Abstract
Candida auris has emerged as a multidrug-resistant yeast implicated in healthcare-associated invasive infections and hospital outbreaks. The aim of the current 38-month period observational study in a multidisciplinary Intensive Care Unit (ICU) was to analyze the epidemiology, potential risk factors, management strategies, and patient outcomes of patients with C. auris. During the study period, 32 patients were identified with C. auris infection (6 patients) or colonization (26 patients) and their clinical characteristics and treatment-related factors were compared. Identification of C. auris isolates was confirmed by MALDI-TOF spectrometry. According to our results, regarding patient-related factors, no significant differences were identified. Regarding treatment-related factors, the proportion of patients already receiving corticosteroids (34.6% vs. 83.3%, p = 0.064) or being on renal replacement treatment (7.7% vs. 33.3%) was higher in infected patients. Median time elapsed from ICU admission to first positive culture was 7 (1–21) days and half of cases were ICU-imported. All strains were resistant to fluconazole and susceptible to echinocandines and amphotericin B. Crude mortality of the study population was 43.75%, similar to other previously reported candidemias. Rapid identification of C. auris, continued surveillance, and infection control practices are important elements for controlling successfully its spread in the hospital setting and for establishing promptly its transition from commensalism to infection.
1. Introduction
Candida auris is a multidrug-resistant fungal pathogen that has emerged as a global threat in the last decade. Since its first report in 2009, after being isolated from the external ear canal discharge of a patient in Japan [1], it has been implicated in nosocomial outbreaks worldwide, often in Intensive Care Units (ICUs) [2,3]. Based on whole-genome sequencing, six distinct clades were classified by region of independent emergence: Clade I (South Asian), Clade II (East Asian), Clade III (African), Clade IV (South American), Clade V (Iranian), and Clade VI (Bangladesh-Singapore) [4,5]. These clades depict different genetic determinants of resistance and resulting antifungal resistance profiles. Although specific clades of C. auris continue to dominate the parts of the world where they originated, transmission in other areas has been reported, for example, in China, where three different genetic clades (I, II, III) have been identified in 18 hospitals during 2018–2023 [6,7,8].
The earliest C. auris isolate was uncovered in South Korea in 1996, as a misidentified isolate from a bloodstream infection in a pediatric surgery patient [9], whereas a 2008 isolate from Pakistan was also recognized [10]. A reanalysis of 20,788 Candida spp. isolates collected from four continents by the SENTRY Antifungal Surveillance Program between 1997 and 2016, did not find C. auris isolates before 2009, and only six misidentified isolates between 2009 and 2016 [11]. These data support the emergence of C. auris as a relatively recent clinical problem rather than its misidentification previously, due to a reliance on conventional phenotypic methods.
C. auris displays characteristics, such as multidrug resistance, that are evocative of other yeasts (e.g., Yarrowia lypolitica, Stephanoascus ciferrii complex, and Candida blankie) or bacteria, as well as exceptional adaptation to the nosocomial environment and spread between patients and hospitals [12,13,14,15,16]. C. auris’ ability to persist on healthcare surfaces and medical equipment, along with its propensity for persistent skin colonization, may account for its high transmissibility [7,17,18]. Consequently, preventing the spread of C. auris depends on prompt and accurate identification of cases and the implementation of infection control precautions. Unfortunately, the identification of C. auris remains challenging since the most common diagnostic platforms available in clinical and public health laboratories (e.g., VITEK 2) often misidentify C. auris [19,20,21,22]. Accurate identification can be accomplished with matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry [19], ribosomal DNA sequencing [4], polymerase chain reaction [23,24], and T2 Magnetic Resonance assay [25].
C. auris’ high-level resistance to antifungals complicates the successful management of their corresponding infections. Further concern causes the lack of C. auris-specific interpretative breakpoints, yet to be reported by both the Clinical Laboratory Standards Institute (CLSI) and the European Committee for Antimicrobial Susceptibility Testing (EUCAST). However, tentative interpretative criteria have been proposed by the U.S.A. Centers for Disease Control and Prevention (CDC) [26]. Based on these breakpoints, most isolates are resistant to fluconazole (90%) and exhibit variable susceptibility to amphotericin B (30%) and echinocandines (5%). Multidrug resistance has been reported in 41% of the strains and pan-resistance in 3–4% [12,27], with clade-specific variations [6,12].
In Greece, the first report of C. auris in 2019 involved a sporadic case of a 20-year-old male cystic fibrosis patient who presented with respiratory exacerbation [28]. Thereafter, the isolation of C. auris strains exhibited exponential growth and involved several hospitals within various prefectures. The aim of the current 38-month period observational study in a multidisciplinary ICU was to analyze the epidemiology, potential risk factors, management strategies, and patient outcomes of patients with C. auris.
2. Materials and Methods
2.1. Setting
Konstantopouleio-Patission is a 330-bed tertiary care hospital in Athens, Greece, which includes a 9-bed multidisciplinary ICU, internal medicine, cardiology, surgical, urology, orthopaedical, and other wards. In this retrospective observational study, we studied the patients who were colonized or infected with C. auris between 10 November 2020 and 31 December 2023. The ICU was converted to a COVID-19 unit on 25 February 2021, during the third wave of COVID-19 in Greece, and operated in this way for 15 consecutive months.
