N-Acetylcysteine as a Host-Directed Therapy Against Clarithromycin-Resistant Mycobacterium abscessus
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymerase Chain Reaction (PCR) Sequencing
2.2. Drug Susceptibility Testing
2.3. Bacterial and Cell Culture
2.4. Macrophage Intracellular Infection
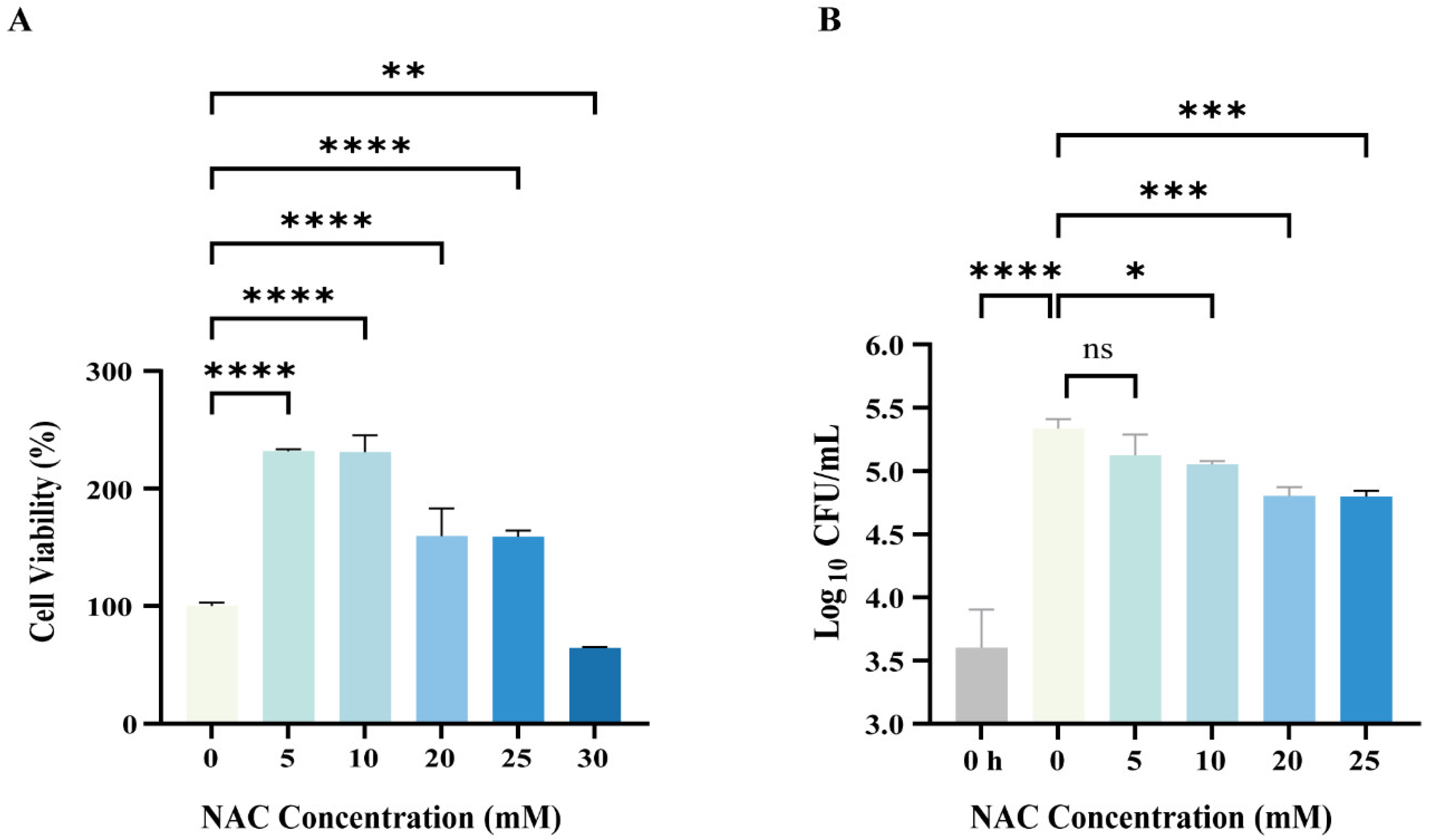
2.5. Cell Viability Assay
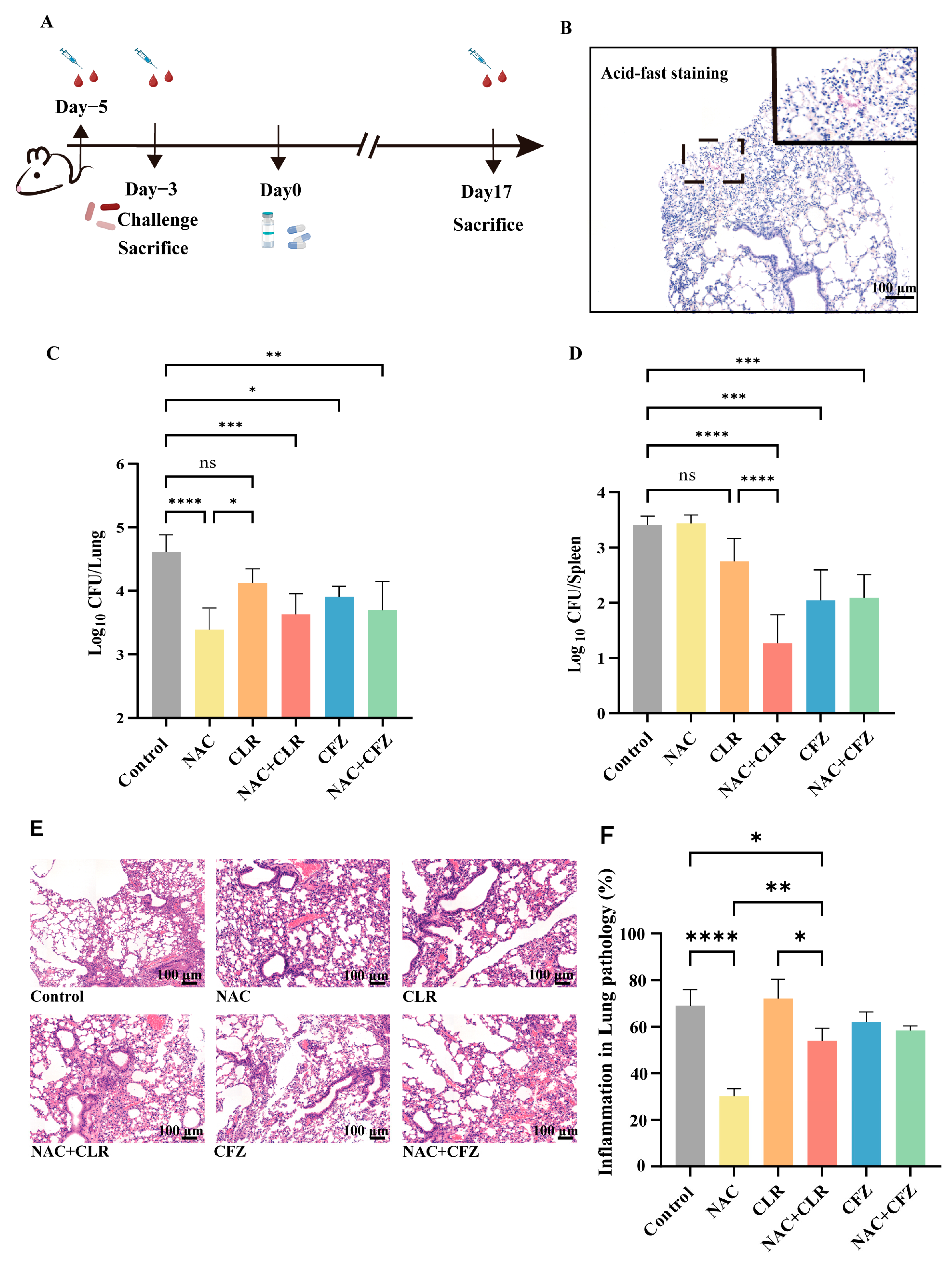
2.6. Mice
2.7. Pathology
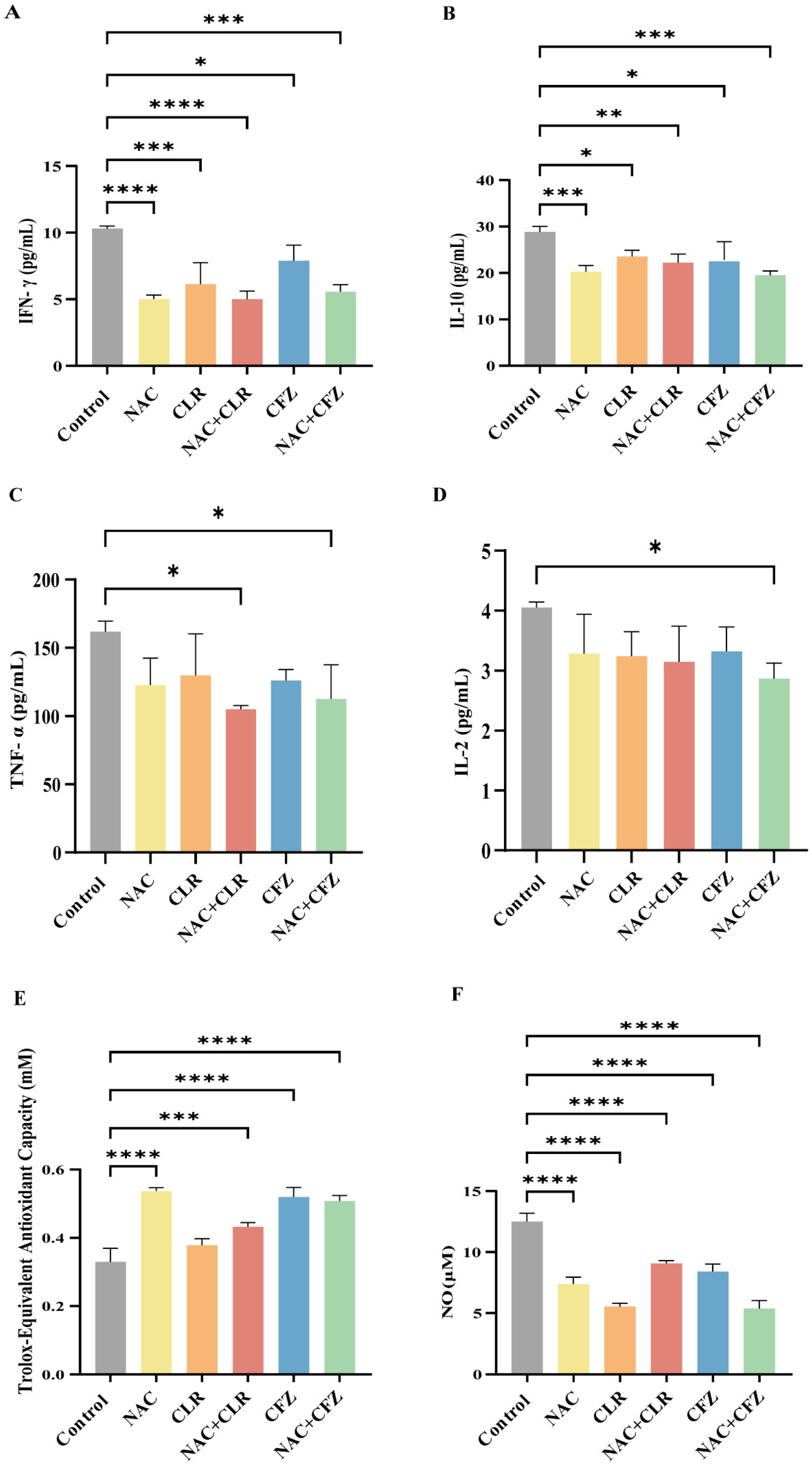
2.8. Cytometric Bead Array (CBA)
2.9. Oxidative Stress-Related Indicators
2.10. RNA Sequencing (RNA-Seq)
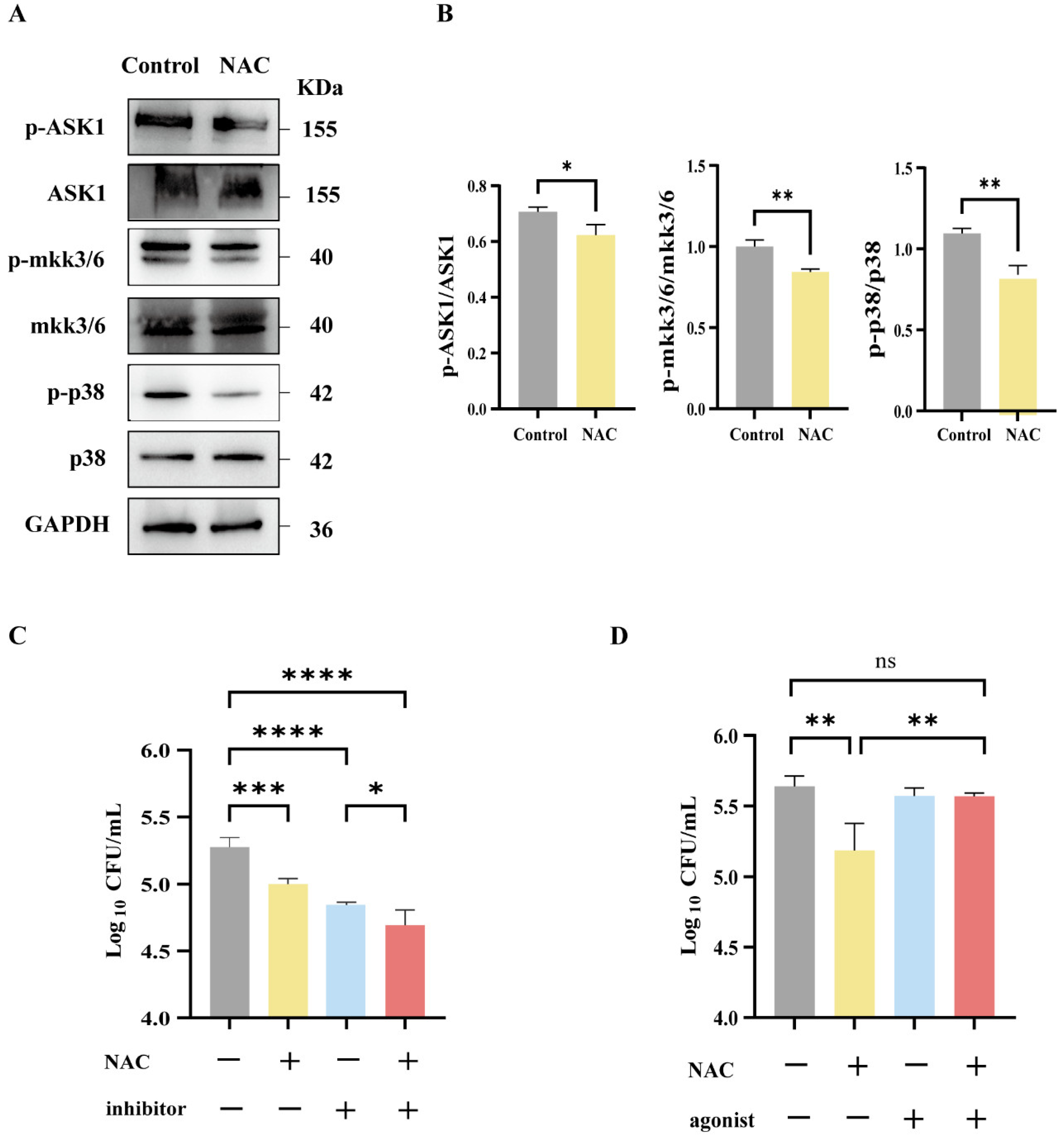
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. Screening of Clinical Isolates of CLR-Resistant M. abscessus
3.2. NAC Exhibited Antimicrobial Activity Against Intracellular M. abscessus Infection
3.3. NAC Exhibited Anti-M. abscessus Activity in Murine Models
3.4. NAC Reduced Proinflammatory Cytokines and Oxidative Stress Caused by M. abscessus Infection
3.5. Suppression of MAPK-Related Pathways Following NAC Therapy in Mice Infected with M. abscessus
3.6. The Antibacterial Effect of NAC Was Mediated by the p38 Signaling Pathway
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bents, S.J.; Mercaldo, R.A.; Powell, C.; Henkle, E.; Marras, T.K.; Prevots, D.R. Nontuberculous mycobacterial pulmonary disease (NTM PD) incidence trends in the United States, 2010–2019. BMC Infect. Dis. 2024, 24, 1094. [Google Scholar]
- Thornton, C.S.; Mellett, M.; Jarand, J.; Barss, L.; Field, S.K.; Fisher, D.A. The respiratory microbiome and nontuberculous mycobacteria: An emerging concern in human health. Eur. Respir. Rev. 2021, 30, 200299. [Google Scholar]
- Guo, W.; Shangguan, Y.; Ji, Z.; Hu, M.; Li, X.; Hu, W.; Zheng, L.; Huang, S.; Wang, Y.; Xia, J.; et al. Clinical characteristics and antimicrobial susceptibility profiles of Mycobacterium abscessus and Mycobacterium massiliense pulmonary infection. J. Glob. Antimicrob. Resist. 2024, 38, 83–89. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Y.; Li, Z.; Xia, S.; Guo, Z.; Li, W.; Yuan, Y.; Gao, J.; Wang, W. Laboratory tests and analysis of drug resistance in non-tuberculous mycobacteria. Heliyon 2024, 10, e28665. [Google Scholar] [CrossRef]
