Multiparametric Immune Profiles and Their Potential Role in HIV-1 Disease Progression and Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. CD4+ T Cell Count and HIV-1 Viral Load
2.3. Multicolor Flow Cytometry
2.4. HIV Reservoir Quantification
2.5. Statistical Analysis
3. Results
3.1. Increased Immune Activation Level During HIV-1 Infection
3.2. Altered Distribution of Memory T Cell Subsets in HIV-1-Infected Individuals
3.3. Treg and Th17 Cell Analysis Revealed Incomplete Immune Recovery in HIV-Infected Patients
3.4. Persistent Immune Activation in T Cell Profiles Underscores the Challenges of Achieving Optimal Immune Restoration and Viral Suppression in HIV Patients
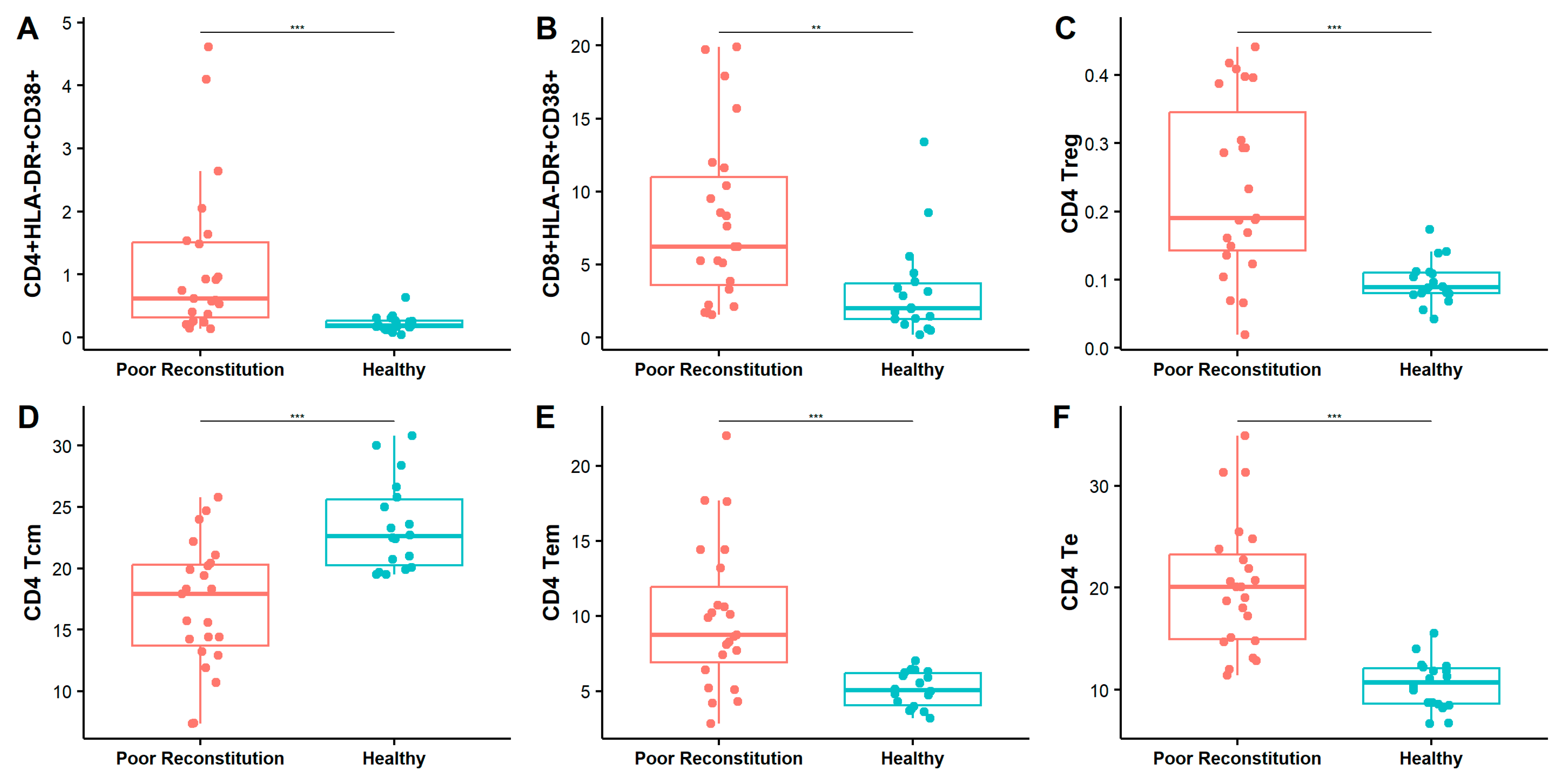
3.5. Elevated Immune Activation and Persistent Viral Replication Contribute to Poor Immune Reconstitution in HAART-Treated HIV Patients with Low CD4 Counts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bekker, L.G.; Beyrer, C.; Mgodi, N.; Lewin, S.R.; Delany-Moretlwe, S.; Taiwo, B.; Masters, M.C.; Lazarus, J.V. HIV infection. Nat. Rev. Dis. Primers 2023, 9, 42. [Google Scholar] [PubMed]
- SeyedAlinaghi, S.; Afsahi, A.M.; Moradi, A.; Parmoon, Z.; Habibi, P.; Mirzapour, P.; Dashti, M.; Ghasemzadeh, A.; Karimi, E.; Sanaati, F.; et al. Current ART, determinants for virologic failure and implications for HIV drug resistance: An umbrella review. AIDS Res. Ther. 2023, 20, 74. [Google Scholar]
- Pathela, P.; Jamison, K.; Braunstein, S.L.; Borges, C.M.; Lazar, R.; Mikati, T.; Daskalakis, D.; Blank, S. Initiating antiretroviral treatment for newly diagnosed HIV patients in sexual health clinics greatly improves timeliness of viral suppression. AIDS 2021, 35, 1805–1812. [Google Scholar]