2.2. Definitions of Cases and Collection of Clinical Data
A case was defined as any patient hospitalized in the ICU and colonized or infected with C. auris during routine surveillance or targeted cultures from various clinical specimens. Colonization was defined as the presence of C. auris in samples obtained from urine, tracheal aspirates, and swab specimens from the skin and rectum. Infection was defined as the growth of C. auris in blood culture or a sample obtained from a sterile site, along with compatible clinical signs of infection. Patients underwent screening on admission to the ICU and then on a weekly basis. Screening involved culture swab specimens from the axilla, groin, and rectum, as well as urine and tracheal aspirates cultures. Blood cultures were aspirated based on clinical criteria of sepsis [29]. When multiple specimens of a patient showed growth of C. auris, only the specimen which was identified as a source of infection was considered. When C. auris was isolated from multiple cultures of a single patient, only the first isolate was included in the study.
Clinical and epidemiological data of ICU patients were reviewed, including gender, age, initial diagnosis, surgical procedures, disease severity at ICU admission as determined by an Acute Physiology Chronic Health Evaluation (APACHE II) score, comorbidities, previous treatment with antibiotics and antifungals, outcome and length of stay (LOS) in the ICU, and outcome on the 28th day after ICU discharge.
At the time of C. auris isolation, we recorded the length of prior hospitalization (in a general ward or ICU), previous or current receipt of antifungals, administration of glucocorticosteroids and application of a central venous catheter (CVC), mechanical ventilation, and renal replacement therapy.
We compared patients’ clinical characteristics and treatment-related factors among patients who were colonized and those infected, in order to determine potential predictors of a C. auris infection.
2.3. Environmental Sampling
The Infectious Disease Control Committee of the hospital was informed whenever a new case of C. auris isolation was identified. Environmental screening was performed when the first cluster of three patients occurred (during August–September 2021). This screening focused on the sampling of high-touch surfaces, such as beds and side tables, and also yielded this pathogen.
2.4. Identification of Isolates and Antifungal Susceptibility Testing
Yeasts from various clinical samples were identified and antifungal susceptibility testing was performed by the VITEK 2 Compact 15 automated system (Biomerieux, Marcy l’ Etoile, France) on a routine basis at the hospital clinical laboratory. Yeasts were grown on Sabouraud Dextrose agar at 35 °C and 42 °C. Additionally, CHROMagarTM Candida Plus agar was used and pale cream colonies with a distinctive blue halo, suspected as C. auris, were sent to the Department of Microbiology, Medical School, National and Kapodistrian University of Athens, Greece for further analysis. Identification of isolates was confirmed by MALDI-TOF spectrometry, which is one of the most efficient diagnostic techniques for accurate identification of C. auris, using the Microflex LT (Bruker Daltonics, Bremen, Germany) platform.
Susceptibility to antifungal agents was evaluated by the EUCAST standardized broth microdilution method [30]. Since there are no established breakpoints, interpretation of minimum inhibitory concentration (MIC) values was based on CDC proposed tentative breakpoints [26]. Therefore, the information below should be considered as a general guide and not as definitive breakpoints for resistance.
2.5. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range). Continuous variables were compared with Student’s t test (for normally distributed variables) or the Mann–Whitney U test (for non-normally distributed variables). Categorical variables were evaluated with the χ2 or Fisher exact test. All tests were 2-tailed, and p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Patients’ Characteristics—Host Risk Factors (Table 1)

Table 1.
Risk factors and characteristics of colonized or infected patients a.
A total of 591 patients were admitted to the ICU and screened for C. auris between 10 November 2020 and 31 December 2023. During this study period, 32 patients were identified as being colonized (26 patients) or infected (6 patients) with C. auris. The mean age was 68 ± 14 years and males predominated (21 patients). Concerning disease severity, mean APACHE II score at ICU admission was 17.4 ± 6.9. All patients had been hospitalized in a general ward and/or in ICU before admission to our ICU [median time 13 (3–27) days]. All patients had at least one CVC, urinary catheter, arterial catheter, and nasogastric catheter and were on invasive mechanical ventilation. Four patients were on renal replacement therapy. All patients had received antibiotic treatment, whereas nine patients (28.1%) had recent history of antifungal therapy. Echinocandins had been administered in 5 (55.6%) patients, while azoles were prescribed in the rest of them (4; 44.4%). Duration of antifungal exposure was more than 8 days in all cases.
Initial diagnosis and reason for ICU admission were mainly medical (23 patients). The most common risk factors were cardiovascular disease (arterial hypertension, coronary artery disease, congestive heart failure) (15; 46.9%) and neuropsychiatric disorders (ischemic or hemorrhagic stroke, dementia, neurotic or psychotic disorders) (13;40.6%), followed by malignancy (11; 34.4%), diabetes (9; 28.1%), and chronic pulmonary disease (5; 15.6%). Two patients suffered from chronic renal failure and a total of five (15.6%) patients were immuno-compromised (on corticosteroids or chemotherapy treatment, or hematologic malignancy). COVID-19 infection and acute respiratory distress syndrome (ARDS) was the primary diagnosis for 11 (34.4%) patients.