- De, K.; Belardinelli, J.M.; Pandurangan, A.P.; Ehianeta, T.; Lian, E.; Palčeková, Z.; Lam, H.; Gonzalez-Juarrero, M.; Bryant, J.M.; Blundell, T.L.; et al. Lipoarabinomannan modification as a source of phenotypic heterogeneity in host-adapted Mycobacterium abscessus isolates. Proc. Natl. Acad. Sci. USA 2024, 121, e2403206121. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, T.; Liu, W.; Zhu, D.; Feng, X.; Chen, Y.; Zheng, H. Prevalence and antimicrobial susceptibility pattern of Mycobacterium abscessus complex isolates in Chongqing, Southwest China. Heliyon 2024, 10, e34546. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, C.A.; Tan, X.; Liang, Y.; Kotey, S.K.; Rogers, J.; Hartson, S.D.; Liu, L.; Cheng, Y. Mycobacterium abscessus extracellular vesicles increase mycobacterial resistance to clarithromycin in vitro. Proteomics 2024, 24, e2300332. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ihara, H.; Takei, S.; Nakamura, A.; Fujimoto, Y.; Handoh, T.; Kurokawa, K.; Arai, Y.; Shibayama, K.; Sumiyoshi, I.; et al. Antimicrobial susceptibility analysis of isepamicin combination treatments in Mycobacterium abscessus species. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 36, 100464. [Google Scholar] [CrossRef]
- Liao, W.; Wang, X.; Wang, Y.; Ma, P.; Chen, K.; Ge, L.; Yang, X.; Zeng, S.; Gao, W.; Zhang, S.; et al. Noncanonical mutations in ribosome nascent peptide exit tunnel confer clarithromycin resistance in Mycobacterium abscessus complex. Int. J. Antimicrob. Agents 2024, 64, 107223. [Google Scholar] [CrossRef]
- Mushatt, D.M.; Witzig, R.S. Successful treatment of Mycobacterium abscessus infections with multidrug regimens containing clarithromycin. Clin. Infect. Dis. 1995, 20, 1441–1442. [Google Scholar] [CrossRef]
