One Health Approach to Brazilian Spotted Fever: Capybaras, Horses, and Rural Areas as Predictors for Human Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
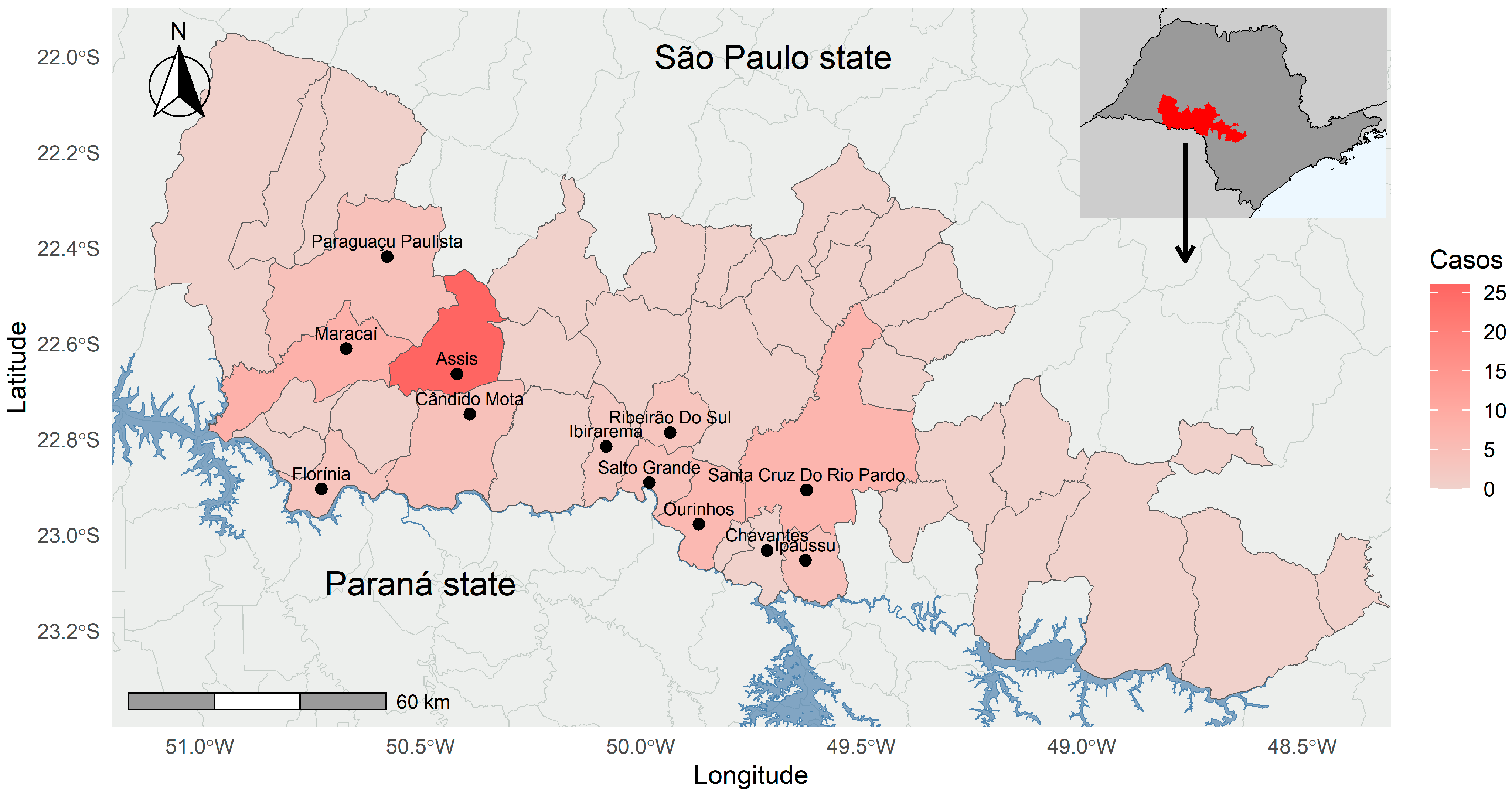
2.3. Study Area
2.4. Data Source
2.5. Statistical Analysis
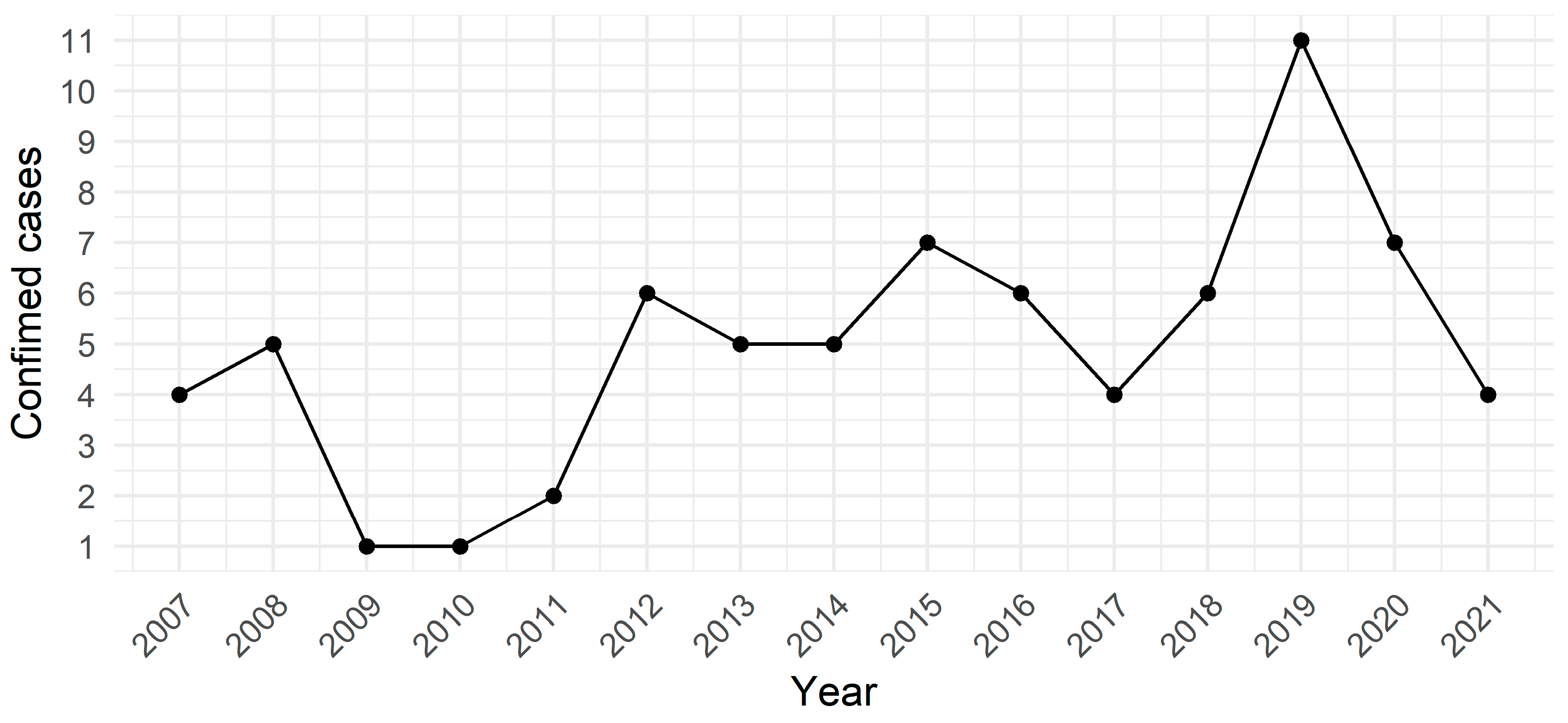
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polo, G.; Labruna, M.B.; Ferreira, F. Basic reproduction number for the Brazilian Spotted Fever. J. Theor. Biol. 2018, 458, 119–124. [Google Scholar] [PubMed]
- Szabó, M.P.J.; Pinter, A.; Labruna, M.B. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front. Cell. Infect. Microbiol. 2013, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.V.; Guimarães, J.N.; Reckziegel, G.C.; Neves, B.M.d.C.; Araújo-Vilges, K.M.d.; Fonseca, L.X.; Pinna, F.V.; Pereira, S.V.C.; de Caldas, E.P.; Gazeta, G.S.; et al. An update on the epidemiological situation of spotted fever in Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 22. [Google Scholar]
- Sekeyová, Z.; Danchenko, M.; Filipčík, P.; Fournier, P.E. Rickettsial infections of the central nervous system. PLoS Neglected Trop. Dis. 2019, 13, e0007469. [Google Scholar]
- de Oliveira, S.V.; Willemann, M.C.A.; Gazeta, G.S.; Angerami, R.N.; Gurgel-Gonçalves, R. Predictive Factors for Fatal Tick-Borne Spotted Fever in Brazil. Zoonoses Public Health 2017, 64, e44–e50. [Google Scholar]
- Polo, G.; Labruna, M.B.; Ferreira, F. Satellite Hyperspectral Imagery to Support Tick-Borne Infectious Diseases Surveillance. PLoS ONE 2015, 10, e0143736. [Google Scholar]
- Ribeiro, C.M.; da Costa, V.M.; de Carvalho, J.L.B.; Mendes, R.G.; Bastos, P.A.d.S.; Katagiri, S.; Amaku, M. Brazilian spotted fever: A spatial analysis of human cases and vectors in the state of São Paulo, Brazil. Zoonoses Public Health 2020, 67, 629–636. [Google Scholar]
