Re-Evaluation of ELISA for the Detection of Bovine Tuberculosis and a New Proposal for Its Use in Eradication Efforts on Outbreak Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diagnostic Procedures Section
2.1.1. Tuberculin Skin Test (TST)
2.1.2. Interferon-Gamma Assay (IFN-γ)
2.1.3. ELISA
2.1.4. Tissue Sample Collection and Real-Time Reverse Transcription Polymerase Chain Reaction (rRT-PCR)
2.1.5. Statistical Analyses
2.2. Experimental Design
2.2.1. Surveillance Period and Target Farms
2.2.2. Selection of the ELISA-Only-Positive Cattle in a bTB Outbreak Farm and PCR Confirmation
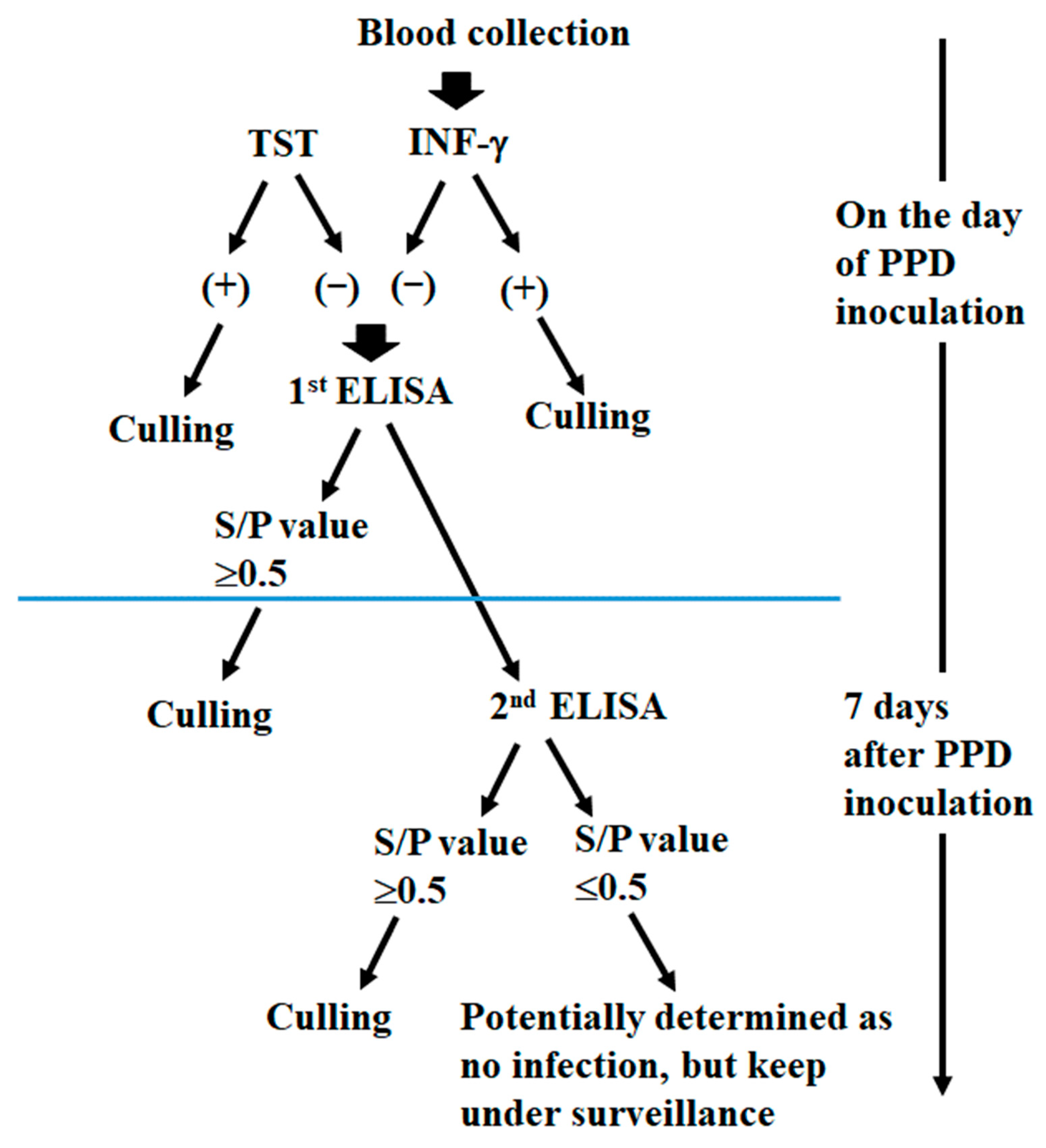
2.2.3. Change of the ELISA Results Seven Days After PPD in TST and/or IFN-γ-Positive Cattle
2.2.4. Changes in the ELISA Antibody Level Seven Days After PPD Inoculation in the Cattle Which Were All Negative for TST, IFN-γ Assay, and ELISA