- Ren, W.; Tan, Y.; Ma, Z.; Shang, Y.; Li, S.; Zhang, X.; Wang, W.; Yao, C.; Yuan, J.; Li, L.; et al. In vitro susceptibility of nontuberculous mycobacteria in China. BMC Infect. Dis. 2024, 24, 118. [Google Scholar] [CrossRef]
- Yusuf, B.; Wang, S.; Alam, M.S.; Zhang, J.; Liu, Z.; Lu, Z.; Ding, J.; Chiwala, G.; Gao, Y.; Fang, C.; et al. Investigating the role of MAB_1915 in intrinsic resistance to multiple drugs in Mycobacterium abscessus. Microbiol. Spectr. 2024, 12, e0397423. [Google Scholar] [CrossRef]
- van den Biggelaar, R.; Walburg, K.V.; van den Eeden, S.J.F.; van Doorn, C.L.R.; Meiler, E.; de Ries, A.S.; Fusco, M.C.; Meijer, A.H.; Ottenhoff, T.H.M.; Saris, A. Identification of kinase inhibitors as potential host-directed therapies for intracellular bacteria. Sci. Rep. 2024, 14, 17225. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.I.; Yazel Eiser, I.E.; Kallianpur, K.J.; Gangcuangco, L.M.; Chow, D.C.; Ndhlovu, L.C.; Paul, R.; Shikuma, C.M. Dynamics of peripheral T cell exhaustion and monocyte subpopulations in neurocognitive impairment and brain atrophy in chronic HIV infection. J. Neurovirol. 2024, 30, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.A.; Bhaduri-McIntosh, S. Inflammation and Epstein-Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses 2023, 15, 949. [Google Scholar] [CrossRef]
- Huang, X.; Lowrie, D.B.; Fan, X.Y.; Hu, Z. Natural products in anti-tuberculosis host-directed therapy. Biomed. Pharmacother. 2024, 171, 116087. [Google Scholar] [CrossRef]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 2018, 24, 439–446.E4. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.; Jahn, A.; Murray, T.; Morikubo, M.; Lim, P.N.; Cervantes, M.M.; Pham, L.K.; Nemeth, J.; Urdahl, K.; Diercks, A.H.; et al. Exposure to Mycobacterium remodels alveolar macrophages and the early innate response to Mycobacterium tuberculosis infection. PLoS Pathog. 2024, 20, e1011871. [Google Scholar] [CrossRef]