After patients’ allocation in two groups according to C. auris colonization or infection, the sex distribution, mean age, and disease severity were similar between the groups. Regarding patient-related factors, no statistically significant differences in comorbidities were identified, despite the 2-fold higher incidence of diabetes and malignancies in the group of infected patients. Regarding treatment-related factors, the proportion of patients already receiving corticosteroids (34.6% vs. 83.3%, p = 0.064) or being on renal replacement treatment (7.7% vs. 33.3%, p = 0.15) were also higher in the group of infected patients. However, the observed differences did not reach statistical significance, probably due to the small size of groups.
3.2. Timeline of Cases
Median time elapsed from ICU admission to first positive culture was 7 (1–21) days, whereas the corresponding time from hospital admission was 26 (19–49) days. Further analysis of the timeline of C. auris isolations revealed that half of cases occurred within 24 h after ICU admission, while the rest of them occurred after the first week of hospitalization in the ICU (Table 1). Out of the 16 imported C. auris cases, eight patients had been transferred from another ICU. The timeline of the 32 C. auris cases is presented in Figure 1.
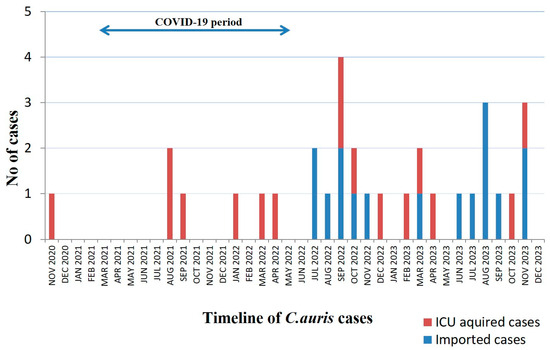
Figure 1.
Timeline of 32 C. auris cases in ICU. The blue arrow denotes the period that the ICU has been functioning as a COVID-19 ICU.
3.3. Microbiological Data and Antifungal Susceptibility Testing
A total of 48 positive samples were recovered and one for each patient was analyzed when the C. auris strain was isolated simultaneously from colonized areas. Six patients had a growth of C. auris in the blood, whereas the rest of the isolates were allocated as follows: swab from axilla/groin 19 (39.58%); urine 9 (18.75%); tracheal aspirates 8 (16.67%); skin-soft tissue samples 3 (6.25%); central venous catheter (CVC) tip 3 (6.25%). Among our study population, 13 patients were colonized at two or more sites, out of which 6 patients C. auris emerged simultaneously at different sites. Out of six infections, C. auris colonization preceded in two cases (19 days and 39 days, respectively). None of the patients turned out negative until ICU discharge. All infected patients had suffered also from co-infections caused by multidrug-resistant bacteria (Acinetobacter baumannii, Klebsiella pneumoniae, Enterococcus faecium). In three cases, bacterial infection preceded the fungal infections, whereas in the rest, they followed.
Since all the strains shared almost identical susceptibilities. Based on tentative CDC MIC breakpoints for fluconazole (≥32 μg/mL), amphotericin B (≥2 μg/mL), micafungin (<4 μg/mL), anidulafungin (<4 μg/mL) and caspofungin (<2 μg/mL), all isolates were resistant to fluconazole (MICs > 32), and susceptible to echinocandines and amphotericin B (Table 2).

Table 2.
Minimum inhibitory concentration values (MICs) of Candida auris strains isolated from the samples of 32 patients a.
3.4. Therapeutic Strategies and Outcome Analysis
Out of a total of 32 patients, 17 did not receive any antifungal treatment after C. auris isolation. Out of 18 patients who were treated with antifungals, 9 received micafungin, 6 received caspofungin, 1 received anidulafungin, while 2 patients received combination therapy (anidulafungin or caspofungin plus amphotericin B).
Regarding source control, in the six cases of candidemia, the CVC was removed within 48 h of positive blood culture. In three cases where C. auris was isolated from skin/soft tissue samples, wound debridement was performed after 48 h of positive culture.
The all-cause mortality during ICU stay was 43.75%, whereas at 28 days after ICU discharge, this was 65.6%. All patients discharged alive from the ICU were transferred to general wards or ICUs within our other hospitals, according to the department where each patient had been initially admitted. Median ICU length of stay was 24 (13–45) days and was significantly longer for infected patients.
The crude mortality for the six patients with C. auris infection was 50%. However, only for one patient with bloodstream infection, death could be attributed to C. auris, whereas in the rest of cases, death was associated with multidrug-resistant bacteria. The mean time between the emergence of infection and ICU discharge was 36 ± 16 days and was also significantly longer when compared to colonized patients.
