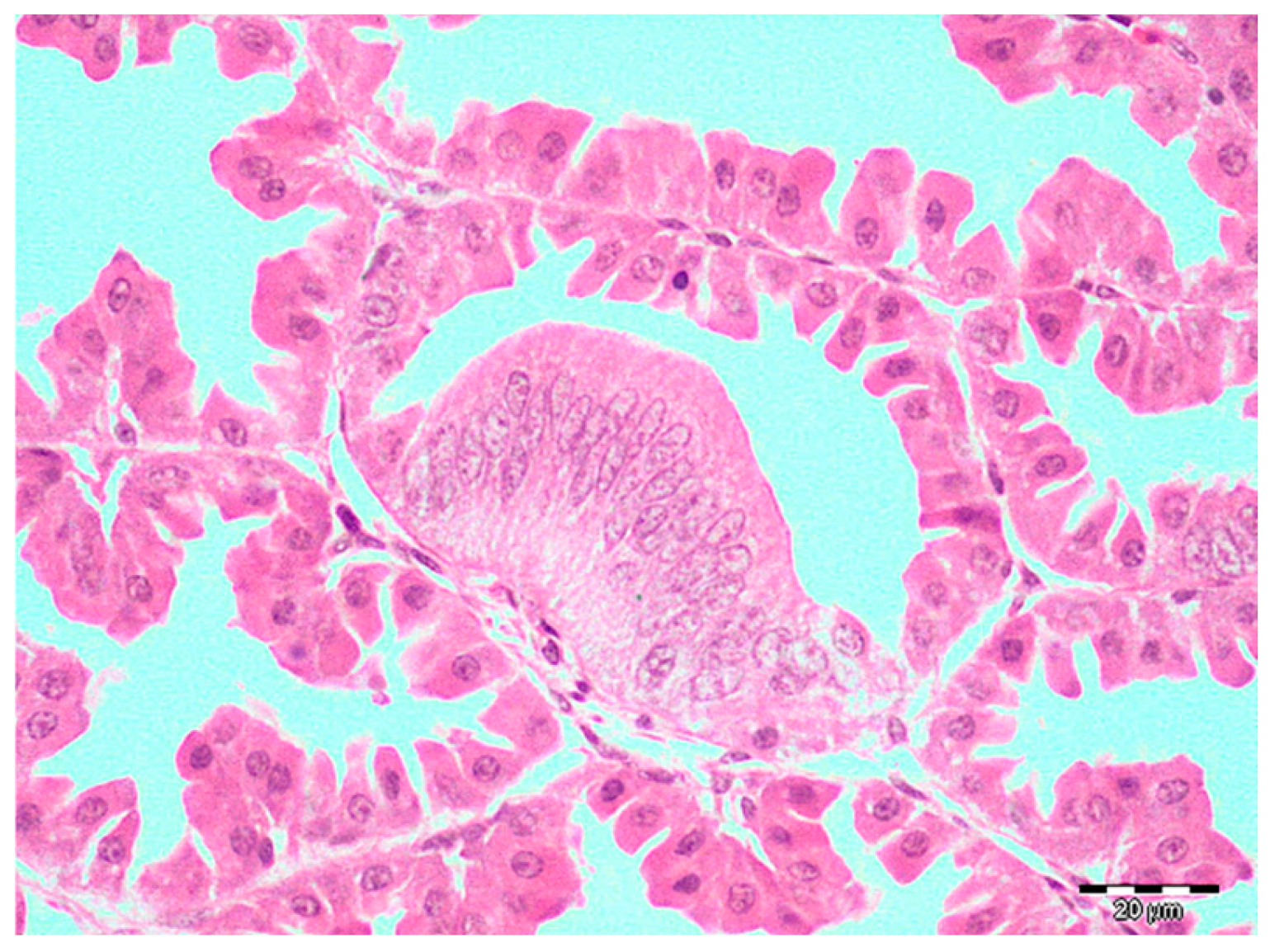
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guy, J.S.; Barnes, H.J.; Smith, L.; Owen, R.; Fuller, F.J. Partial characterization of an adenovirus-like virus isolated from broiler chickens with transmissible viral proventriculitis. Avian Dis. 2005, 49, 344–351. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Hafner, S.; Bounous, D.I.; Latimer, K.S.; Player, E.C.; Niagro, F.D.; Brown, J. Viral proventriculitis in chickens. Avian Pathol. 1996, 25, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Grau-Roma, L.; Schock, A.; Nofrarías, M.; Ali Wali, N.; de Fraga, A.P.; Garcia-Rueda, C.; de Brot, S.; Majó, N. Retrospective study on transmissible viral proventriculitis and chicken proventricular necrosis virus (CPNV) in the UK. Avian Pathol. 2020, 49, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.A.; Amaral, C.I.; Santos, W.H.; Moreira, M.V.; de Oliveira, L.B.; Costa, E.A.; Resende, M.; Wenceslau, R.; Ecco, R. Retrospective and prospective studies of transmissible viral proventriculitis in broiler chickens in Brazil. J. Vet. Diag. Invest. 2021, 33, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven, B.; Davelaar, F.G.; Van Walsum, J. Infectious proventriculitis causing runting in broilers. Avian Pathol. 1978, 7, 183–187. [Google Scholar] [CrossRef]
- Wu, Y.; Du, Y.; Zhu, W.; Liu, P.; Wang, Y.; Wang, Y.; Qu, Z.; Yang, H. A reticuloendotheliosis virus isolated from transmissible proventriculitis in chickens. Chin. J. Vet. Sci. 1999, 19, 434–436. [Google Scholar]
- Jones, R.C. Avian reovirus infections. Rev. Sci. Tech. 2000, 19, 614–625. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, Y.; Low, S.; Wang, Z.; Nam, S.J.; Liu, W.; Kwang, J. Characterization of three infectious bronchitis virus isolates from China associated with proventriculus in vaccinated chickens. Avian Dis. 2001, 45, 416–424. [Google Scholar] [CrossRef]
- Huff, G.R.; Zheng, Q.; Newberry, L.A.; Huff, W.E.; Balog, J.M.; Rath, N.C.; Kim, K.S.; Martin, E.M.; Goeke, S.C.; Skeeles, J.K. Viral and bacterial agents associated with experimental transmission of infectious proventriculitis of broiler chickens. Avian Dis. 2001, 45, 828–843. [Google Scholar] [CrossRef]
- Kim, H.R.; Yoon, S.J.; Lee, H.S.; Kwon, Y.K. Identification of a picornavirus from chickens with transmissible viral proventriculitis using metagenomic analysis. Archiv. Virol. 2015, 160, 701–709. [Google Scholar] [CrossRef]