- Chen, G.; Shen, L.; Hu, H.; Feng, Y.; Wen, D.; Liu, Y.; Zhai, H.; Sun, W.; Wang, M.; Lei, X.; et al. Sulforaphane Inhibits Oxidative Stress and May Exert Anti-Pyroptotic Effects by Modulating NRF2/NLRP3 Signaling Pathway in Mycobacterium tuberculosis-Infected Macrophages. Microorganisms 2024, 12, 1191. [Google Scholar] [CrossRef]
- Murphy, D.M.; Walsh, A.; Stein, L.; Petrasca, A.; Cox, D.J.; Brown, K.; Duffin, E.; Jameson, G.; Connolly, S.A.; O’Connell, F.; et al. Human Macrophages Activate Bystander Neutrophils’ Metabolism and Effector Functions When Challenged with Mycobacterium tuberculosis. Int. J. Mol. Sci. 2024, 25, 2898. [Google Scholar] [CrossRef]
- Tasci, T.; Orta-Yilmaz, B.; Aydin, Y.; Caliskan, M. N-acetylcysteine attenuates sodium arsenite-induced oxidative stress and apoptosis in embryonic fibroblast cells. Toxicol. Res. 2024, 13, tfae128. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Zhao, R.; Wang, P.; Jin, M.; Xu, J. Antioxidant N-acetylcysteine removing ROS: An antifouling strategy inspired by mussels. Environ. Sci. Process. Impacts 2023, 25, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Safe, I.P.; Amaral, E.P.; Araújo-Pereira, M.; Lacerda, M.V.G.; Printes, V.S.; Souza, A.B.; Beraldi-Magalhães, F.; Monteiro, W.M.; Sampaio, V.S.; Barreto-Duarte, B.; et al. Adjunct N-Acetylcysteine Treatment in Hospitalized Patients With HIV-Associated Tuberculosis Dampens the Oxidative Stress in Peripheral Blood: Results from the RIPENACTB Study Trial. Front. Immunol. 2020, 11, 602589. [Google Scholar] [CrossRef]
- Wallis, R.S.; Sabi, I.; Lalashowi, J.; Bakuli, A.; Mapamba, D.; Olomi, W.; Siyame, E.; Ngaraguza, B.; Chimbe, O.; Charalambous, S.; et al. Adjunctive N-Acetylcysteine and Lung Function in Pulmonary Tuberculosis. NEJM Evid. 2024, 3, EVIDoa2300332. [Google Scholar] [CrossRef]
