Occurrence of Endoparasites in Creole Goats Under an Extensive Production System on the Southern Coast of Peru
Abstract
1. Introduction
2. Materials and Methods
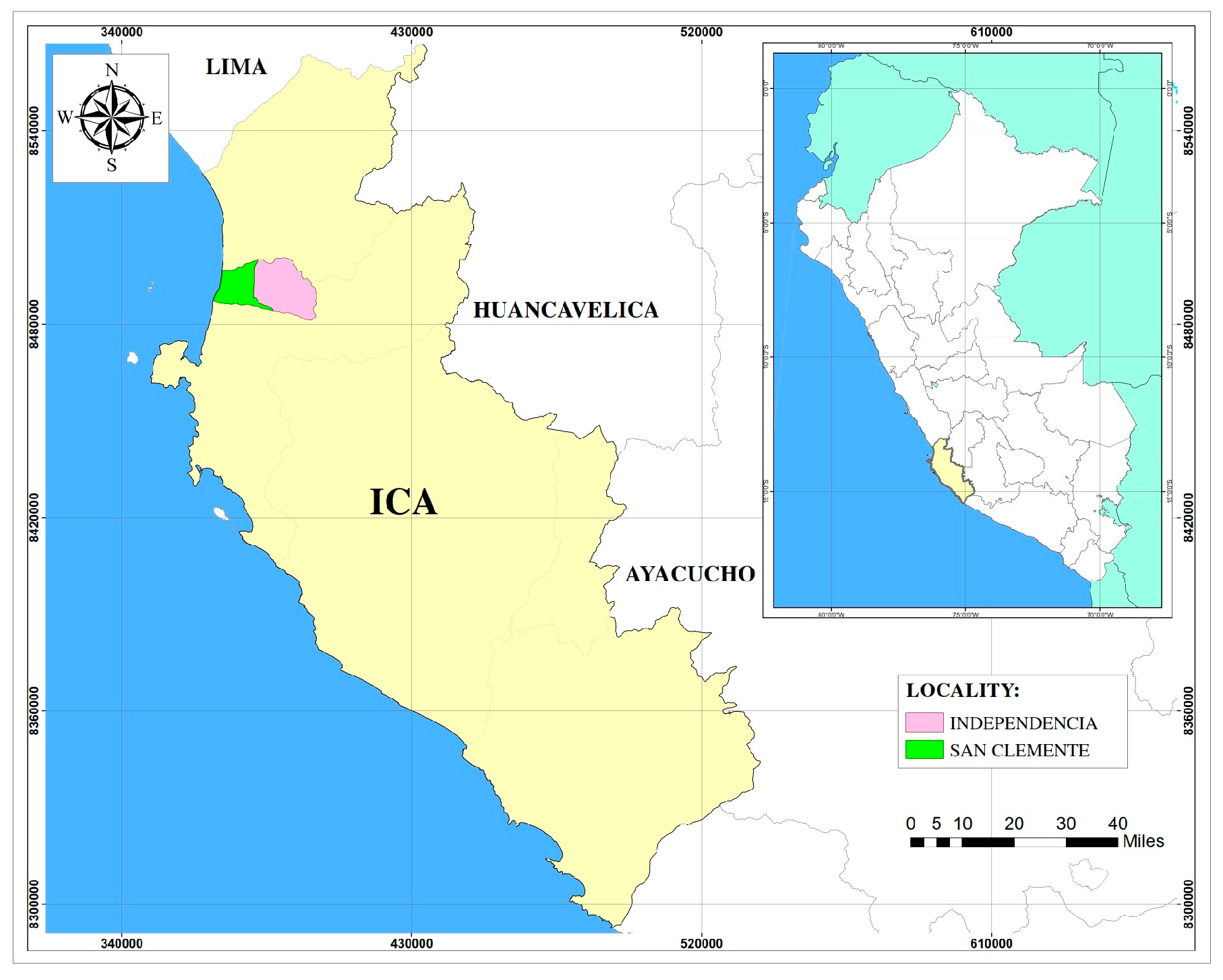
2.1. Study Area
2.2. System of Production and Management
2.3. Study Population
2.4. Sample Collection
2.5. Coprological Examination
2.6. Statistical Analysis
3. Results
3.1. Occurrence of Endoparasites
3.2. Risk Factors for Endoparasite Presence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertolini, F.; Servin, B.; Talenti, A.; Rochat, E.; Kim, E.S.; Oget, C.; Palhière, I.; Crisà, A.; Catillo, G.; Steri, R.; et al. AdaptMap Consortium: Signatures of selection and environmental adaptation across the goat genome post-domestication. Genet. Sel. Evol. 2018, 50, 57. [Google Scholar] [CrossRef]
- Ministerio de Desarrollo Agrario y Riego (MIDAGRI). Boletín Estadístico Mensual “EL AGRO EN CIFRAS”-2021, pp. 8–165. Available online: https://www.gob.pe/institucion/midagri/informes-publicaciones/1763886-boletin-estadistico-mensual-el-agro-en-cifras-2021 (accessed on 10 January 2025).