4. Discussion
In the present study, we reported an ongoing outbreak of C. auris colonization and infection in a Greek ICU. The introduction of C. auris in our ICU occurred before ICU’s operation as ICU-COVID -19 and represented a single case, since no other contemporary patients, nor the environmental samples, revealed C. auris. The next three cases occurred 10 months later, during the fourth wave of COVID-19 in Greece, when the ICU was operated as a COVID-19 ICU and clustered within a 25-day period. After a 4-month period, a new case of C. auris appeared and, thence, strains were isolated almost every month. After the introduction of C. auris in the healthcare setting in Greece in 2019, a nationwide outburst was observed. The National Public Health Organization (NPHO) of Greece recorded 429 cases during a 3-year period (November 2019 till December 2022) [31]. These isolates involved 45 public and private hospitals in Greece; 27 of them (60%) belonged to the district of Attiki. The majority of cases concerned colonization (314; 73.2%) and the crude mortality of affected patients was 28.4%. These data, however, might underestimate C. auris dissemination, given that reporting C. auris cases to the NHPO is still not mandatory. In a previous work [32], we have reported for the first time a series of five cases with C. auris that were clonally related, belonged to clade I (clustered with South-Asian strains).
One of the major problems regarding C. auris is its misidentification with conventional methodologies with other yeasts (e.g., C. haemulonii, C. famata, C. sake, C. catenulata, Rhodotorula glutinis, or Saccharomyces cerevisiae) [33]. The gold standard for C. auris identification is either advanced molecular methods, such as PCR and DNA sequencing, or MALDI-TOF mass spectrometry (MS). This latter methodology detects especially ribosomal proteins from the Candida surface and compares them to proteins from other yeast species using a large database and identifies C. auris with excellent specificity and sensitivity in a few minutes [33,34]. Of note, the US Food and Drug Administration (FDA) approved the Bruker MALDI Biotyper system (20 April 2018) and the bioMérieux Vitek MS (21 December 2018) for C. auris identification [34]. In our study, all the isolated yeasts strains were further analyzed by the aforementioned Bruker system.
The recent evolution of C auris can be associated with the increase in global temperatures, but also with the pandemic. Indeed, a major driver of C. auris healthcare-associated dissemination in Greece was COVID-19. Outbreaks of C. auris have been reported in COVID-19 patients worldwide, resulting in colonization or infection rates as high as 50% [35,36,37,38]. During the COVID-19 pandemic, extended use of the underlayer protective equipment, double gloving, poor adherence to hand hygiene, lapses in cleaning and disinfection procedures, along with low nurse-to-patient ratio, and inadequately trained staff recruited to work in ICUs may have contributed to widespread transmission of C. auris [37]. C. auris can form dense biofilms with up to 30-fold higher cellular burden than C. albicans [39], which are highly resistant to desiccation, osmotic stress, and disinfectants like chlorhexidine and can contaminate the skin of patients and healthcare workers [39,40]. Indeed, new patients become colonized with C. auris after a 4 h contact time with a carrier, while invasive infections have been described in patients within 48 h of ICU admission [41,42]. In our ICU, C. auris patients are isolated in single-person rooms and strict contact precautions are followed. However, the assignation of dedicated healthcare staff is not feasible and the low nurse-to-patient ratio, especially during night shifts, might have contributed to the horizontal transmission of C. auris.
An alarming issue in our series is the observed gap of 4 and 3 months between the discharge of a C. auris carrier and the subsequent emergence of a new one, during the time course of cases. Persistence of C. auris in the patient environment, along with undetected carriage by colonized patients or healthcare workers, might explain this time gap. C. auris biofilms can persist in viable colonies for ≥2 weeks and as viable nonculturable cells for ≥ 4 weeks [13]. In a recent observational multicenter study, positivity rates for colonization were 56% and 27% for groin and axilla, respectively [43]. Colonization of nares, groin, axilla, skin, urinary tract, vagina, and rectum with C. auris can last from 1 month to 3 years, and perhaps indefinitely [13,16,44]. Patients have been found to be colonized for several months after active infection has been resolved or may have intermittent negative results followed by a positive one. However, it has not been established yet if this timeframe differs between clinical cases and screening cases.
Through the present study, we investigated various host- and treatment-related factors, in order to identify potential risk factors that could predetermine C. auris infection. Similarly to previous workers [43,45], the most frequent comorbidities/risk factors in our case series were cardiovascular and neuropsychiatric disorders, malignancy, diabetes, COVID-19 infection, invasive mechanical ventilation, the presence of a CVC and urinary catheter, and a recent history of antibiotic and antifungal agents and corticosteroids. However, chronic comorbidities did not prove to affect patients’ colonization or infection status. Despite the fact that infected patients showed almost 2.5-fold higher rates of corticosteroid administration and 4.5-fold higher rates of renal replacement therapy compared with colonized patients, these differences did not reach statistical significance, probably due to the small size sample.
Nearly 10% of C. auris colonized patients develop invasive infections, particularly those with mechanical ventilation and placement of invasive devices in ICU settings [42,46,47]. Other investigators reported higher prevalence of candidemia (17%) among colonized patients, with an estimated cumulative incidence up to over 25% with increasing length of stay in critically ill patients [41,48,49]. In our case series, only two patients among the 28 already colonized patients develop C. auris bloodstream infection. However, the considerable time that elapsed before these patients became infected indicates this cumulative risk with extended hospitalization, especially in ICU setting.