- Safe, I.P.; Lacerda, M.V.G.; Printes, V.S.; Praia Marins, A.F.; Rebelo Rabelo, A.L.; Costa, A.A.; Tavares, M.A.; Jesus, J.S.; Souza, A.B.; Beraldi-Magalhães, F.; et al. Safety and efficacy of N-acetylcysteine in hospitalized patients with HIV-associated tuberculosis: An open-label, randomized, phase II trial (RIPENACTB Study). PLoS ONE 2020, 15, e0235381. [Google Scholar] [CrossRef]
- Shiozawa, A.; Kajiwara, C.; Ishii, Y.; Tateda, K. N-acetyl-cysteine mediates protection against Mycobacterium avium through induction of human β-defensin-2 in a mouse lung infection model. Microbes Infect. 2020, 22, 567–575. [Google Scholar] [CrossRef]
- Vilchèze, C.; Jacobs, W.R., Jr. The promises and limitations of N-acetylcysteine as a potentiator of first-line and second-line tuberculosis drugs. Antimicrob. Agents Chemother. 2021, 65, 10-1128. [Google Scholar] [CrossRef]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef]
- Xu, J.C.; Hu, Z.; Fan, X.Y. Protocol for analyzing BCG-induced trained immunity in murine bone marrow-derived macrophages. STAR Protoc. 2024, 5, 103267. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Wang, L.; Di Stefano, A.F.D.; Zanin, V.; Magrone, P.; Yuan, Y. Phase I study of the pharmacokinetics and safety of single and multiple doses of intravenous N-acetylcysteine in healthy Chinese subjects. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 12103–12111. [Google Scholar] [CrossRef]
- Jeon, M.; Bae, S. In vitro effects of N-acetylcysteine in combination with antifungal agents against Malassezia pachydermatis isolated from canine otitis externa. Vet. Med. Sci. 2024, 10, e1479. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Mascellino, M.T.; Miele, M.C.; Al Ismail, D.; Colone, M.; Stringaro, A.; Vullo, V.; Venditti, M.; Mastroianni, C.M.; Oliva, A. High Activity of N-Acetylcysteine in Combination with Beta-Lactams against Carbapenem-Resistant Klebsiella pneumoniae and Acinetobacter baumannii. Antibiotics 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Rosain, J.; Neehus, A.L.; Manry, J.; Yang, R.; Le Pen, J.; Daher, W.; Liu, Z.; Chan, Y.H.; Tahuil, N.; Türel, Ö.; et al. Human IRF1 governs macrophagic IFN-γ immunity to mycobacteria. Cell 2023, 186, 621–645.E33. [Google Scholar] [CrossRef] [PubMed]