- Sessarego, E.A.; Trillo, F.C.; Godoy, D.J.; Palomino-Guerrera, W.; Cruz, J.A. Characterization and typology of goat production systems in the Southern highlands of Peru. Vet. World. 2025, 18, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Guerrera, W.; Huaman, M.R.; Flores-Prado, V.; Padilla, D.G.; Zárate-Rendón, D.A. Gastrointestinal parasites in free grazing goats from Ayacucho, Peru: Prevalence and risk factors associated with infection in herds. Trop. Anim. Health Prod. 2024, 56, 365. [Google Scholar] [CrossRef]
- Monau, P.; Raphaka, K.; Zvinorova-Chimboza, P.; Gondwe, T. Sustainable utilization of indigenous goats in Southern Africa. Diversity 2020, 12, 20. [Google Scholar] [CrossRef]
- Tumusiime, M.; Ndayisenga, F.; Ntampaka, P. Prevalence of gastrointestinal Nematodes, Cestodes, and Protozoans of goats in Nyagatare district, Rwanda. Vet. Med. 2022, 13, 339–349. [Google Scholar] [CrossRef]
- Maurizio, A.; Stancampiano, L.; Tessarin, C.; Pertile, A.; Pedrini, G.; Asti, C.; Terfa, W.; Di Regalbono, A.F.; Cassini, R. Survey on endoparasites of dairy goats in North-Eastern Italy using a Farm-tailored monitoring approach. Vet. Sci. 2021, 8, 69. [Google Scholar] [CrossRef]
- Vasilevich, F.I.; Tsepilova, I.I.; Gorchakova, V.I. Spread of endoparasites of small cattle in conditions of private farms. Russ. J. Parasitol. 2020, 14, 29–31. [Google Scholar] [CrossRef]
- Weny, G.; Okwee-Acai, J.; Okech, S.G.; Tumwine, G.; Ndyanabo, S.; Abigaba, S.; Goldberg, T.L. Prevalence and risk factors associated with Hemoparasites in cattle and goats at the edge of Kibale National Park, Western Uganda. J. Parasitol. 2017, 103, 69–74. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewics, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Ortiz-Pineda, M.C.; López-Buitrago, M.E.; Bulla-Castañeda, D.M.; Lopez-Buitrago, H.A.; Lancheros-Buitrago, D.J.; Díaz-Anaya, A.M.; Giraldo Florero, J.C.; Garcia-Corredor, D.J.; Pulido-Medellín, M.O. Diagnóstico coproparasitológico de fascioliasis en ovinos y caprinos de Boavita, Boyacá (Colombia). Rev. Investig. Vet. Perú. 2022, 33, e23340. [Google Scholar] [CrossRef]
- de Sá Guimarães, A.; Guimarães Gouveia, A.M.; do Carmo, F.B.; Gouveia, G.C.; Silva, M.X.; da Silva Vieira, L.; Molento, M.B. Management practices to control gastrointestinal parasites in dairy and beef goats in Minas Gerais; Brazil. Vet. Parasitol. 2011, 176, 265–269. [Google Scholar] [CrossRef]
- Panta, K.; Pathak, C.R.; Dahal, A.; Pandey, S.; Shrestha, R. Prevalence and risk factors of Haemonchus contortus infection in goats: Linked to FAMACHA, BCS, and biochemical markers. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 1429–1435. [Google Scholar] [CrossRef]
- Moiloa, M.; Phoofolo, M.; Matebesi-Ranthimo, P.; Molapo, S.; Phalatsi, M.; Mahlehla, M. Angora goat gastrointestinal parasite knowledge and control practices among Lesotho farming communities. Trop. Anim. Health Prod. 2020, 52, 3077–3083. [Google Scholar] [CrossRef]