- Ford, N.; Migone, C.; Calmy, A.; Kerschberger, B.; Kanters, S.; Nsanzimana, S.; Mills, E.J.; Meintjes, G.; Vitoria, M.; Doherty, M.; et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018, 32, 17–23. [Google Scholar] [CrossRef]
- Shahmohamadi, E.; SeyedAlinaghi, S.; Karimi, A.; Behnezhad, F.; Mehraeen, E.; Dadras, O. HIV/HTLV-1 co-infection: A systematic review of current evidence. HIV AIDS Rev. Int. J. HIV Relat. Probl. 2021, 20, 158–165. [Google Scholar]
- Wu, V.H.; Nordin, J.M.; Nguyen, S.; Joy, J.; Mampe, F.; del Rio Estrada, P.M.; Torres-Ruiz, F.; González-Navarro, M.; Luna-Villalobos, Y.A.; Ávila-Ríos, S.; et al. Profound phenotypic and epigenetic heterogeneity of the HIV-1-infected CD4+ T cell reservoir. Nat. Immunol. 2023, 24, 359–370. [Google Scholar]
- Chomont, N.; DaFonseca, S.; Vandergeeten, C.; Ancuta, P.; Sekaly, R.P. Maintenance of CD4+ T-cell memory and HIV persistence: Keeping memory, keeping HIV. Curr. Opin. HIV AIDS 2011, 6, 30–36. [Google Scholar] [PubMed]
- Salwe, S.; Singh, A.; Padwal, V.; Velhal, S.; Nagar, V.; Patil, P.; Deshpande, A.; Patel, V. Immune signatures for HIV-1 and HIV-2 induced CD4+ T cell dysregulation in an Indian cohort. BMC Infect. Dis. 2019, 19, 135. [Google Scholar]
- Mirzapour, P.; Motlagh, F.; SeyedAlinaghi, S.; Mehraeen, E. Comparison of the effectiveness of positive thinking training and acceptance and commitment therapy on quality of life and resilience of people living with HIV. HIV AIDS Rev. Int. J. HIV Relat. Probl. 2021, 21, 50–57. [Google Scholar]
- Singh, A.K.; Salwe, S.; Padwal, V.; Velhal, S.; Sutar, J.; Bhowmick, S.; Mukherjee, S.; Nagar, V.; Patil, P.; Patel, V. Delineation of homeostatic immune signatures defining viremic non-progression in HIV-1 infection. Front. Immunol. 2020, 11, 182. [Google Scholar]