- Lutzky, V.P.; Ratnatunga, C.N.; Smith, D.J.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Thomson, R.M.; Bell, S.C.; Miles, J.J. Anomalies in T Cell Function Are Associated with Individuals at Risk of Mycobacterium abscessus Complex Infection. Front. Immunol. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Park, H.E.; Lee, W.; Choi, S.; Jung, M.; Shin, M.K.; Shin, S.J. Modulating macrophage function to reinforce host innate resistance against Mycobacterium avium complex infection. Front. Immunol. 2022, 13, 931876. [Google Scholar] [CrossRef]
- Anidi, I.U.; Olivier, K.N. Host-Directed Therapy in Nontuberculous Mycobacterial Pulmonary Disease: Preclinical and Clinical Data Review. Clin. Chest Med. 2023, 44, 839–845. [Google Scholar] [CrossRef]
- Gotoh, Y.; Cooper, J.A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem. 1998, 273, 17477–17482. [Google Scholar] [CrossRef]
- Kim, A.H.; Khursigara, G.; Sun, X.; Franke, T.F.; Chao, M.V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell Biol. 2001, 21, 893–901. [Google Scholar] [CrossRef]
- Huang, C.; Li, J.; Ding, M.; Leonard, S.S.; Wang, L.; Castranova, V.; Vallyathan, V.; Shi, X. UV Induces phosphorylation of protein kinase B (Akt) at Ser-473 and Thr-308 in mouse epidermal Cl 41 cells through hydrogen peroxide. J. Biol. Chem. 2001, 276, 40234–40240. [Google Scholar] [CrossRef]
- Wang, X.; McCullough, K.D.; Franke, T.F.; Holbrook, N.J. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J. Biol. Chem. 2000, 275, 14624–14631. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Ichijo, H.; Giannakakou, P.; Foster, J.S.; Fojo, T.; Wimalasena, J. Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J. Biol. Chem. 1998, 273, 4928–4936. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.; Xu, T.; Ren, M.; Gao, M.; Lin, H. Quercetin antagonizes apoptosis, autophagy and immune dysfunction induced by di(2-ethylhexyl) phthalate via ROS/ASK1/JNK pathway. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 285, 109991. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Y.; Xing, Z.; Gong, T.; Yang, L.; Yang, T.; Chang, B.; Wang, X.; Yu, B.; Guo, R. ABT-737 increases cisplatin sensitivity through the ROS-ASK1-JNK MAPK signaling axis in human ovarian cancer cisplatin-resistant A2780/DDP cells. Oncol. Rep. 2024, 52, 122. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Chen, K.; Wang, Y.; Zhu, Y.; Zou, X.; Xu, X.; Jiang, Y. Astragalus polysaccharides ameliorate osteoarthritis via inhibiting apoptosis by regulating ROS-mediated ASK1/p38 MAPK signaling pathway targeting on TXN. Int. J. Biol. Macromol. 2024, 258, 129004. [Google Scholar] [CrossRef]
- Lombardi, A.; Villa, S.; Castelli, V.; Bandera, A.; Gori, A. T-Cell Exhaustion in Mycobacterium tuberculosis and Nontuberculous Mycobacteria Infection: Pathophysiology and Therapeutic Perspectives. Microorganisms 2021, 9, 2460. [Google Scholar] [CrossRef] [PubMed]
- Oberley-Deegan, R.E.; Rebits, B.W.; Weaver, M.R.; Tollefson, A.K.; Bai, X.; McGibney, M.; Ovrutsky, A.R.; Chan, E.D.; Crapo, J.D. An oxidative environment promotes growth of Mycobacterium abscessus. Free Radic. Biol. Med. 2010, 49, 1666–1673. [Google Scholar] [CrossRef]
- Oh, J.; Bowling, J.J.; Zou, Y.; Chittiboyina, A.G.; Doerksen, R.J.; Ferreira, D.; Leininger, T.D.; Hamann, M.T. Configurational assignments of conformationally restricted bis-monoterpene hydroquinones: Utility in exploration of endangered plants. Biochim. Biophys. Acta 2013, 1830, 4229–4234. [Google Scholar] [CrossRef]

| CLR MIC (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Strain | Source | Certain Disease | Day 3 | Day 5 | Day 7 | Day 14 | erm(41) |