2.2.5. Application of ELISA for Eradication of bTB in the Chronic Outbreak Farms
3. Results
3.1. Detection Rate and Agreement of Three Diagnostic Methods in Thirty-Two bTB Outbreak Farms
3.2. The Twelve ELISA-Only-Positive Cattle in Chronic bTB Outbreak Farms
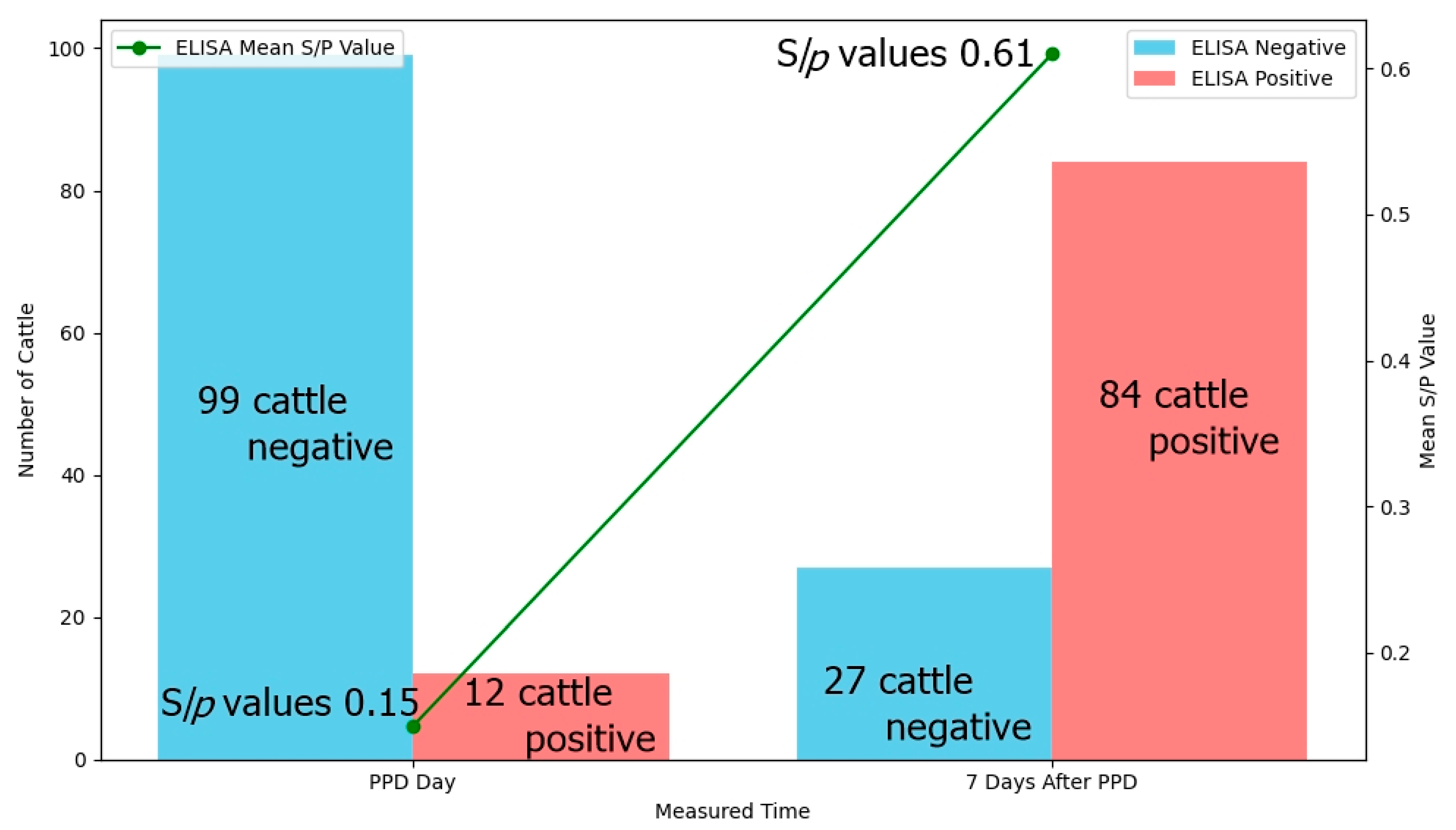
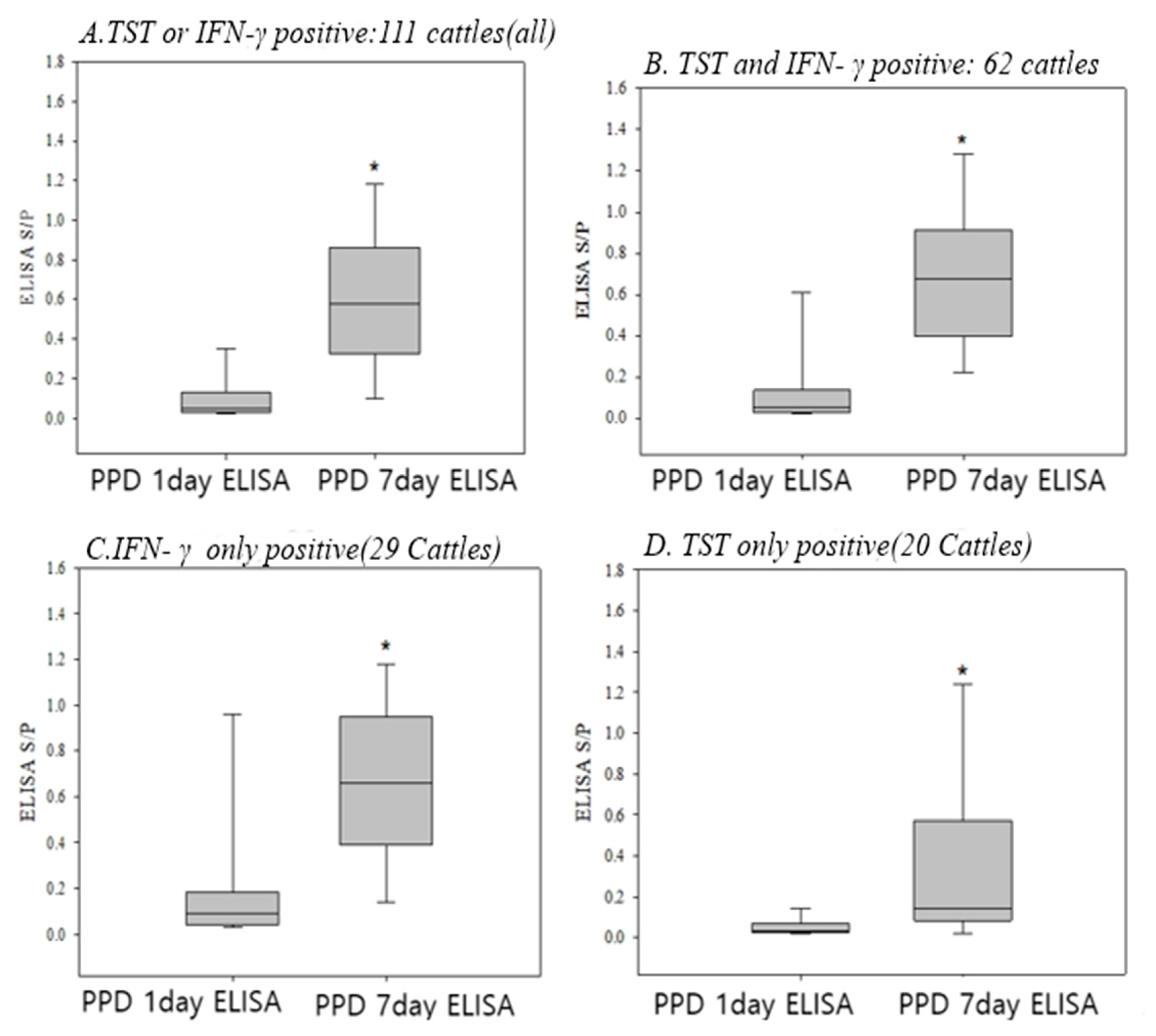
3.3. Change of the ELISA Results Seven Days After PPD in the TST and/or IFN-γ-Positive Cattle
3.4. Conversion to Positive in the ELISA Results Seven Days After PPD in All Negative Cattle for TST, IFN-γ, and ELISA
3.5. Eradication of bTB in the Outbreak Farms Using the ELISA Test Seven Days After PPD Inoculation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, J.M.; Neill, S.D. Mycobacterium bovis infection and tuberculosis in cattle. Vet. J. 2002, 163, 115–127. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE). Bovine tuberculosis. In OIE Terrestrial Manual; World Organisation for Animal Health: Paris, France, 2018; Chapter 3.4.6. [Google Scholar]