C. auris has been implicated in a variety of infections, such as urinary tract infections, otitis, surgical wound infections, peritonitis, skin and bone infections, myocarditis, meningitis, and bloodstream infections [50,51]. However, most of the reported cases referred to bloodstream infections, as in our study.
Antifungal treatment management of C. auris infections is similar to other Candida species infections. Nevertheless, C. auris high-level resistance to antifungals is a major obstacle to successful management of the corresponding infections. Multidrug and pan drug resistance are seen in 41% and 3–4% of the strains [11,26,27]. Moreover, regional and clade-specific resistance variations have been reported. For example, clade II isolates show the highest fluconazole sensitivity rates of up to 86% [6]. On the contrary, clade I isolates, as in our case series, show the highest overall resistance with 97% to fluconazole, 54% to amphotericin B, and 49% presenting multidrug resistance [6]. Amphotericin B resistance has been observed in clades I and IV, with resistance rates as high as 50% [6,12]. C. auris employs multiple resistance mechanisms, such as drug target mutation or overexpression and biofilm formation. Both resistant and sensitive strains can coexist in the same population and genetically related isolates may convey different resistance alleles. Considering these clade-specific resistance variations, it is assumable that its high-level antifungal resistance is more likely to be an acquired trait rather than an intrinsic property [6,12,52]. Based on the aforementioned data, echinocandins are recommended as initial therapy for treatment of C. auris infections [26]. Similarly to recommendations for other Candida species, treatment of C. auris isolated from non-invasive sites (such as respiratory tract, urine, and skin colonization) is not encouraged when there is no evidence of infection [26]. The duration of antifungal treatment is also similar to those prescribed for infections caused by other Candida species and depends on clinical cure and source control, along with microbiological clearance [43]. In our study, infected patients received antifungal treatment for a median time of 27 days, along with source removal, such as CVC removal and wound debridement. Adequate and prompt source control is an essential intervention to improve treatment success, to reduce antifungal duration and possibly to prevent induction or selection of resistance. Indeed, there have been alarming reports of emerging resistance to echinocandins after a first treatment course with these antifungals, especially in cases of catheter-related infections [49,53,54]. As resistance can develop while patients are receiving therapy, repeat susceptibility testing is recommended to evaluate the need for an alternative agent [55].
Two of our infected patients, due to clinical deterioration, received combination therapy of an echinocandin (caspofungin or anidulafungin) with amphotericin B and a favorable outcome was achieved for one of them. Combinations of antifungals have been tested mostly in vitro. Synergism was noted for echinocandins and azoles combinations, especially for anidulafungin or micafungin with isavuconazole [56]. Jaggavarapu et al. demonstrated synergy between amphotericin B and micafungin in 8 among 10 tested strains [57]. Despite the limitations of in vitro synergy models, the activity of antifungal combinations may not be only species-specific but also strain-specific [56].
The crude ICU mortality of our case series was 43.75% and was similar for patients colonized (42.3%) and infected (50%), suggesting that C. auris may not have been directly implicated in an unfavorable outcome. Indeed, almost all infected patients died due to infection caused by multidrug-resistant bacteria. Biran et al. have also reported no differences in mortality among colonized and infected patients [45]. Crude mortality rates of 0 to 72% have been reported for C. auris infections, indicating heterogeneity of patients and studies [58]. In a meta-analysis, Chen et al. [59], reported that the pooled crude mortality of C. auris infection was 39%, with an overall mortality of bloodstream infections of 45%. Comparing this mortality with that of candidemia in Europe (38%) and that of multidrug-resistant p. aeruginosa (44.6%) and carbapenem-resistant K. pneumoniae (54.3%), they concluded that the mortality of C. auris candidemia was similar to other candidemias and some drug-resistant Gram-negative bacteremias. Since crude mortality might reflect the multiple acute and chronic comorbidities of critically ill patients, well-designed case–control studies should be carried out to estimate attributable mortality of C. auris more accurately.
Nevertheless, C. auris infections are associated with increased length of hospital stay and, in this way, may indirectly affect mortality [60]. Indeed, in our case series, a significant difference regarding the time elapsed from C. auris isolation to ICU discharge was observed among colonized and infected patients. Consequently, infection control policies are of paramount significance in order to contain the spreading of C. auris in the healthcare environment. Identifying patients or healthcare workers colonized with C. auris is the first step in impeding fungus transmission. High-risk patients and close healthcare contacts of patients with C. auris should be considered for screening for C. auris colonization. Patients on contact precautions should be either isolated in a single room or in a cohort with other C. auris patients and dedicated staff should be assigned. Strict contact precautions should include rigorous hand hygiene with alcohol or chlorhexidine rubs, personal protective equipment for healthcare staff, and dedicated medical equipment or single-use items. It is crucial to decontaminate regularly the high-touch areas along with the terminal cleaning and disinfection of patient environment after discharge [61,62] with high-strength (>1000 ppm) chlorine disinfectants or hydrogen peroxide with silver nitrate [26,40]
Additionally, there is no cumulative evidence regarding the effectiveness of protocols for the decolonization of patients with C. auris [26]. The antimicrobial stewardship team could check for the unnecessary management of cases. The cooperation of the antimicrobial stewardship team with the infection control team is essential in order to limit C. auris transmission [63]. Finally, information on C. auris infection or colonization should be communicated whenever patients are transferred to lower levels of care, in order to ensure all appropriate infection control measures are continued.