| P-1 | Mab-1 | respiratory tract | AIDS | <0.25 | <0.25 | <0.25 | <0.25 | C28 |
| P-2 | Mab-2 | respiratory tract | / | 0.25 | 0.5 | 2 | 4 | T28 |
| P-3 | Mab-3 | respiratory tract | AIDS, DLBCL | 0.5 | 1 | 2 | 8 | T28 |
| P-4 | Mab-4 | respiratory tract | AIDS | 4 | 32 | 128 | 256 | T28 |
| P-5 | Mab-5 | respiratory tract | COPD | 0.5 | 4 | 4 | 64 | T28 |
| P-6 | Mab-6 | respiratory tract | / | 0.5 | 0.5 | 1 | 2 | C28 |
| P-7 | Mab-7 | cerebrospinal fluid | AIDS, syphilis | <0.25 | <0.25 | 0.25 | 1 | T28 |
| P-8 | Mab-8 | respiratory tract | PTB, CHB | 0.25 | 0.5 | 0.5 | 1 | C28 |
| Isolate | MIC (mg/L) | FICI | ||||
|---|---|---|---|---|---|---|
| NAC | CFZ | AMK | AZM | NAC + CFZ | NAC + CLR | |
| Mab-4 | 16000 | 8 | 64 | 128 | 0.3125 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, Y.; Xu, J.; Chen, Z.; Ren, Y.; Long, Y.; Huang, X.; Liu, J.; Huang, H.; Xie, S.; et al. N-Acetylcysteine as a Host-Directed Therapy Against Clarithromycin-Resistant Mycobacterium abscessus. Pathogens 2025, 14, 302. https://doi.org/10.3390/pathogens14040302
Yang S, Zhang Y, Xu J, Chen Z, Ren Y, Long Y, Huang X, Liu J, Huang H, Xie S, et al. N-Acetylcysteine as a Host-Directed Therapy Against Clarithromycin-Resistant Mycobacterium abscessus. Pathogens. 2025; 14(4):302. https://doi.org/10.3390/pathogens14040302
Chicago/Turabian StyleYang, Shuqi, Ying Zhang, Jinchuan Xu, Zhenyan Chen, Yang Ren, Yujiao Long, Xuejiao Huang, Juanxi Liu, Huan Huang, Shiqi Xie, and et al. 2025. "N-Acetylcysteine as a Host-Directed Therapy Against Clarithromycin-Resistant Mycobacterium abscessus" Pathogens 14, no. 4: 302. https://doi.org/10.3390/pathogens14040302
APA StyleYang, S., Zhang, Y., Xu, J., Chen, Z., Ren, Y., Long, Y., Huang, X., Liu, J., Huang, H., Xie, S., Ma, R., Dong, Y., Fan, X., Hu, Z., & Li, F. (2025). N-Acetylcysteine as a Host-Directed Therapy Against Clarithromycin-Resistant Mycobacterium abscessus. Pathogens, 14(4), 302. https://doi.org/10.3390/pathogens14040302