- Eke, S.S.; Omalu, I.C.J.; Ochaguba, J.E.; Urama, A.C.; Hassan, S.C.; Otuu, C.A.; Okafor, I.D. Prevalence of gastrointestinal parasites of sheep and goats slaughtered in Minna modern abattoir, Niger state, Nigeria. J. Anim. Sci. Vet. Med. 2019, 4, 65–70. [Google Scholar] [CrossRef]
- Arroyo, Ó. Situación actual y proyecciones de la crianza de caprinos en el Perú. Asoc. Latinoam. Prod. Anim. 2007, 15, 290–293. [Google Scholar]
- Várady, M.; Papadopoulos, E.; Dolinská, M.; Königová, A. Anthelmintic resistance in parasites of small ruminants: Sheep versus goats. Helmin 2011, 48, 137–144. [Google Scholar] [CrossRef]
- Baudinette, E.; O’Handley, R.; Trengove, C. Anthelmintic resistance of gastrointestinal nematodes in goats: A systematic review and meta-analysis. Vet. Parasitol. 2022, 312, 109809. [Google Scholar] [CrossRef]
- Barriga, O.O. Las Enfermedades Parasitarias de los Animales Domésticos en la América Latina; Editorial Germinal: Santiago de Chile, Chile, 2002. [Google Scholar]
- Sohail, M.; Nauman-ul-Islam, M.; Shah, S.S.A.; Shah, I.A.; Raziq, A.; Khan, M.I. Incidence of gastrointestinal parasites in beetal goats at district Peshawar, Pakistan. Adv. Anim. Vet. Sci. 2017, 5, 205–207. [Google Scholar] [CrossRef]
- Urquhart, G.M.; Armour, J.; Duncan, J.L.; Dunn, A.M.; Jennings, F.W. Parasitología Veterinaria; Editorial Acribia: Zaragoza, Spain, 2001. [Google Scholar]
- Correa, S.; Correa, S.; Martínez, Y.L.; Martínez, Y.L.; López, J.L.; López, J.L.; Velásquez, L.E. Evaluación de la técnica parasitológica Dennis modificada para el diagnóstico de fasciolosis bovina. Biomédica 2016, 36, 64–68. [Google Scholar] [CrossRef]
- Rafiq, M.; Khan, N.U.; Khan, I.; Ahmad, M.; Bibi, A.; Ben Said, M.; Belkahia, H.; Tariq, M.; Saeed, S.; Abdel-Maksoud, M.A.; et al. Evaluating prevalence, risk factors, and diagnostic techniques for Cryptosporidium infection in goats and surrounding water sources. Front. Vet. Sci. 2024, 11, 1498682. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- R Core Team. Version 4.4.2. The R Project for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Tariq, K.A.; Chishti, M.Z.; Ahmad, F. Gastro-intestinal nematode infections in goats relative to season, host sex and age from the Kashmir valley, India. J. Helminthol. 2010, 84, 93–97. [Google Scholar] [CrossRef]
- La O-Arias, M.A.; Guevara-Hernández, F.; Rodríguez-Larramendi, L.A.; Reyes-Muro, L.; Nahed-Toral, J.; Mandujano-Camacho, H.O.; Pinto-Ruiz, R. Factores epizootiológicos de las estrongilosis gastrointestinales en cabras Criollas Cubanas: Bases para un manejo integrado. Rev. Mex. Cienc. Pecu. 2022, 12, 1208–1220. [Google Scholar] [CrossRef]
- Cavalcante, A.C.; Teixeira, M.; Monteiro, J.P.; Lopes, C.W. Eimeria species in dairy goats in Brazil. Vet. Parasitol. 2012, 183, 356–358. [Google Scholar] [CrossRef]
