Retrospective Epidemiological Analysis of Influenza A Infections in a Single Hospital in Korea (2007–2024): Age, Sex, and Seasonal Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. RNA Extraction and Real-Time PCR
2.4. Data Preprocessing
2.5. Data Analysis
2.6. Ethical Considerations
3. Results
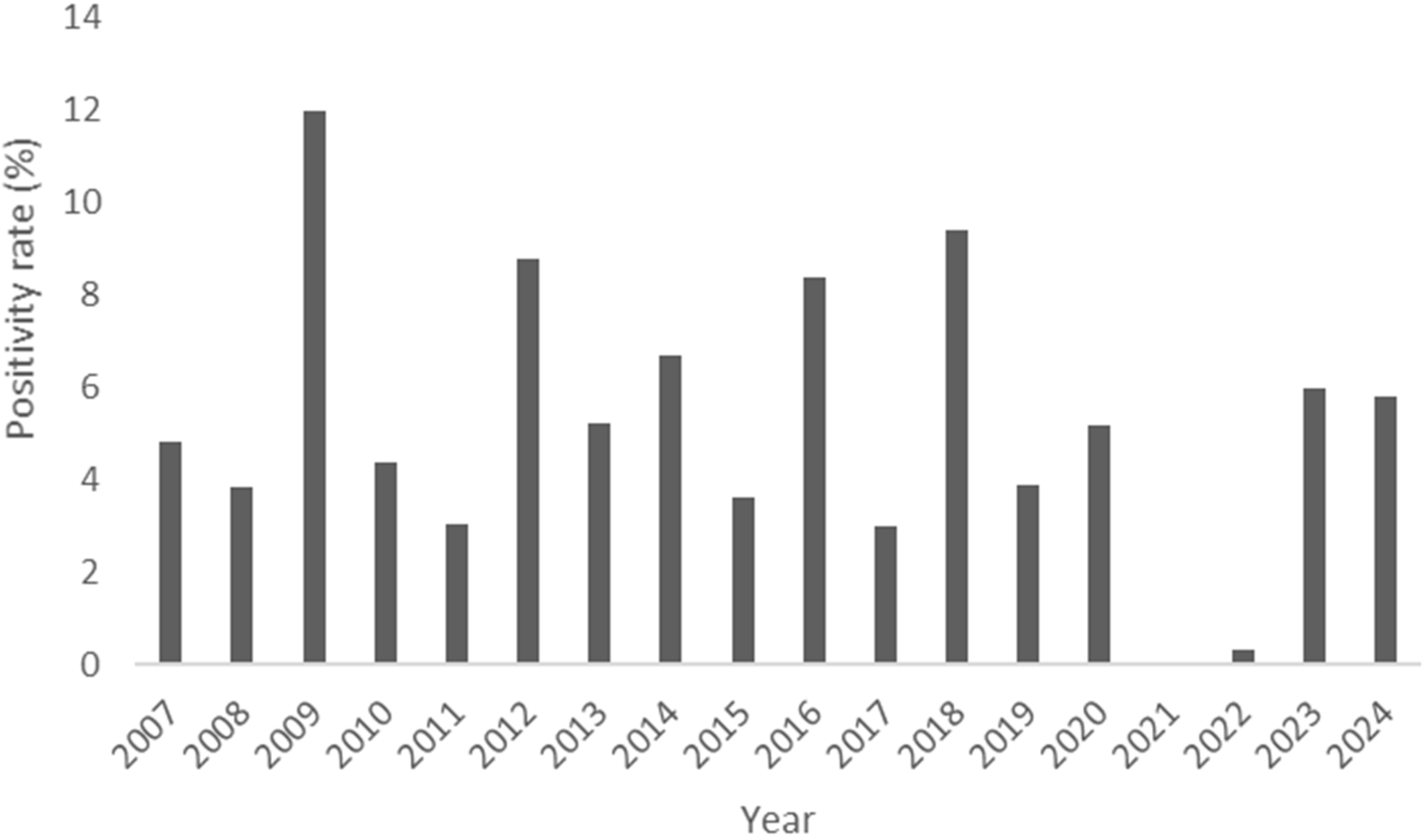
3.1. Annual Trends of Incidence
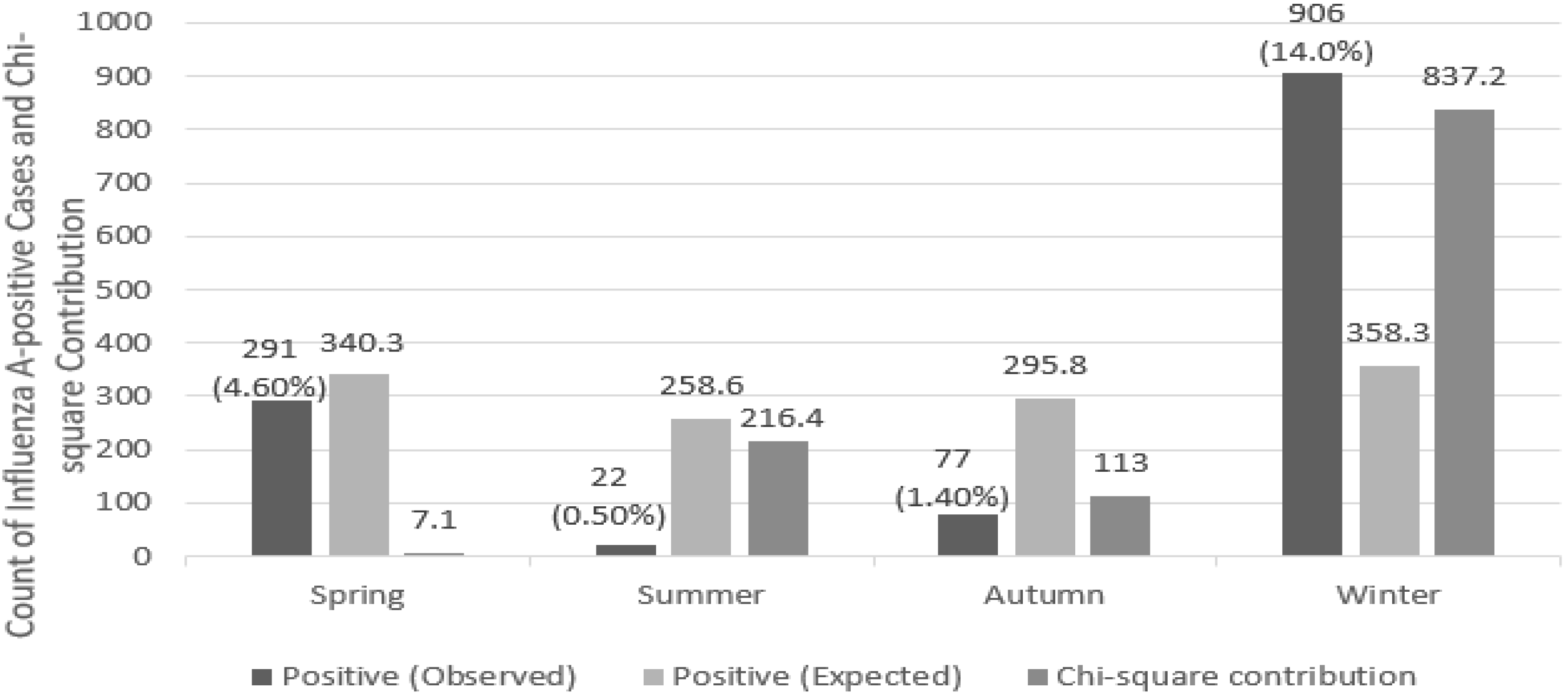
3.2. Seasonal Patterns
3.3. Sex-Based Analysis
3.4. Age-Based Analysis
4. Discussion
4.1. Annual Trends of Incidence
4.2. Seasonal Patterns
4.3. Sex-Based Analysis
4.4. Age-Based Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| CT | Cycle threshold |
References
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Bridges, C.B.; Cox, N.J.; Fukuda, K. Influenza-associated hospitalizations in the United States. JAMA 2004, 292, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Nichol, K.L. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch. Intern. Med. 2001, 161, 441–446. [Google Scholar] [CrossRef]
- Keilich, S.R.; Bartley, J.M.; Haynes, L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell. Immunol. 2019, 345, 103992. [Google Scholar] [CrossRef]
- Wong, K.K.; Jain, S.; Blanton, L.; Dhara, R.; Brammer, L.; Fry, A.M.; Finelli, L. Influenza-associated pediatric deaths in the United States, 2004–2012. Pediatrics 2013, 132, 796–804. [Google Scholar] [CrossRef]
- Coletti, P.; Poletto, C.; Turbelin, C.; Blanchon, T.; Colizza, V. Shifting patterns of seasonal influenza epidemics. Sci. Rep. 2018, 8, 12786. [Google Scholar] [CrossRef] [PubMed]
- Musau, J.; Baumann, A.; Kolotylo, C.; O’Shea, T.; Bialachowski, A. Infectious disease outbreaks and increased complexity of care. Int. Nurs. Rev. 2015, 62, 404–411. [Google Scholar] [CrossRef]