- Lundgren, J.D.; Babiker, A.G.; Sharma, S.; Grund, B.; Phillips, A.N.; Matthews, G.; Kan, V.L.; Aagaard, B.; Abo, I.; Alston, B.; et al. INSIGHT Strategic Timing of AntiRetroviral Treatment (START) Study Group. Long-term benefits from early antiretroviral therapy initiation in HIV infection. NEJM Evid. 2023, 2, EVIDoa2200302. [Google Scholar]
- Baisley, K.; Orne-Gliemann, J.; Larmarange, J.; Plazy, M.; Collier, D.; Dreyer, J.; Mngomezulu, T.; Herbst, K.; Hanekom, W.; Dabis, F.; et al. Early HIV treatment and survival over six years of observation in the ANRS 12249 treatment as prevention trial. HIV Med. 2022, 23, 922–928. [Google Scholar] [PubMed]
- Mdluli, T.; Li, Y.; Pinyakorn, S.; Reeves, D.B.; Cardozo-Ojeda, E.F.; Yates, A.; Intasan, J.; Tipsuk, S.; Phanuphak, N.; Sacdalan, C.; et al. Acute HIV-1 infection viremia associate with rebound upon treatment interruption. Med 2022, 3, 622–635. [Google Scholar] [PubMed]
- Rasmussen, T.A.; Ahuja, S.K.; Kuwanda, L.; Vjecha, M.J.; Hudson, F.; Lal, L.; Rhodes, A.; Chang, J.; Palmer, S.; Auberson-Munderi, P.; et al. Antiretroviral initiation at ≥ 800 CD4+ cells/mm3 associated with lower human immunodeficiency virus reservoir size. Clin. Infect. Dis. 2022, 75, 1781–1791. [Google Scholar]
- Wang, X.; Xu, H. Residual proviral reservoirs: A high risk for HIV persistence and driving forces for viral rebound after analytical treatment interruption. Viruses 2021, 13, 335. [Google Scholar] [CrossRef]
- Aamer, H.A.; McClure, J.; Ko, D.; Maenza, J.; Collier, A.C.; Coombs, R.W.; Mullins, J.I.; Frenkel, L.M. Cells producing residual viremia during antiretroviral treatment appear to contribute to rebound viremia following interruption of treatment. PLoS Pathog. 2020, 16, e1008791. [Google Scholar]