- Guy, J.S.; West, M.A.; Fuller, F.J. Physical and genomic characteristics identify chicken proventricular necrosis virus (R11/3 virus) as a novel birnavirus. Avian Dis. 2011, 55, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yuan, S.; He, M.; Zhao, M.; Hao, X.; Song, M.; Zhang, L.; Qiao, C.; Huang, L.; Zhang, L.; et al. Emergence of gyrovirus 3 in commercial broiler chickens with transmissible viral proventriculitis. Transbound. Emerg. Dis. 2018, 65, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, G.; Zhou, D.; Yang, X.; Hu, L.; Cheng, Z. Novel cyclovirus identified in broiler chickens with transmissible viral proventriculitis in China. Front. Vet. Sci. 2020, 7, 569098. [Google Scholar] [CrossRef] [PubMed]
- Grau-Roma, L.; Reid, K.; de Brot, S.; Jennison, R.; Barrow, P.; Sánchez, R.; Nofrarías, M.; Clark, M.; Majó, N. Detection of transmissible viral proventriculitis and chicken proventricular necrosis virus in the UK. Avian Pathol. 2017, 46, 68–75. [Google Scholar] [CrossRef]
- Marguerie, J.; Leon, O.; Albaric, O.; Guy, J.S.; Guerin, J.L. Birnavirus-associated proventriculitis in French broiler chickens. Vet. Rec. 2011, 169, 394–396. [Google Scholar] [CrossRef]
- Noiva, R.; Guy, J.S.; Hauck, R.; Shivaprasad, H.L. Runting stunting syndrome associated with transmissible viral proventriculitis in broiler chickens. Avian Dis. 2015, 59, 384–387. [Google Scholar] [CrossRef]
- Śmiałek, M.; Gesek, M.; Dziewulska, D.; Niczyporuk, J.S.; Koncicki, A. Transmissible viral proventriculitis caused by chicken Proventricular necrosis virus displaying serological cross-reactivity with IBDV. Animals 2021, 11, 8. [Google Scholar] [CrossRef]
- Li, G.; Zhou, D.; Zhao, M.; Liu, Q.; Hao, X.; Yan, T.; Yuan, S.; Zhang, S.; Cheng, Z. Kinetic analysis of pathogenicity and tissue tropism of gyrovirus 3 in experimentally infected chickens. Vet. Res. 2021, 52, 120. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Q.; Bi, X.; Shi, H.; Yang, J.; Cheng, X.; Yan, T.; Zhang, H.; Cheng, Z. The synergy of chicken Anemia virus and gyrovirus homsa 1 in chickens. Viruses 2023, 15, 515. [Google Scholar] [CrossRef]
- Niu, J.T.; Yi, S.S.; Dong, G.Y.; Guo, Y.B.; Zhao, Y.L.; Huang, H.L.; Wang, K.; Hu, G.X.; Dong, H. 2019. Genomic characterization of diverse gyroviruses identified in the feces of domestic cats. Sci. Rep. 2019, 9, 13303. [Google Scholar] [CrossRef]
- Tomás, G.; Hernández, M.; Marandino, A.; Techera, C.; Grecco, S.; Hernández, D.; Banda, A.; Panzera, Y.; Pérez, R. Development of an RT-qPCR assay for the specific detection of a distinct genetic lineage of the infectious bursal disease virus. Avian Pathol. 2017, 46, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, K.K.; Zuki, A.B.Z.; Noordin, M.M.; Babjee, S.M.A. Histomorphology of the stomach, proventriculus and ventriculus of the red jungle fowl. Anatom. Histol. Embryo. 