- Rao, X.; Chen, Z.; Dong, H.; Zhu, C.; Yan, Y. Epidemiology of influenza in hospitalized children with respiratory tract infection in Suzhou area from 2016 to 2019. J. Med. Virol. 2020, 92, 3038–3046. [Google Scholar] [CrossRef]
- Stark, J.H.; Sharma, R.; Ostroff, S.; Cummings, D.A.T.; Ermentrout, B.; Stebbins, S.; Burke, D.S.; Wisniewski, S.R. Local spatial and temporal processes of influenza in Pennsylvania, USA: 2003–2009. PLoS ONE 2012, 7, e34245. [Google Scholar] [CrossRef]
- Baguelin, M.; Flasche, S.; Camacho, A.; Demiris, N.; Miller, E.; Edmunds, W.J. Assessing optimal target populations for influenza vaccination programmes: An evidence synthesis and modelling study. PLoS Med. 2013, 10, e1001527. [Google Scholar] [CrossRef]
- Basta, N.E.; Chao, D.L.; Halloran, M.E.; Matrajt, L.; Longini, I.M., Jr. Strategies for pandemic and seasonal influenza vaccination of schoolchildren in the United States. Am. J. Epidemiol. 2009, 170, 679–686. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Huang, H.M.; Lan, Y.C. On temporal patterns and circulation of influenza virus strains in Taiwan, 2008–2014: Implications of 2009 pH1N1 pandemic. PLoS ONE 2016, 11, e0154695. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Kawaoka, Y. The first influenza pandemic of the new millennium. Influenza Other Respir. Viruses 2011, 5, 157–166. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Shen, C.; Luo, J.; Yu, W. Decreased incidence of influenza during the COVID-19 pandemic. Int. J. Gen. Med. 2022, 15, 2957–2962. [Google Scholar] [CrossRef]
- Bonacina, F.; Boëlle, P.Y.; Colizza, V.; Lopez, O.; Thomas, M.; Poletto, C. Global patterns and drivers of influenza decline during the COVID-19 pandemic. Int. J. Infect. Dis. 2023, 128, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.J.; Azziz-Baumgartner, E.; Budd, A.P.; Brammer, L.; Sullivan, S.; Pineda, R.F.; Cohen, C.; Fry, A.M. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, E.; Fefferman, N.H.; Naumov, Y.N.; Gorski, J.; Naumova, E.N. Influenza seasonality: Underlying causes and modeling theories. J. Virol. 2007, 81, 5429–5436. [Google Scholar] [CrossRef]