- bepa_213.pdf. Available online: https://saude.sp.gov.br/resources/ccd/homepage/bepa/edicoes-2021/bepa_213.pdf (accessed on 28 February 2025).
- IBGE|Portal do IBGE|IBGE. Available online: https://www.ibge.gov.br/ (accessed on 5 June 2024).
- Monteiro, G.G.; Peronti, A.L.B.G.; Martinelli, N.M. Distribution, abundance and seasonality of scale insects in sugarcane crops in the state of São Paulo. Braz. J. Biol. Rev. Brasleira Biol. 2021, 83, e250879. [Google Scholar]
- Molijn, R.A.; Iannini, L.; Rocha, J.V.; Hanssen, R.F. Author Correction: Ground reference data for sugarcane biomass estimation in São Paulo state, Brazil. Sci. Data 2020, 7, 193. [Google Scholar]
- SAGE Publications Ltd. An R Companion to Applied Regression. Available online: https://uk.sagepub.com/en-gb/eur/an-r-companion-to-applied-regression/book246125 (accessed on 28 February 2025).
- Robinson, D.; Hayes, A.; Couch, S. broom: Convert Statistical Objects into Tidy Tibbles. Available online: https://cran.r-project.org/web/packages/broom/index.html (accessed on 28 February 2025).
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 5 June 2024).
- de Lemos, E.R.; Ravagnani, R.C.; Zaki, S.R.; Ferebee, T.L.; Ferreira, F.C.; Coura, J.R.; Guimarães, M.A.; Paddock, C.D.; Cintra, M.L.; Ramos, M.C.; et al. Spotted fever in Brazil: A seroepidemiological study and description of clinical cases in an endemic area in the state of São Paulo. Am. J. Trop. Med. Hyg. 2001, 65, 329–334. [Google Scholar]