C. auris is capable of colonizing up to 90% of asymptomatic humans, while adopting commensal lifestyle or the pathogen (pathobiont). The detection of its passage into pathological mode is very difficult and depends on various host factors, along with C. auris genetic potential and its relationships with neighboring microbes. Also, C.auris undergoes micro-diversification on its host on the timescale of months [55]. Further genomic and clinical studies could elucidate the unanswered questions and promote therapeutic modalities that will keep C. auris out of its pathogenic state.
5. Limitations—Conclusions
The present study has some limitations that should be considered. The observational and retrospective nature of the study brings about an intrinsic limitation. Since it is a single-center study, susceptibility patterns and management practices might have influenced our conclusions, and the results may differ according to the settings of different ICUs. The sample size to analyze the anti-fungal MIC distribution of C. auris isolates is not large. The low number of infected patients may have underestimated the causal role of certain risk factors and hampered further mortality analysis. Nevertheless, this study represents a real-life clinical experience that provides useful data regarding this emerging yeast. It demonstrates several clinical characteristics that render critically ill patients vulnerable for C. auris acquisition. It appoints the high resistance of C. auris to fluconazole and the excellent sensitivity of echinocandins, leading to administration of these antifungals as the first choice. The crude mortality of our study population was similar to other candidemias and some Gram-negative drug-resistant bacteria previously reported, and the attributable mortality was even lower. C. auris may not be so scary, however it should not be underestimated. Rapid identification of C. auris, continued surveillance, and infection control practices, such as screening of close contacts, contact precautions, and proper terminal cleaning, are the most important elements for controlling successfully its spread in the hospital setting. Each patient’s reaction to C. auris may differ according to host inner immunity, different C. auris strains and local environment. Implementation of individualized and prompt therapy is necessary in order to avoid antifungal consumption and potential micro-diversification of C. auris.
Author Contributions
Conceptualization, G.V., K.T. and M.K. methodology; M.K., C.N., E.P., K.T. and G.V.; formal analysis, investigation and writing—original draft preparation, G.V. and M.K.; writing—review, editing, and supervision, A.T., G.V. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval for the study were in accordance to the Ethic Committee of Konstantopoulos- Petition General Hospital (Act 29321/02-11-2021).
Informed Consent Statement
Patients’ consent was waived due to the observational nature of this study. The anonymity of the patients was guaranteed during the whole process of data analysis and results reporting.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors due to ethical reasons
Acknowledgments
The authors would like to thank Theodora Koliou, Stavroula Kritikou, and Afroditi Milioni for their excellent technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Suphavilai, C.; Ko, K.K.K.; Lim, K.M.; Tan, M.G.; Boonsimma, P.; Chu, J.J.K.; Goh, S.S.; Rajandran, P.; Lee, L.C.; Tan, K.Y.; et al. Detection and characterisation of a sixth Candida auris clade in Singapore: A genomic and phenotypic study. Lancet Microbe 2024, 5, 100878. [Google Scholar] [CrossRef]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 2020, 11, e03364-19. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Kallen, A.; Tsay, S.; Chow, N.; Welsh, R.; Kerins, J.; Kemble, S.K.; Pacilli, M.; Black, S.R.; Landon, E.; et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus-United States, May 2013–August 2016. Am. J. Transplant. 2017, 17, 296–299. [Google Scholar] [CrossRef]
- Bing, J.; Du, H.; Guo, P.; Hu, T.; Xiao, M.; Lu, S.; Nobile, C.J.; Chu, H.; Huang, G. Candida auris-associated hospitalizations and outbreaks, China, 2018–2023. Emerg. Microbes Infect. 2024, 13, 2302843. [Google Scholar] [CrossRef]
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, J.Q.; Jabeen, K.; Saeed, N.; Iqbal, N.; Malik, B.; Lockhart, S.R.; Zafar, A.; Brandt, M.E.; Hasan, R. Invasive candidiasis in Pakistan: Clinical characteristics, species distribution and antifungal susceptibility. J. Med. Microbiol. 2013, 62, 259–268. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY antifungal surveillance program: Results for Candida species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; Ramage, G. Combined antifungal resistance and biofilm tolerance: The global threat of Candida auris. mSphere 2019, 4, e00458-19. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 2017, 55, 2996–3005. [Google Scholar] [CrossRef]