2011, 40, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.S.; West, M.A.; Fuller, F.J.; Marusak, R.A.; Shivaprasad, H.L.; Davis, J.L.; Fletcher, O.J. Detection of chicken proventricular necrosis virus (R11/3 virus) in experimental and naturally occurring cases of transmissible viral proventriculitis with the use of a reverse transcriptase-PCR procedure. Avian Dis. 2011, 55, 70–75. [Google Scholar] [CrossRef]
- Hauck, R.; Stoute, S.; Senties-Cue, C.G.; Guy, J.S.; Shivaprasad, H.L. A Retrospective Study of Transmissible Viral Proventriculitis in Broiler Chickens in California: 2000–18. Avian Dis. 2020, 64, 525–531. [Google Scholar] [CrossRef]
- Randall, C.J.; Reece, R.L. Alimentary system. In Color Atlas of Avian Histopathology; Randall, C.J., Reece, R.L., Eds.; Mosby-Wolfe: London, UK, 1996; pp. 47–74. [Google Scholar]
- Fletcher, O.J.; Abdul-Aziz, T. Alimentary System. In Avian Histopathology, 4th ed.; Tahseen, A.-A., Fletcher, O.J., Barnes, H.J., Eds.; American Association of Avian Pathologists: Jacksonville, FL, USA, 2016; pp. 354–571. [Google Scholar]
- Miller, M.A.; Lyle, L.T.; Zachary, J.F. Mechanisms and morphology of cellular injury, Adaptation, and death. In Pathologic Basis of Veterinary Disease, 7th ed.; Zachary, J.F., Ed.; Elsevier: St. Louis, MO, USA, 2022; pp. 16–74. [Google Scholar]
- Grau-Roma, L.; Marco, A.; Martínez, J.; Chaves, A.; Dolz, R.; Majó, N. Infectious bursal disease-like virus in cases of transmissible viral proventriculitis. Vet. Rec. 2010, 167, 836. [Google Scholar] [CrossRef]
- Guy, J.S.; Smith, L.G.; Evans, M.E.; Barnes, H.J. Experimental reproduction of transmissible viral proventriculitis by infection of chickens with a novel adenovirus-like virus (isolate R11/3). Avian Dis. 2007, 51, 58–65. [Google Scholar] [CrossRef]
- Yan, T.; Li, G.; Zhou, D.; Hu, L.; Hao, X.; Li, R.; Wang, G.; Cheng, Z. Long read sequencing revealed proventricular virome of broiler chicken with transmission viral proventriculitis. BMC Vet. Res. 2022, 18, 253. [Google Scholar] [CrossRef]
- Santen, V.L.V.; Joiner, K.S.; Murray, C.; Petrenko, N.; Hoerr, F.J.; Toro, H. Pathogenesis of chicken anemia virus: Comparison of the oral and the intramuscular routes of infection. Avian Dis. 2004, 48, 494–504. [Google Scholar] [CrossRef]
- Dormitorio, T.V.; Giambrone, J.J.; Hoerr, F.J. Transmissible proventriculitis in broilers. Avian Pathol. 2007, 36, 87–91. [Google Scholar] [CrossRef]