- Kmetiuk, L.B.; Paula WV de, F.; Pádua, G.T.; Delai, R.R.; Freitas, A.R.; Farinhas, J.H.; de Paula, L.G.F.; Giuffrida, R.; Pimpão, C.T.; Santarém, V.Á.; et al. Epidemiology of Rickettsia spp. in Atlantic rainforest areas of island and seashore mainland, southern Brazil. Transbound. Emerg. Dis. 2022, 69, 3597–3605. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.E.; Camargo, L.B.; Pinter, A.; Donalisio, M.R. High Seroprevalence for Rickettsia rickettsii in Equines Suggests Risk of Human Infection in Silent Areas for the Brazilian Spotted Fever. PLoS ONE 2016, 11, e0153303. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Aléssio, F.M.; Siqueira, D.B.; Mauffrey, J.-F.; Marvulo, M.F.V.; Martins, T.F.; Moraes-Filho, J.; Camargo, M.C.G.O.; D’Auria, S.R.N.; Labruna, M.B.; et al. Exposure of small mammals to ticks and rickettsiae in Atlantic Forest patches in the metropolitan area of Recife, North-eastern Brazil. Parasitology 2012, 139, 83–91. [Google Scholar] [CrossRef]
- de Paula, L.G.F.; Nascimento, R.M.D.; Franco, A.d.O.; Szabó, M.P.J.; Labruna, M.B.; Monteiro, C.; Krawczak, F.d.S. Seasonal dynamics of Amblyomma sculptum: A review. Parasit Vectors 2022, 15, 193. [Google Scholar] [CrossRef]
- Labruna, M.B.; Kasai, N.; Ferreira, F.; Faccini, J.L.H.; Gennari, S.M. Seasonal dynamics of ticks (Acari: Ixodidae) on horses in the state of São Paulo, Brazil. Vet. Parasitol. 2002, 105, 65–77. [Google Scholar] [CrossRef]
- de Siqueira, S.M.; da Costa Maia, R.; do Nascimento Ramos, V.; da Silva Rodrigues, V.; Szabó, M.P.J. Rhipicephalus microplus and Amblyomma sculptum (Ixodidae) infestation of Nellore cattle (Bos taurus indicus) in a farm of the Brazilian Cerrado: Seasonality and infestation patterns. Exp. Appl. Acarol. 2021, 84, 659–672. [Google Scholar] [CrossRef]
- Reck, J.; Souza, U.; Souza, G.; Kieling, E.; Dall’agnol, B.; Webster, A.; Michel, T.; Doyle, R.; Martins, T.F.; Labruna, M.B.; et al. Records of ticks on humans in Rio Grande do Sul state, Brazil. Ticks Tick-Borne Dis. 2018, 9, 1296–1301. [Google Scholar] [CrossRef]