- Cosio, T.; Pica, F.; Fontana, C.; Pistoia, E.S.; Favaro, M.; Valsecchi, I.; Zarabian, N.; Campione, E.; Botterel, F.; Gaziano, R. Stephanoascus ciferrii Complex: The Current State of Infections and Drug Resistance in Humans. J. Fungi 2024, 10, 294. [Google Scholar] [CrossRef]
- Magrì, C.; De Carolis, E.; Ivagnes, V.; Di Pilato, V.; Spruijtenburg, B.; Marchese, A.; Meijer, E.F.J.; Chowdhary, A.; Sanguinetti, M. “CLADE-FINDER”: Candida auris Lineage Analysis Determination by Fourier Transform Infrared Spectroscopy and Artificial Neural Networks. Microorganisms 2024, 12, 2153. [Google Scholar] [CrossRef]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: Impact and lessons learned. J. Clin. Microbiol. 2020, 58, e01503–e01519. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris outbreak and its control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef]
- Edwards, J.E., Jr.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Elsevier: Philadelphia, PA, USA, 2015; pp. 2879–2894. [Google Scholar]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kweon, O.J.; Kim, H.R.; Lee, M.-K. Identification of uncommon Candida species using commercial identification systems. J. Microbiol. Biotechnol. 2016, 26, 2206–2213. [Google Scholar] [CrossRef]
- Kordalewska, M.; Zhao, Y.; Lockhart, S.R.; Chowdhary, A.; Berrio, I.; Perlin, D.S. Rapid and accurate molecular identification of the emerging multidrug-resistant pathogen Candida auris. J. Clin. Microbiol. 2017, 55, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Leach, L.; Zhu, Y.; Chaturvedi, S. Development and validation of a real-time PCR assay for rapid detection of Candida auris from surveillance samples. J. Clin. Microbiol. 2018, 56, e01223-17. [Google Scholar] [CrossRef] [PubMed]
- Sexton, D.J.; Kordalewska, M.; Bentz, M.L.; Welsh, R.M.; Perlin, D.S.; Litvintseva, A.P. Direct detection of emergent fungal pathogen Candida auris in clinical skin swabs by SYBR Green-Based Quantitative PCR Assay. J. Clin. Microbiol. 2018, 56, e01337-18. [Google Scholar] [CrossRef]
- Sexton, D.J.; Bentz, M.L.; Welsh, R.M.; Litvintseva, A.P. Evaluation of a new T2 Magnetic Resonance assay for rapid detection of emergent fungal pathogen Candida auris on clinical skin swab samples. Mycoses 2018, 61, 786–790. [Google Scholar] [CrossRef]
- U.S.A Centers for Disease Control and Prevention (CDC). Candida auris Antifungal Susceptibility Testing and Interpretation. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 24 December 2024).
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere 2018, 3, e00334-18. [Google Scholar] [CrossRef]
- Stathi, A.; Loukou, I.; Kirikou, H.; Petrocheilou, A.; Moustaki, M.; Velegraki, A.; Zachariadou, L. Isolation of Candida auris from cystic fibrosis patient, Greece, April 2019. Eurosurveillance 2019, 24, 1900400. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 11.0. 2024. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 4 February 2025).
- Politi, L. The European Programme for Intervention Epidemiology Training (EPIET), 2021 Cohort National Public Health Organization (NPHO); Greece European Centre for Disease Prevention and Control: Athens, Greece, 2023. [Google Scholar]
- Katsiari, M.; Mavroidi, A.; Kesesidis, N.; Palla, E.; Zourla, K.; Ntorlis, K.; Konstantinidis, K.; Laskou, M.; Strigklis, K.; Sakkalis, A.; et al. Emergence of Clonally-Related South Asian Clade I Clinical Isolates of Candida auris in a Greek COVID-19 Intensive Care Unit. J. Fungi 2023, 9, 243. [Google Scholar] [CrossRef]
- Long, B.; Lacy, A.J.; Koyfman, A.; Liang, S.Y. Candida auris: A focused review for emergency clinicians. Am. J. Emerg. Med. 2024, 84, 162–167. [Google Scholar] [CrossRef]
- Dennis, E.K.; Chaturvedi, S.; Chaturvedi, V. So Many Diagnostic Tests, So Little Time: Review and Preview of Candida auris Testing in Clinical and Public Health Laboratories. Front. Microbiol. 2021, 12, 757835. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef]
- Villanueva-Lozano, H.; Treviño-Rangel, R.d.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 2021, 73, e2836–e2837. [Google Scholar] [CrossRef]
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida auris forms high-burden biofilms in skin niche conditions and on porcine skin. mSphere 2020, 5, e00910-19. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; McKloud, E.; Townsend, E.M.; Sherry, L.; Delaney, C.; Jones, B.L.; Williams, C.; Ramage, G. The comparative efficacy of antiseptics against Candida auris biofilms. Int. J. Antimicrob. Agents 2018, 52, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, A.; Wang, Y.; Hi van Haren, M.; Singh, A.; de Groot, T.; Meis, J.F.; Xu, J.; Chowdhary, A. Colonisation and transmission dynamics of Candida auris among chronic respiratory diseases patients hospitalised in a Chest Hospital, Delhi, India: A comparative analysis of whole genome sequencing and microsatellite typing. J. Fungi 2021, 7, 81. [Google Scholar] [CrossRef]