- Klepp, L.I.; Blanco, F.C.; Bigi, M.M.; Vázquez, C.L.; García, E.A.; Sabio y García, J.; Bigi, F. B Cell and Antibody Responses in Bovine Tuberculosis. Antibodies 2024, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Choi, J.S.; Kim, T.W.; Jeong, M.K.; Seo, Y.J.; Kim, Y.H.; Choi, E.J.; Yoon, S.S. Research on Risk Factors for Transmission and Bio-Security Measures Associated with Bovine Tuberculosis Breakdowns; QIA: Gimcheon-si, Republic of Korea, 2019; pp. 1442–1485. [Google Scholar]
- Waters, W.R.; Buddle, B.M.; Vordermeier, H.M.; Gormley, E.; Palmer, M.V.; Thacker, T.C.; Bannantine, J.P.; Stabel, J.R.; Linscott, R.; Martel, E.; et al. Development and evaluation of an enzyme-linked immunosorbent assay for use in the detection of bovine tuberculosis in cattle. Clin. Vaccine Immunol. 2011, 18, 1882–1888. [Google Scholar] [CrossRef]
- Plackett, P.; Ripper, J.; Corner, L.A.; Small, K.; de Witte, K.; Melville, L.; Hides, S.; Wood, P.R. An ELISA for the detection of anergic tuberculous cattle. Aust. Vet. J. 1989, 66, 15–19. [Google Scholar] [CrossRef]
- Welsh, M.D.; Cunningham, R.T.; Corbett, D.M.; Girvin, R.M.; McNair, J.; Skuce, R.A.; Bryson, D.G.; Pollock, J.M. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 2005, 114, 101–111. [Google Scholar] [CrossRef]
- Choi, K.Y.; Choi, E.S. A Comparison Study of Tuberculin, IFN-γ, and Antibody ELISA Assay for the Diagnosis of Bovin Tuberculosis. Master’s Thesis, College of Veterinary Medicine, Chonbuk National University, Cheongju, Republic of Korea, 2014. Available online: http://www.riss.kr/link?id=T13576874&outLink=K (accessed on 15 March 2023).