- Sáez-Cirión, A.; Bacchus, C.; Hocqueloux, L.; Avettand-Fenoel, V.; Girault, I.; Lecuroux, C.; Potard, V.; Versmisse, P.; Melard, A.; Prazuck, T.; et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy: ANRS VISCONTI Study. PLoS Pathog. 2013, 9, e1003211. [Google Scholar]
- Salwe, S.; Padwal, V.; Nagar, V.; Patil, P.; Patel, V. T cell functionality in HIV-1, HIV-2, and dually infected individuals: Correlates of disease progression and immune restoration. Clin. Exp. Immunol. 2019, 198, 233–250. [Google Scholar]
- Deeks, S.G.; Kitchen, C.M.; Liu, L.; Guo, H.; Gascon, R.; Narváez, A.B.; Hunt, P.; Martin, J.N.; Kahn, J.O.; Levy, J.; et al. Immune activated set point during early HIV infection predicts subsequent CD4+ T cell changes independent of viral load. Blood 2004, 104, 942–947. [Google Scholar]
- Migueles, S.A.; Connors, M. Success and failure of the cellular immunity response against HIV-1. Nat. Immunol. 2015, 16, 563–570. [Google Scholar]
- Lederman, M.M.; Calabrese, L.H.; Funderburg, N.T.; Clagett, B.; Medvik, K.; Bonilla, H.; Gripshover, B.; Salata, R.A.; Taege, A.; Lisgaris, M.; et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J. Infect. Dis. 2011, 204, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Sauce, D.; Larsen, M.; Fastenackels, S.; Duperrier, A.; Keller, M.; Grubeck-Loebenstein, B.; Ferrand, C.; Debré, P.; Sidi, D.; Appay, V. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Investig. 2009, 119, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.M.; Singh, A.K.; Padwal, V.; Nagar, V.; Patil, P.; Patel, V. Monocyte-based correlates of immune activation and viremia in HIV-infected long-term non-progressors. Front Immunol. 2019, 10, 2849. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Hui, H.; Agrawal, K.; Karris, M.A.; Rana, T.M. An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerg. Microbes Infect. 2020, 9, 2333–2347. [Google Scholar] [CrossRef]