- Diao, N.-C.; Zhao, B.; Chen, Y.; Wang, Q.; Chen, Z.-Y.; Yang, Y.; Sun, Y.-H.; Shi, J.-F.; Li, J.-M.; Shi, K.; et al. Prevalence of Eimeria spp. among goats in China: A systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 2022, 12, 806085. [Google Scholar] [CrossRef]
- Mpofu, T.J.; Nephawe, K.A.; Mtileni, B. Prevalence of gastrointestinal parasites in communal goats from different agro-ecological zones of South Africa. Vet. World. 2020, 13, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, G. Fasciola. In Molecular Medical Microbiology, 3rd ed.; Tang, Y.-W., Hindiyeh, M.Y., Liu, D., Sails, A., Spearman, P., Zhang, J.-R., Eds.; Academic Press: New York, NY, USA, 2024; pp. 3249–3259. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Papadopoulos, E. Impact of parasitism in goat production. Small Rumin. Res. 2018, 163, 21–23. [Google Scholar] [CrossRef]
- Henrioud, A.N. Towards sustainable parasite control practices in livestock production with emphasis in Latin America. Vet. Parasitol. 2011, 180, 2–11. [Google Scholar] [CrossRef]
- dos Santos Oliveira, L.L.; Vieira e Silva, F.; dos Santos Lima, W.; Farias Batista, L.; de Oliveira Castro, A.L.; Rocha Júnior, V.R. Effect of climatic conditions on the occurrence of sheep endoparasites. Acta Vet. Bras. 2019, 13, 37–42. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Bombik, E.; Seremak, B.; Udała, J.; Sadowska, N. A comparison of the prevalence of the parasites of the digestive tract in goats from organic and conventional farms. Animals 2021, 11, 2581. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.S.; Motta, S.P.; dos Santos, C.C.; da Silva Moreira, A.; Evaristo, T.A.; da Rosa Farias, N.A.; Ruas, J. Ovine Eimeria infections in Southern Brazil–prevalence and risk factors. Semin. Cienc. Agrar. 2022, 43, 229–240. [Google Scholar] [CrossRef]
- Alcala-Canto, Y.; Figueroa-Castillo, J.A.; Ibarra-Velarde, F.; Vera-Montenegro, Y.; Cervantes-Valencia, M.E.; Alberti-Navarro, A. First database of the spatial distribution of Eimeria species of cattle, sheep and goats in Mexico. Parasitol. Res. 2020, 119, 1057–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhytova, O.P.; Shelyuk, Y.V.; Andreeva, O.Y.; Lehka, A.S. Ecological and parasitological characteristics of the “Fasciola hepatica L.–Lymnaea (Galba) subangulata” system. Bíol. Stud. 2022, 16, 35–46. [Google Scholar] [CrossRef]
- Martínez-Valladares, M.; Robles-Pérez, D.; Martínez-Pérez, J.M.; Cordero-Pérez, C.; del Rosario Famularo, M.; Fernández-Pato, N.; Gonzáles-Lanza, C.; Castañón-Ordóñez, L.; Rojo-Vázquez, F.A. Prevalence of gastrointestinal nematodes and Fasciola hepatica in sheep in the Northwest of Spain: Relation to climatic conditions and/or man-made environmental modifications. Parasites Vectors 2013, 6, 282. [Google Scholar] [CrossRef] [PubMed]
- Kandelous, M.M.; Kamai, T.; Vrugt, J.A.; Šimůnek, J.; Hanson, B.; Hopmans, J.W. Evaluation of subsurface drip irrigation design and management parameters for alfalfa. Agric. Water. Manag. 2012, 109, 81–93. [Google Scholar] [CrossRef]