- Jeong, C.; Yun, G.R.; Ra, D.K.; Kim, K.M.; Lee, J.G.; Kim, K.H.; Lee, S.M. Case of bovine tuberculosis diagnosis in a slaug terhouse confirmed as PPD negative. Korean J. Vet. Serv. 2014, 37, 131–136. [Google Scholar] [CrossRef]
- Harboe, M.; Wiker, H.G.; Duncan, J.R.; Garcia, M.M.; Dukes, T.W.; Brooks, B.W.; Turcotte, C.; Nagai, S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 1990, 28, 913–921. [Google Scholar] [CrossRef]
- Harboe, M.; Nagai, S.; Wiker, H.G.; Sletten, K.; Haga, S. Homology between the MPB70 and MPB83 proteins of Mycobacterium bovis BCG. Scand. J. Immunol. 1995, 42, 46–51. [Google Scholar] [CrossRef]
- Moens, C.; Saegerman, C.; Fretin, D.; Marché, S. Field Evaluation of Two Commercial Serological Assays for Detecting Bovine Tuberculosis. Res. Vet. Sci. 2023, 159, 125–132. [Google Scholar] [CrossRef]
- Carneiro, P.A.M.; de Moura Sousa, E.; Viana, R.B.; Monteiro, B.M.; do Socorro Lima Kzam, A.; de Souza, D.C.; Coelho, A.S.; Filho, J.D.R.; Jordao, R.S.; Tavares, M.R.M.; et al. Study on Supplemental Test to Improve the Detection of Bovine Tuberculosis in Individual Animals and Herds. BMC Vet. Res. 2021, 17, 137. [Google Scholar] [CrossRef]
- Lyashchenko, K.; Whelan, A.O.; Greenwald, R.; Pollock, J.M.; Andersen, P.; Hewinson, R.G.; Vordermeier, H.M. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect. Immun. 2004, 72, 2462–2467. [Google Scholar] [CrossRef]
- Waters, W.R.; Palmer, M.V.; Stafne, M.R.; Bass, K.E.; Maggioli, M.F.; Thacker, T.C.; Linscott, R.; Lawrence, J.C.; Nelson, J.T.; Esfandiari, J.; et al. Effects of serial skin testing with purified protein derivative on the level and quality of antibodies to complex and defined antigens in Mycobacterium bovis-infected cattle. Clin. Vaccine Immunol. 2015, 22, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Waters, W.R.; Thacker, T.C.; Greenwald, R.; Esfandiari, J.; Lyashchenko, K.P. Effects of different tuberculin skin-testing regimens on gamma interferon and antibody responses in cattle experimentally infected with Mycobacterium bovis. Clin. Vaccine Immunol. 2006, 13, 377–384. [Google Scholar] [CrossRef]
- Koni, A.; Juma, A.; Morini, M.; Nardelli, S.; Connor, R.; Koleci, X. Assessment of an ELISA method to support surveillance of bovine tuberculosis in Albania. Ir. Vet. J. 2016, 69, 11. [Google Scholar] [CrossRef]
- Silva, E. Evaluation of an enzyme-linked immunosorbent assay in the diagnosis of bovine tuberculosis. Vet. Microbiol. 2001, 78, 111–117. [Google Scholar] [CrossRef] [PubMed]
- de la Rua-Domenech, R.; Goodchild, A.T.; Vordermeier, H.M.; Hewinson, R.G.; Christiansen, K.H.; Clifton-Hadley, R.S. Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 2006, 81, 190–210. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, D.S.; Lee, J.H.; Lee, C.S. A Comparative Study on the Diagnosis of ELISA Test and PPD Test of the Bovine Tuberculosis. Korean J. Vet. Serv. 2010, 33, 335–340. [Google Scholar]