- Ragland, W.L.; Mazija, H.; Cvelić-Čabrilo, V.; Savić, V.; Novak, R.; Pogaćnik, M. Immune suppression of commercial broilers in Croatia, Slovenia, and Bosnia and Herzegovina from 1981 to 1991. Avian Pathol. 1998, 27, 200–204. [Google Scholar] [CrossRef]
- Biđin, M.; Savić, V.; Biđin, Z.; Balenović, M.; Majnarić, D. The prevalence of antibodies against chicken anemia virus in unvaccinated broilers and broiler breeders in Croatia. Vet. Arhiv. 2010, 80, 753–760. [Google Scholar]
- Smuts, H.E.M. Novel gyroviruses, including chicken anaemia virus, in clinical and chicken samples from South Africa. Adv. Virol. 2014, 2014, 321284. [Google Scholar] [CrossRef] [PubMed]
- Belova, A.V.; Smutka, L.; Rosochatecká, E. World chicken meat market–its development and current status. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 15–30. [Google Scholar] [CrossRef]
- Zenunović, A.; Glavić, M. The Situation in the Poultry Sector in Bosnia and Herzegovina. Internat. J. Adv. Agricult. Sci. Technol. 2017, 4, 1–6. [Google Scholar]

| Town/Location of the Farm | n of Sampled Broiler Chickens | Age (Days) |
|---|---|---|
| Kalesija * | 1. Floor—10 | 21 |
2. Floor—5 | 21 | |
| Kalesija | 9 | 15 |
| Gračanica | 4 | 22 |
| Stjepan Polje | 10 | 15 |
| Kalesija * | 8 | 41 |
| Tešanj | 8 | 7 |
| Tarčin | 11 | 21–22 |
| Tarčin | 6 | 34–35 |
| Gradačac | 5 | 31 |
| Gradačac | 3 | 31 |
| Gradačac | 5 | 31 |
| Gradačac | 5 | 31 |
| Gradačac | 5 | 31 |
| Brijesnica Mala, Opština Doboj istok | 1. Floor—10 | 20 |
2. Floor—12 | 16 | |
| Piskavica, Opština Gračanica | 1. Floor—5 | 23 |
2. Floor—6 | 23 | |
| Tolisa, Opština Orašje | 1. Floor—5 | 21 |
2. Floor—5 | 21 | |
| Ilijaš | 6 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervović, J.; Goletić, Š.; Šeho-Alić, A.; Prašović, S.; Goletić, T.; Alić, A. Transmissible Viral Proventriculitis in Broiler Chickens from Bosnia and Herzegovina. Pathogens 2025, 14, 438. https://doi.org/10.3390/pathogens14050438
Dervović J, Goletić Š, Šeho-Alić A, Prašović S, Goletić T, Alić A. Transmissible Viral Proventriculitis in Broiler Chickens from Bosnia and Herzegovina. Pathogens. 2025; 14(5):438. https://doi.org/10.3390/pathogens14050438
Chicago/Turabian StyleDervović, Jovana, Šejla Goletić, Alma Šeho-Alić, Senad Prašović, Teufik Goletić, and Amer Alić. 2025. "Transmissible Viral Proventriculitis in Broiler Chickens from Bosnia and Herzegovina" Pathogens 14, no. 5: 438. https://doi.org/10.3390/pathogens14050438
APA StyleDervović, J., Goletić, Š., Šeho-Alić, A., Prašović, S., Goletić, T., & Alić, A. (2025). Transmissible Viral Proventriculitis in Broiler Chickens from Bosnia and Herzegovina. Pathogens, 14(5), 438. https://doi.org/10.3390/pathogens14050438