- Dube, A.; Kalinda, C.; Manyangadze, T.; Mindu, T.; Chimbari, M.J. Effects of temperature on the life history traits of intermediate host snails of fascioliasis: A systematic review. PLoS Negl. Trop. Dis. 2023, 17, e0011812. [Google Scholar] [CrossRef]
- Chandra Deb, L.; Ahmed, S.S.U.; Baidhya, C.C.; Deb Nath, N.; Ghosh, S.; Paul, S. Prevalence of Eimeria spp. with associated risk factors in dairy calves in Sylhet, Bangladesh. Vet. Med. Sci. 2022, 8, 1250–1257. [Google Scholar] [CrossRef]
- Ntonifor, H.; Mbunkur, G.; Shei, S.; Ndaleh, N. Epidemiological studies of gastrointestinal parasitic infections in ruminants in Jakiri, Bui Division, North West Region of Cameroon. J. Vet. Med. Anim. Health 2013, 5, 344–352. [Google Scholar] [CrossRef]
- Nwosu, C.O.; Madu, P.P.; Richards, W.S. Prevalence and seasonal changes in the population of gastrointestinal nematodes of small ruminants in the semi-arid zone of North-eastern Nigeria. Vet. Parasitol. 2007, 144, 118–124. [Google Scholar] [CrossRef]
- Sarkar, V.K.; Kamalla, R.; Solanki, P.; Mehra, S.; De, U.K.; Pateer, D.P.; Saxena, H.; Sakshi. Analysis of gastrointestinal parasitic impact on goats: Insights into infection rates, hematological and biochemical changes, and oxidative stress responses. Int. J. Adv. Biochem. Res. 2024, 8, 880–885. [Google Scholar] [CrossRef]
- Challaton, K.P.; Boko, A.C.; Akouedegni, C.G.; Alowanou, G.G.; Kifouly, A.H.; Hounzangbé-Adoté, M.S. Common infectious and parasitic diseases in goats of tropical Africa and their impacts on production performance: A review. World Vet. J. 2023, 13, 425–440. [Google Scholar] [CrossRef]
- Charlier, J.; Hoste, H.; Sotiraki, S. COMBAR–Combatting anthelmintic resistance in ruminants. Parasite 2023, 30, E1. [Google Scholar] [CrossRef]
- Griffiths, H.; Carr, A.N. Potential for silvopastoral systems to control nematode burden in livestock farming in winter rainfall areas of South Australia, Australia. Int. J. Vet. Sci. Res. 2022, 8, 118–126. [Google Scholar] [CrossRef]
| Parasites | Occurrence: % (n+/n; 95% CI) | ||
|---|---|---|---|
| Independencia (n = 58) | San Clemente (n = 71) | Total (n = 129) | |
| By type | |||
| Eimeria spp. | 94.8 (55/58; 85.9–98.2) | 78.9 (56/71; 85.9–98.2) | 86.0 (110/129; 79.0–91.0) |
| Trichostrongyles | 62.1 (36/58; 49.2–73.4) | 67.6 (48/71; 56.1–77.3) | 65.1 (84/129; 56.6–72.8) |
| Skrjabinema spp. | 0.0 (0/58; 0.0–6.2) | 12.7 (9/71; 6.8–22.4) | 7.0 (9/129; 3.7–12.7) |
| Trichuris spp. | 1.7 (1/58; 0.3–9.7) | 2.8 (2/71; 0.7–9.7) | 2.3 (3/129; 0.7–6.6) |
| Fasciola hepatica | 31.0 (18/58; 20.6–43.8) | 0.0 (0/71; 0.0–5.1) | 14.0 (18/129; 9.0–21.0) |
| By infestation type | |||
| Simple | 22.4 (13/58; 13.6–34.7) | 36.6 (26/71; 26.4–48.2) | 30.2 (39/129; 23.0–38.6) |
| Eimeria spp. | 20.7(12/58; 12.3–32.8) | 21.1(15/71; 13.2–40.0) | 20.9(27/129; 14.8–28.7) |
| Trichostrongyles | 0.0 (0/58; 0.0–6.2) | 15.5(11/71; 8.9–25.7) | 8.5 (11/129; 4.8–14.6) |