- Pandya, N.; Cag, Y.; Pandak, N.; Pekok, A.U.; Poojary, A.; Ayoade, F.; Fasciana, T.; Giammanco, A.; Caskurlu, H.; Rajani, D.P.; et al. International multicentre study of Candida auris infections. J. Fungi 2021, 7, 878. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef]
- Biran, R.; Cohen, R.; Finn, T.; Brosh-Nissimov, T.; Rahav, G.; Yahav, D.; Amit, S.; Shachor-Meyouhas, Y.; Atamna, A.; Bishara, J.; et al. Nationwide outbreak of Candida auris infections driven by COVID-19 hospitalizations, Israel, 2021–2022. Emerg. Infect. Dis. 2023, 29, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Shastri, P.S.; Shankarnarayan, S.A.; Oberoi, J.; Rudramurthy, S.M.; Wattal, C.; Chakrabarti, A. Candida auris candidaemia in an intensive care unit-prospective observational study to evaluate epidemiology, risk factors, and outcome. J. Crit. Care 2020, 57, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kenters, N.; Kiernan, M.; Chowdhary, A.; Denning, D.W.; Pemán, J.; Saris, K.; Schelenz, S.; Tartari, E.; Widmer, A.; Meis, J.F.; et al. Control of Candida auris in healthcare institutions: Outcome of an International Society for Antimicrobial Chemotherapy expert meeting. Int. J. Antimicrob. Agents 2019, 54, 400–406. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Salavert, M.; Ruiz-Gaitán, A.C.; Cabanero-Navalon, M.D.; Sigona-Giangreco, I.A.; Pemán, J. A clinical predictive model of candidaemia by Candida auris in previously colonized critically ill patients. Clin. Microbiol. Infect. 2020, 26, 1507–1513. [Google Scholar] [CrossRef]
- Briano, F.; Magnasco, L.; Sepulcri, C.; Dettori, S.; Dentone, C.; Mikulska, M.; Ball, L.; Vena, A.; Robba, C.; Patroniti, N.; et al. Candida auris candidemia in critically ill, colonized patients: Cumulative incidence and risk factors. Infect. Dis. Ther. 2022, 11, 1149–1160. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Farooqi, J.; Jabeen, K.; Awan, S.; Mahmood, S.F. Clinical spectrum and factors impacting outcome of Candida auris: A single center study from Pakistan. BMC Infect. Dis. 2019, 19, 384. [Google Scholar] [CrossRef]
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef]
- Burrack, L.S.; Todd, R.T.; Soisangwan, N.; Wiederhold, N.P.; Selmecki, A. Genomic diversity across Candida auris clinical isolates shapes rapid development of antifungal resistance in vitro and in vivo. mBio 2022, 13, e00842-22. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Bayona, J.V.; Salvador-Garcia, C.; Tormo-Palop, N.; Gimeno-Cardona, C. Recurrent candidemia and isolation of echinocandin-resistant Candida auris in a patient with a long-term central catheter. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2022, 40, 334–335. [Google Scholar] [CrossRef]
- Biagi, M.J.; Wiederhold, N.P.; Gibas, C.; Wickes, B.L.; Lozano, V.; Bleasdale, S.C.; Danziger, L. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect. Dis. 2019, 6, ofz262. [Google Scholar] [CrossRef]
- Proctor, D.M.; Drummond, R.A.; Lionakis, M.S.; Segre, J.A. One population, multiple lifestyles: Commensalism and pathogenesis in the human mycobiome. Cell Host Microbe 2023, 31, 539–553. [Google Scholar] [CrossRef]
- Caballero, U.; Kim, S.; Eraso, E.; Quindós, G.; Vozmediano, V.; Schmidt, S.; Jauregizar, N. In vitro synergistic interactions of isavuconazole and echinocandins against Candida auris. Antibiotics 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Jaggavarapu, S.; Burd, E.M.; Weiss, D.S. Micafungin and amphotericin b synergy against Candida auris. Lancet Microbe 2020, 1, e314–e315. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Asadzadeh, M. Strategies to prevent transmission of Candida auris in healthcare settings. Curr. Fungal Infect. Rep. 2023, 17, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef]
- Taori, S.K.; Khonyongwa, K.; Hayden, I.; Athukorala, G.D.A.; Letters, A.; Fife, A.; Desai, N.; Borman, A.M. Candida auris outbreak: Mortality, interventions and cost of sustaining control. J. Infect. 2019, 79, 601–611. [Google Scholar] [CrossRef]
- ECDC Rapid Risk Assessment. Candida Auris in Healthcare Settings-Europe. European Centre for Disease Prevention and Control; Stockholm. 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-Candida-auris-European-Union-countries.pdf (accessed on 20 August 2020).
- Tsay, S.; Kallen, A.; Jackson, B.R.; Chiller, T.M.; Vallabhaneni, S. Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin. Infect. Dis. 2018, 66, 306–311. [Google Scholar] [CrossRef]
- Sabino, R.; Veríssimo, C.; Pereira, Á.A.; Antunes, F. Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind? Microorganisms 2020, 8, 181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).