- Shete, A.; Dhayarkar, S.; Sangale, S.; Medhe, U.; Panchal, N.; Rahane, G.; Yelgate, R.; Dhamanage, A.; Gangakhedkar, R. Incomplete functional T-cell reconstitution in immunological non-responders at one year after initiation of antiretroviral therapy possibly predisposes them to infectious diseases. Int. J. Infect. Dis. 2019, 81, 114–122. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Paiardini, M.; Ceccarelli, G.; Silvestri, G.; Vullo, V. HIV-associated immune activation: From bench to bedside. AIDS Res. Hum. Retroviruses 2011, 27, 355–364. [Google Scholar] [CrossRef]
- Piconi, S.; Trabattoni, D.; Gori, A.; Parisotto, S.; Magni, C.; Meraviglia, P.; Bandera, A.; Capetti, A.; Rizzardini, G.; Clerici, M. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS 2010, 24, 1991–2000. [Google Scholar] [CrossRef]
- Mazzuti, L.; Turriziani, O.; Mezzaroma, I. The many faces of immune activation in HIV-1 infection: A multifactorial interconnection. Biomedicines 2023, 11, 159. [Google Scholar] [CrossRef]
- Mathur, P.; Kottilil, S.; Pallikkuth, S.; Frasca, D.; Ghosh, A. Persistent CD38 expression on CD8+ T lymphocytes contributes to altered mitochondrial function and chronic inflammation in people with HIV, despite ART. J. Acquir. Immune Defic. Syndr. 2022, 91, 410–418. [Google Scholar] [PubMed]
- Guo, X.Y.; Qu, M.M.; Wang, X.; Wang, Z.R.; Song, J.W.; Yang, B.P.; Guo, Y.T.; Zhang, Y.; Zhang, C.; Fan, X.; et al. Characteristics of blood immune cell profile and their correlation with disease progression in patients infected with HIV-1. BMC Infect. Dis. 2023, 23, 51. [Google Scholar]