- Valente, J.D.; Silva, P.W.; Arzua, M.; Barros-Battesti, D.M.; Martins, T.F.; Silva, A.M.; Vieira, T.S.; Labruna, M.B.; Vieira, R.F. Records of ticks (Acari: Ixodidae) on humans and distribution of spotted-fever cases and its tick vectors in Paraná State, southern Brazil. Ticks Tick-Borne Dis. 2020, 11, 101510. [Google Scholar] [CrossRef]
- Aguirre, A.; Garcia, M.V.; da Costa, I.N.; Csordas, B.G.; Rodrigues, V.d.S.; Medeiros, J.F.; Andreotti, R. New records of tick-associated spotted fever group Rickettsia in an Amazon-Savannah ecotone, Brazil. Ticks Tick-Borne Dis. 2018, 9, 1038–1044. [Google Scholar] [CrossRef]
- Souza CEd Pinter, A.; Donalisio, M.R. Risk factors associated with the transmission of Brazilian spotted fever in the Piracicaba river basin, State of São Paulo, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 11–17. [Google Scholar] [CrossRef]
- Durães, L.S.; Bitencourth, K.; Ramalho, F.R.; Nogueira, M.C.; Nunes, E.d.C.; Gazêta, G.S. Biodiversity of Potential Vectors of Rickettsiae and Epidemiological Mosaic of Spotted Fever in the State of Paraná, Brazil. Front. Public Health 2021, 9, 577789. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Labruna, M.B. Isolation of Rickettsia rickettsii and Rickettsia bellii in Cell Culture from the Tick Amblyomma aureolatum in Brazil. Ann. N. Y. Acad. Sci. 2006, 1078, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.F.; Soares, H.S.; Barbieri, A.M.; Labruna, M.B. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Med. Vet. Entomol. 2012, 26, 139–151. [Google Scholar] [CrossRef]
- STROBE. Available online: https://www.strobe-statement.org/ (accessed on 28 February 2025).