- Jang, Y.H.; Kim, T.W.; Jeong, M.K.; Seo, Y.J.; Ryoo, S.; Park, C.H.; Kang, S.S.; Lee, Y.J.; Yoon, S.S.; Kim, J.M. Introduction and application of the interferon-γ assay in the national bovine tuberculosis control program in South Korea. Front. Vet. Sci. 2020, 7, 222. [Google Scholar] [CrossRef]
- Goodchild, A.V.; Clifton-Hadley, R.S. Cattle-to-cattle transmission of Mycobacterium bovis. Tuberculosis 2001, 81, 23–41. [Google Scholar] [CrossRef]
- Cho, B.J.; Chu, K.S.; Cho, Y.S.; Kang, M.S.; Lee, J.W. Epidemiological study on bovine tuberculosis outbreaks in endemic areas. Korean J. Vet. Serv. 2009, 32, 119–124. [Google Scholar]
- Chai, J.; Wang, Q.; Qin, B.; Wang, S.; Wang, Y.; Shahid, M.; Liu, K.; Zhang, Y.; Qu, W. Association of NOS2A gen polymorphisms with susceptibility to bovine tuberculosis in Chinese Holstein cattle. PLoS ONE 2021, 16, e0253339. [Google Scholar] [CrossRef]
- Kumar, R.; Gandham, S.; Rana, A.; Maity, H.K.; Sarkar, U.; Dey, B. Divergent proinflammatory immune responses associated with the differential susceptibility of cattle breeds to tuberculosis. Front. Immunol. 2023, 14, 1199092. [Google Scholar] [CrossRef]
- Raphaka, K.; Matika, O.; Sánchez-Molano, E.; Mrode, R.; Coffey, M.P.; Riggio, V.; Glass, E.J.; Woolliams, J.A.; Bishop, S.C.; Banos, G. Genomic regions underlying susceptibility to bovine tuberculosis in Holstein-Friesian cattle. BMC Genet. 2017, 18, 27. [Google Scholar] [CrossRef]
- Schiller, I.; Oesch, B.; Vordermeier, H.M.; Palmer, M.V.; Harris, B.N.; Orloski, K.A.; Buddle, B.M.; Thacker, T.C.; Lyashchenko, K.P.; Waters, W.R. Bovine tuberculosis: A review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 2010, 57, 205–220. [Google Scholar] [CrossRef]
| Group | Positivity for Bovine Tuberculosis | No. of Cattle (%) | No. of Positive Cattle for Each Method (Detection Rate, %) | ||||
|---|---|---|---|---|---|---|---|
| TST | IFN-γ | ELISA | TST | IFN-γ | ELISA | ||
| Negative | − | − | − | 2150 (91.3) | 0 | 0 | 0 |
| TST only | + | − | − | 44 (1.9) | 44 | 0 | 0 |
| IFN-γ only | − | + | − | 38 (1.6) | 0 | 38 | 0 |
| ELISA only | − | − | + | 40 (1.7) | 0 | 0 | 40 |
| TST and IFN-γ | + | + | − | 79 (3.4) | 79 | 79 | 0 |
| TST and ELISA | + | − | + | 8 (0.3) | 8 | 0 | 8 |
| IFN-γ and ELISA | − | + | + | 12 (0.5) | - | 12 | 12 |
| TST and IFN-γ and ELISA | + | + | + | 8 (0.3) | 8 | 8 | 8 |
| Total positivity 205 (8.7) * | 2355 (100) * | 123 (5.2) * | 121 (5.1) * | 52 (2.2) * | |||
| TST | ELISA | ELISA | |||||||||
| − | + | − | + | − | + | ||||||
| IFN-γ | − | 2190 | 44 | TST | − | 2188 | 44 | IFN-γ | − | 2194 | 40 |
| + | 42 | 79 | + | 115 | 8 | + | 109 | 12 | |||
| Overall agreement 96.3% | Overall agreement 93.2% | Overall agreement 93.7% | |||||||||
| Kappa value 0.628 | Kappa value 0.062 | Kappa value = 0.111 | |||||||||
| Animal ID | TST | IFN-γ | ELISA * | Range of the ELISA S/p Values | ELISA Mean S/p Value | rRT-PCR |
|---|---|---|---|---|---|---|
| H2 | − | − | + | 0.68~1.62 | 1.17 | + |
| H12 | − | − | + | 0.81~1.12 | 1.04 | + |
| H5 | − | − | + | 0.53~1.27 | 0.95 | + |
| H39 | − | − | + | 0.67~1.12 | 0.91 | + |
| H20 | − | − | + | 0.74~0.98 | 0.88 | + |
| H14 | − | − | + | 0.33~0.81 | 0.61 | + |