- Munier, M.L.; Kelleher, A.D. Acutely dysregulated, chronically disabled by the enemy within: T-cell responses to HIV-1 infection. Immunol. Cell Biol. 2007, 85, 6–15. [Google Scholar]
- Kohler, S.; Wagner, U.; Pierer, M.; Kimmig, S.; Oppmann, B.; Möwes, B.; Jülke, K.; Romagnani, C.; Thiel, A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur. J. Immunol. 2005, 35, 1987–1994. [Google Scholar]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C.; et al. A human memory T cell subset with stem cell–like properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar]
- Rallón, N.; Sempere-Ortells, J.M.; Soriano, V.; Benito, J.M. Central memory CD4 T cells are associated with incomplete restoration of the CD4 T cell pool after treatment-induced long-term undetectable HIV viraemia. J. Antimicrob. Chemother. 2013, 68, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Letvin, N.L.; Mascola, J.R.; Sun, Y.; Gorgone, D.A.; Buzby, A.P.; Xu, L.; Yang, Z.Y.; Chakrabarti, B.; Rao, S.S.; Schmitz, J.E.; et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 2006, 312, 1530–1533. [Google Scholar]
- Kelley, C.F.; Kitchen, C.M.; Hunt, P.W.; Rodriguez, B.; Hecht, F.M.; Kitahata, M.; Crane, H.M.; Willig, J.; Mugavero, M.; Saag, M.; et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin. Infect. Dis. 2009, 48, 787–794. [Google Scholar] [PubMed]
- Jin, C.; Zhang, F.; Wu, L.; Xie, T.; Cheng, Y.; Tang, Z.; Wu, N. Immune activation and CD127 expression on T lymphocyte subsets of a Chinese cohort of pediatric AIDS patients with different viral responses. Curr. HIV Res. 2012, 10, 584–591. [Google Scholar]
- Simonetta, F.; Bourgeois, C. CD4+FOXP3+ regulatory T-cell subsets in human immunodeficiency virus infection. Front Immunol. 2013, 4, 215. [Google Scholar]