| Fasciola hepatica | 1.7 (1/58; 0.3–9.1) | 0.0 (0/71; 0.0–5.1) | 0.7 (1/129; 0.1–4.2) |
| Mixed | 75.9 (44/58; 63.5–85.0) | 57.7 (41/71; 46.2–68.5) | 65.9 (85/129; 57.4–73.5) |
| Eimeria spp. + trichostrongyles | 44.8 (26/58; 32.7–57.5) | 42.3 (30/71; 31.5–53.8) | 43.4 (56/129; 35.2–52.0) |
| Eimeria spp. + Skrjabinema spp. | 0.0 (0/58; 0.0–6.2) | 4.2 (3/71; 1.4–11.7) | 2.3 (3/129; 0.8–6.6) |
| Eimeria spp. + Trichuris spp. | 0.0 (0/58; 0.0–6.2) | 1.4 (1/71; 0.2–7.6) | 0.7 (1/129; 0.1–4.2) |
| Eimeria spp. + Fasciola hepatica. | 13.8 (8/58; 7.2–24.9) | 0.0 (0/71; 0.0–5.1) | 6.2 (8/129; 3.2–11.8) |
| Trichostrongyles + Fasciola hepatica | 1.7 (1/58; 0.3–9.1) | 0.0 (0/71; 0.0–5.1) | 0.7 (1/129; 0.1–4.2) |
| Eimeria spp. + trichostrongyles + Fasciola hepatica | 13.8 (8/58; 7.2–24.9) | 0.0 (0/71; 0.0–5.1) | 6.2 (8/129; 3.2–11.8) |
| Eimeria spp. + trichostrongyles + Skrjabinema spp. | 0.0 (0/58; 0.0–6.2) | 8.4 (6/71; 3.9–17.2) | 4.7 (6/129; 2.1–9.8) |
| Eimeria spp. + trichostrongyles + Trichuris spp. | 1.7 (1/58; 0.3–9.1) | 1.4 (1/71; 0.2–7.6) | 1.6 (2/129; 0.4–5.5) |
| Factors | Eimeria spp. | Trichostrongyles | Skrjabinema spp. | Trichuris spp. | Fasciola hepatica | Mixed |
|---|---|---|---|---|---|---|
| Location | ||||||
| Independencia/San Clemente | 5.72 ** | - | 0.06 | - | 61.4 ** | 2.26 * |
| Type of feed | ||||||
| Alfalfa/alfalfa + stubble | - | 1.89 | - | - | 0.06 * | - |
| Age | ||||||
| ≤4 years/>4 years | - | - | - | 0.07 * | - | - |
| Body condition | 0.084 * | - | - | - | - | - |
| AIC | 98.17 | 168.63 | 56.19 | 27.24 | 67.57 | 164.82 |
| BIC | 106.74 | 174.35 | 70.49 | 32.96 | 76.16 | 170.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sessarego, E.; Soca-Jorge, J.; Teran, J.; Dávalos-Almeyda, M.; Valdivia-Zevallos, J.; Ruiz, J.; Cruz, J.; Cruz, D.J. Occurrence of Endoparasites in Creole Goats Under an Extensive Production System on the Southern Coast of Peru. Pathogens 2025, 14, 437. https://doi.org/10.3390/pathogens14050437
Sessarego E, Soca-Jorge J, Teran J, Dávalos-Almeyda M, Valdivia-Zevallos J, Ruiz J, Cruz J, Cruz DJ. Occurrence of Endoparasites in Creole Goats Under an Extensive Production System on the Southern Coast of Peru. Pathogens. 2025; 14(5):437. https://doi.org/10.3390/pathogens14050437
Chicago/Turabian StyleSessarego, Emmanuel, Jhony Soca-Jorge, Jose Teran, María Dávalos-Almeyda, Justo Valdivia-Zevallos, Jose Ruiz, Juancarlos Cruz, and Danny Julio Cruz. 2025. "Occurrence of Endoparasites in Creole Goats Under an Extensive Production System on the Southern Coast of Peru" Pathogens 14, no. 5: 437. https://doi.org/10.3390/pathogens14050437
APA StyleSessarego, E., Soca-Jorge, J., Teran, J., Dávalos-Almeyda, M., Valdivia-Zevallos, J., Ruiz, J., Cruz, J., & Cruz, D. J. (2025). Occurrence of Endoparasites in Creole Goats Under an Extensive Production System on the Southern Coast of Peru. Pathogens, 14(5), 437. https://doi.org/10.3390/pathogens14050437