- Lowen, A.C.; Steel, J. Roles of humidity and temperature in shaping influenza seasonality. J. Virol. 2014, 88, 7692–7695. [Google Scholar] [CrossRef]
- Kudo, E.; Song, E.; Yockey, L.J.; Rakib, T.; Wong, P.W.; Homer, R.J.; Iwasaki, A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. USA 2019, 116, 10905–10910. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Cantorna, M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc. Nutr. Soc. 2010, 69, 286–289. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Xiao, Y.; Charland, N.; Moghadas, S.M. Strategies for early vaccination during novel influenza outbreaks. Sci. Rep. 2015, 5, 18062. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Stefanizzi, P.; Cuscianna, E.; Di Lorenzo, A.D.; Martinelli, A.; Tafuri, S. Effectiveness of on-site influenza vaccination strategy in Italian healthcare workers: A systematic review and statistical analysis. Expert Rev. Vaccines 2023, 22, 17–24. [Google Scholar] [CrossRef]
- Hakim, F.T.; Gress, R.E. Immunosenescence: Deficits in adaptive immunity in the elderly. Tissue Antigens 2007, 70, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Bowdish, D.M. Infection in an aging population. Curr. Opin. Microbiol. 2016, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.E.; Brown, C.S.; Nguyen-Van-Tam, J.S. Influenza in long-term care facilities. Influenza Other Respir. Viruses 2017, 11, 356–366. [Google Scholar] [CrossRef]
- Jackson, M.L.; Chung, J.R.; Jackson, L.A.; Phillips, C.H.; Benoit, J.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N. Engl. J. Med. 2017, 377, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef]
- Allen, J.C.; Toapanta, F.R.; Chen, W.; Tennant, S.M. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 2020, 38, 8264–8272. [Google Scholar] [CrossRef]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef] [PubMed]