- Card, C.M.; McLaren, P.J.; Wachihi, C.; Kimani, J.; Plummer, F.A.; Fowke, K.R. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J. Infect. Dis. 2009, 199, 1318–1322. [Google Scholar] [PubMed]
- Rueda, C.M. Chronically HIV-1 infected patients exhibit low frequencies of CD25+ regulatory T cells. Open Virol. J. 2012, 6, 49–58. [Google Scholar]
- Hunt, P.W.; Landay, A.L.; Sinclair, E.; Martinson, J.A.; Hatano, H.; Emu, B.; Norris, P.J.; Busch, M.P.; Martin, J.N.; Brooks, C.; et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS ONE 2011, 6, e15924. [Google Scholar]
- Rueda, C.M.; Velilla, P.A.; Chougnet, C.A.; Rugeles, M.T. Incomplete normalization of regulatory T-cell frequency in the gut mucosa of Colombian HIV-infected patients receiving long-term antiretroviral treatment. PLoS ONE 2013, 8, e71062. [Google Scholar]
- Presicce, P.; Orsborn, K.; King, E.; Pratt, J.; Fichtenbaum, C.J.; Chougnet, C.A. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS ONE 2011, 6, e28118. [Google Scholar]
- Valverde-Villegas, J.M.; Matte, M.C.C.; Medeiros, R.M.; Chies, J.A. New insights about Treg and Th17 cells in HIV infection and disease progression. J. Immunol. Res. 2015, 2015, 647916. [Google Scholar]
- Yan, L.; Xu, K.; Xiao, Q.; Tuo, L.; Luo, T.; Wang, S.; Yang, R.; Zhang, F.; Yang, X. Cellular and molecular insights into incomplete immune recovery in HIV/AIDS patients. Front Immunol. 2023, 14, 1152951. [Google Scholar]
- Prendergast, A.; Prado, J.G.; Kang, Y.H.; Chen, F.; Riddell, L.A.; Luzzi, G.; Goulder, P.; Klenerman, P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 2010, 24, 491–502. [Google Scholar]
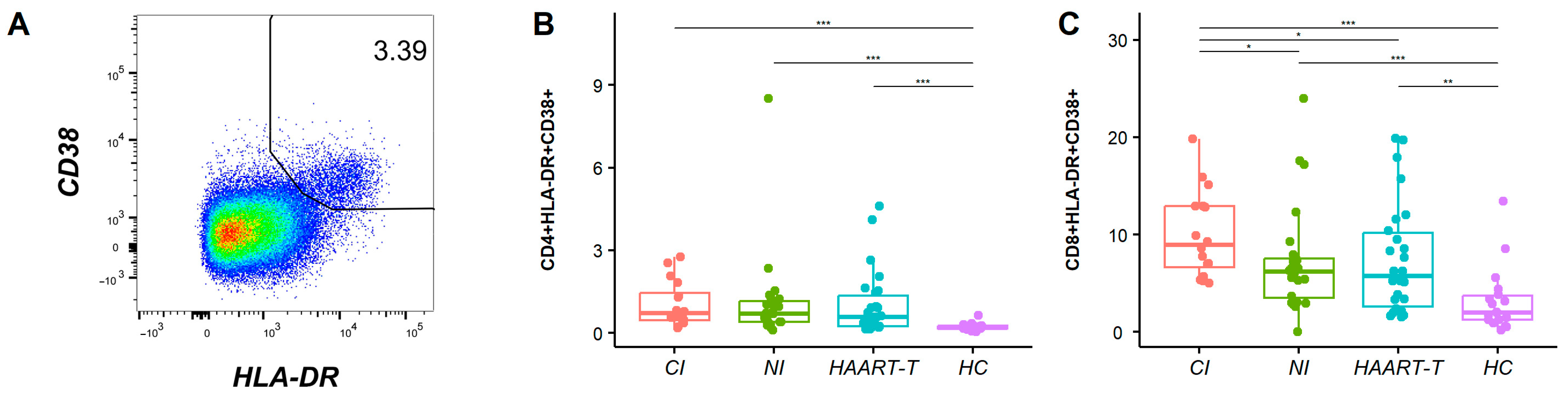
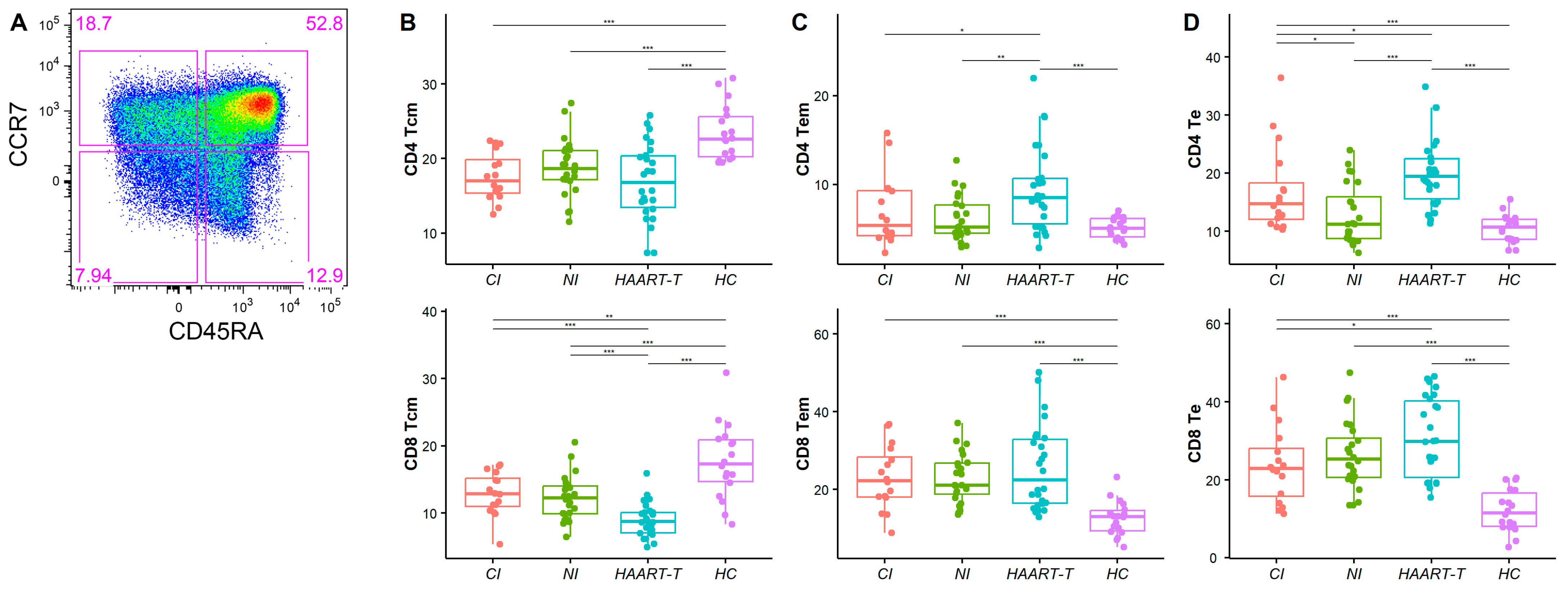
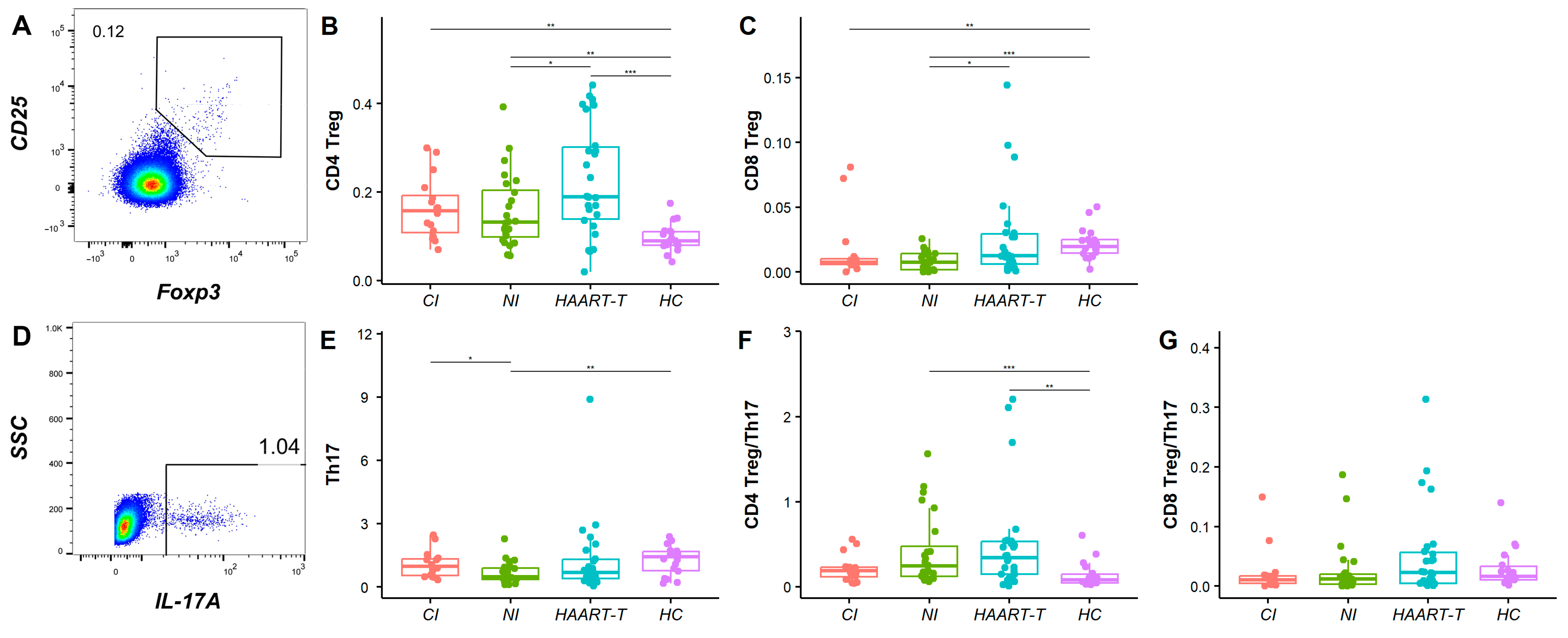
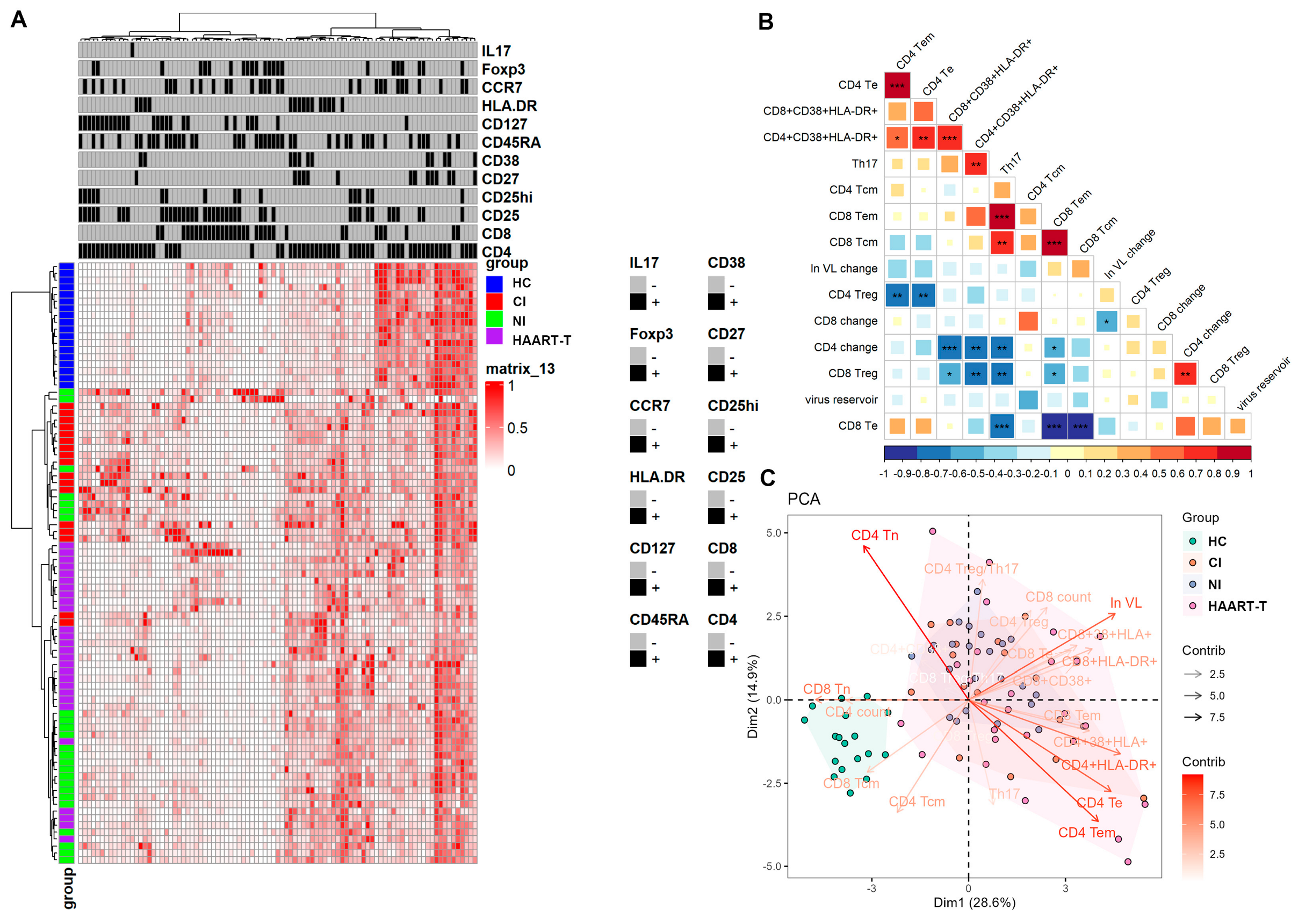