| H1 | − | − | + | 0.49~0.70 | 0.60 | + |
| H9 | − | − | + | 0.32~0.68 | 0.55 | + |
| H38 | − | − | + | 0.35~0.71 | 0.54 | + |
| H17 | − | − | ± | 0.36~0.67 | 0.42 | + |
| H22 | − | − | ± | 0.31~0.66 | 0.41 | + |
| H54 | − | − | ± | 0.3~0.74 | 0.39 | + |
| Total | − | − | 12 | 0.3~1.62 | 0.71 | 12 positive |
| Farm ID | Positive Cattle Age | TST Result | IFN-γ Result | ELISA S/p Value (PPD Inoculation Day) | ELISA S/p Value (Seven Days After PPD) | rRT-PCR |
|---|---|---|---|---|---|---|
| J○○ | 89 months | − | − | 0.47 | 1.11 (+) | + |
| L○○ | 48 months | − | − | 0.36 | 1.21 (+) | + |
| L○○ | 15 months | − | − | 0.49 | 0.77 (+) | + |
| Y○○ | 35 months | − | − | 0.29 | 0.51 (+) | + |
| U○○ | 69 months | − | − | 0.02 | 1.33 (+) | + |
| U○○ | 65 months | − | − | 0.01 | 0.67 (+) | + |
| K○○ | 54 months | − | − | 0.03 | 0.97 (+) | + |
| Study Period | Area | Farm ID | Breed | Herd Size | No. of bTB-Cattle | Application Protocol | No. of bTB Recurrences |
|---|---|---|---|---|---|---|---|
| until 2013.11 | HS-1 | U○○ | Hanwoo | 71 | 38 | TST only confirm (ELISA monitoring) | 8 |
| P○○ | 69 | 18 | 5 | ||||
| 2013.12~2015.11 | HC | J○○ | 74 | 3 | 3 | ||
| HC | J○ | 16 | 7 | Simultaneous 3 Test TST, IFN-γ and ELISA (seven days post-PPD) | 4 | ||
| J○○ | 34 | 22 | 4 | ||||
| HS-2 | S○○ | Dairy cow | 42 | 8 | 4 | ||
| SUM | 306 | 96 | MEAN | 4.7 | |||
| 2015.12~2017.06 | HC | J○○ | Hanwoo | 124 | 8 | Simultaneous 3 Test TST, IFN-γ and ELISA (seven days post-PPD) | 2 |
| HS-2 | B○○ | 75 | 6 | 1 | |||
| Y○○ | 66 | 4 | 1 | ||||
| U○○ | 157 | 19 | 2 | ||||
| HS-3 | L○○ | 142 | 43 | 2 | |||
| HS-4 | K○○ | 47 | 34 | 2 | |||
| SUM | 611 | 114 | MEAN | 1.7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-H.; Kim, J.; Jang, Y.-H.; Son, S.; Ryoo, S.; Kim, J.-H.; Won, S.-M.; Kim, K.-W.; Hong, S.; Jeon, B.-Y.; et al. Re-Evaluation of ELISA for the Detection of Bovine Tuberculosis and a New Proposal for Its Use in Eradication Efforts on Outbreak Farms. Pathogens 2025, 14, 331. https://doi.org/10.3390/pathogens14040331
Park C-H, Kim J, Jang Y-H, Son S, Ryoo S, Kim J-H, Won S-M, Kim K-W, Hong S, Jeon B-Y, et al. Re-Evaluation of ELISA for the Detection of Bovine Tuberculosis and a New Proposal for Its Use in Eradication Efforts on Outbreak Farms. Pathogens. 2025; 14(4):331. https://doi.org/10.3390/pathogens14040331
Chicago/Turabian StylePark, Chan-Ho, Jaemung Kim, Yun-Ho Jang, Sehyun Son, Sungweon Ryoo, Jung-Ho Kim, Sang-Min Won, Kyu-Wook Kim, Sungwon Hong, Bo-Young Jeon, and et al. 2025. "Re-Evaluation of ELISA for the Detection of Bovine Tuberculosis and a New Proposal for Its Use in Eradication Efforts on Outbreak Farms" Pathogens 14, no. 4: 331. https://doi.org/10.3390/pathogens14040331
APA StylePark, C.-H., Kim, J., Jang, Y.-H., Son, S., Ryoo, S., Kim, J.-H., Won, S.-M., Kim, K.-W., Hong, S., Jeon, B.-Y., Pak, S.-I., & Yoon, B.-I. (2025). Re-Evaluation of ELISA for the Detection of Bovine Tuberculosis and a New Proposal for Its Use in Eradication Efforts on Outbreak Farms. Pathogens, 14(4), 331. https://doi.org/10.3390/pathogens14040331