| Suspected Cases | Univariate Analysis | |||
|---|---|---|---|---|
| Confirmed (%) | Not Confirmed (%) | Odds Ratio (95% CI) | p-Value | |
| Variables | 74 (6.6) | 1047 (93.4) | ||
| Age | n = 1121 | 0.563 | ||
| 1 to 18 | 17 (23.0) | 256 (24.5) | 1 | |
| 19 to 35 | 24 (32.4) | 291 (27.8) | 1.24 (0.65–2.40) | |
| 36 to 51 | 14 (18.9) | 265 (25.3) | 0.80 (0.38–1.66) | |
| 52 to 85 | 19 (25.7) | 235 (22.4) | 1.22 (0.61–2.43) | |
| Gender | n = 1121 | 1.0 | ||
| Female | 21 (28.4) | 299 (28.6) | 1 | |
| Male | 53 (71.6) | 748 (71.4) | 1.00 (0.60–1.73) | |
| Ethnicity | n = 1102 | 0.824 | ||
| White | 59 (81.9) | 862 (83.7) | 1 | |
| Non-white | 13 (18.1) | 168 (16.3) | 1.14 (0.59–2.07) | |
| Household location * | n = 1108 | 0.033 | ||
| Urban | 55 (74.3) | 882 (84.5) | 1 | |
| Rural | 19 (25.7) | 162 (15.5) | 1.89 (1.06–3.22) | |
| Previous tick infestation * | n = 976 | 0.073 | ||
| No | 21 (33.3) | 418 (45.8) | 1 | |
| Yes | 42 (66.7) | 495 (54.2) | 1.68 (0.99–2.94) | |
| Previous capybara contact * | n = 984 | 0.001 | ||
| No | 35 (59.3) | 731 (79.0) | 1 | |
| Yes | 24 (40.7) | 194 (21.0) | 2.59 (1.48–4.44) | |
| Dog or cat owner | n = 996 | 0.731 | ||
| No | 30 (48.4) | 481 (51.5) | 1 | |
| Yes | 32 (51.6) | 453 (48.5) | 1.13 (0.67–1.90) | |
| Cattle raising | 0.671 | |||
| No | 48 (81.4) | 781 (84.3) | 1 | |
| Yes | 11 (18.6) | 145 (15.7) | 1.25 (0.60–2.38) | |
| Previous visit to forest * | n = 985 | 0.064 | ||
| No | 10 (15.4) | 244 (26.6) | 1 | |
| Yes | 55 (84.6) | 673 (73.4) | 1.97 (1.03–4.18) | |
| Horse raising * | n = 986 | 0.045 | ||
| No | 44 (73.3) | 779 (84.1) | 1 | |
| Yes | 16 (26.7) | 147 (15.9) | 1.94 (1.03–3.47) | |
| Coefficients | β Estimate | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|
| Intercept | −3.406 | 0.361 | NA |
| Rural household location | 0.687 | 0.037 | 2.0 (1.02–3.72) |
| Previous tick infestation | 0.637 | 0.665 | 1.15 (0.62–2.16) |
| Previous capybara contact | 0.637 | 0.051 | 1.9 (1.0–3.57) |
| Previous visit to forest | 0.168 | 0.683 | 1.18 (0.55–2.78) |
| Horse raising | 0.313 | 0.375 | 1.37 (0.66–2.67) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa-Xavier, I.G.; Pinter, A.; Giuffrida, R.; Biondo, A.W.; Kmetiuk, L.B.; Santarém, V.A. One Health Approach to Brazilian Spotted Fever: Capybaras, Horses, and Rural Areas as Predictors for Human Disease. Pathogens 2025, 14, 305. https://doi.org/10.3390/pathogens14040305
Rosa-Xavier IG, Pinter A, Giuffrida R, Biondo AW, Kmetiuk LB, Santarém VA. One Health Approach to Brazilian Spotted Fever: Capybaras, Horses, and Rural Areas as Predictors for Human Disease. Pathogens. 2025; 14(4):305. https://doi.org/10.3390/pathogens14040305
Chicago/Turabian StyleRosa-Xavier, Iara Giordano, Adriano Pinter, Rogério Giuffrida, Alexander Welker Biondo, Louise Bach Kmetiuk, and Vamilton Alvares Santarém. 2025. "One Health Approach to Brazilian Spotted Fever: Capybaras, Horses, and Rural Areas as Predictors for Human Disease" Pathogens 14, no. 4: 305. https://doi.org/10.3390/pathogens14040305
APA StyleRosa-Xavier, I. G., Pinter, A., Giuffrida, R., Biondo, A. W., Kmetiuk, L. B., & Santarém, V. A. (2025). One Health Approach to Brazilian Spotted Fever: Capybaras, Horses, and Rural Areas as Predictors for Human Disease. Pathogens, 14(4), 305. https://doi.org/10.3390/pathogens14040305