| Basic Features | Chronic Infection (CI) | Newly Infection (NI) | HAART Treated (HAART-T) | Healthy Control (HC) |
|---|---|---|---|---|
| Sample cases | 16 | 24 | 26 | 18 |
| Gender: male/female | 9/7 | 24/0 | 13/13 | 9/9 |
| Age (years, IQR) | 41 (38, 45) | 27 (24, 30) | 34 (29, 44) | 29 (27, 34) |
| Time of infection (years) | 19.3 ± 1.4 | 0.4 ± 0.2 | 6.3 ± 0.9 | - |
| CD4+ T cell count (cells/µL) | 632 ± 174 | 379 ± 164 | 355 ± 146 | 715 ± 182 |
| CD8+ T cell count (cells/µL) | 1010 ± 575 | 1082 ± 506 | 962 ± 354 | 473 ± 163 |
| CD4+/CD8+ T cell ratio | 0.59 ± 0.24 | 0.39 ± 0.21 | 0.37 ± 0.22 | 1.52 ± 0.42 |
| Viral load (HIV-1 RNA copies/mL) | 15,800 ± 119,439 | 13,700 ± 60,492 | 7150 ± 28,371 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Zhang, J.; Wang, Q.; Liu, X.; Wang, S.; Ruan, Y.; Li, D. Multiparametric Immune Profiles and Their Potential Role in HIV-1 Disease Progression and Treatment. Pathogens 2025, 14, 347. https://doi.org/10.3390/pathogens14040347
Su J, Zhang J, Wang Q, Liu X, Wang S, Ruan Y, Li D. Multiparametric Immune Profiles and Their Potential Role in HIV-1 Disease Progression and Treatment. Pathogens. 2025; 14(4):347. https://doi.org/10.3390/pathogens14040347
Chicago/Turabian StyleSu, Junwei, Junjie Zhang, Qianying Wang, Xiaojing Liu, Shuo Wang, Yuhua Ruan, and Dan Li. 2025. "Multiparametric Immune Profiles and Their Potential Role in HIV-1 Disease Progression and Treatment" Pathogens 14, no. 4: 347. https://doi.org/10.3390/pathogens14040347
APA StyleSu, J., Zhang, J., Wang, Q., Liu, X., Wang, S., Ruan, Y., & Li, D. (2025). Multiparametric Immune Profiles and Their Potential Role in HIV-1 Disease Progression and Treatment. Pathogens, 14(4), 347. https://doi.org/10.3390/pathogens14040347