| Age Group | Male (n) | Female (n) | Total (n) |
|---|---|---|---|
| Infants (0) | 2709 | 1844 | 4556 |
| Children (1–19) | 6341 | 4796 | 11,137 |
| Adults (20–64) | 1872 | 1027 | 2899 |
| Older adults (>65) | 3036 | 1656 | 4692 |
| Total | 13,958 | 9323 | 23,284 |
| Season | Positive (Observed) | Positive (Expected) | Negative (Observed) | Negative (Expected) | Positivity Rate (%) |
|---|---|---|---|---|---|
| Spring | 291 (4.6%) | 340.3 | 6100 (95.4%) | 6050.7 | 4.6% |
| Summer | 22 (0.5%) | 258.6 | 4788 (99.5%) | 4551.4 | 0.5% |
| Autumn | 77 (1.4%) | 295.8 | 5530 (98.6%) | 5311.2 | 1.4% |
| Winter | 906 (14.0%) | 358.3 | 5570 (86.0%) | 6117.7 | 14.0% |
| Age Group | Total Individuals | Positive | Negative | Positivity Rate (%) |
|---|---|---|---|---|
| Infants (0) | 4556 | 142 | 4414 | 3.1 |
| Children (1–19) | 11,137 | 559 | 10,578 | 5.0 |
| Adults (20–64) | 2899 | 221 | 2678 | 7.6 |
| Older adults (>65) | 4692 | 374 | 4318 | 7.9 |
| Age Group | Positive (Expected) | Negative (Expected) |
|---|---|---|
| Infants (0 years) | 255.1 | 4301 |
| Children (1–19 years) | 624 | 10,513 |
| Adults (20–64 years) | 162.3 | 2737 |
| Older adults (65 years and above) | 263 | 4429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.S.; Kim, H.H.; Jeon, J.-S.; Chung, Y.N.; Kim, J.K. Retrospective Epidemiological Analysis of Influenza A Infections in a Single Hospital in Korea (2007–2024): Age, Sex, and Seasonal Patterns. Pathogens 2025, 14, 282. https://doi.org/10.3390/pathogens14030282
Han JS, Kim HH, Jeon J-S, Chung YN, Kim JK. Retrospective Epidemiological Analysis of Influenza A Infections in a Single Hospital in Korea (2007–2024): Age, Sex, and Seasonal Patterns. Pathogens. 2025; 14(3):282. https://doi.org/10.3390/pathogens14030282
Chicago/Turabian StyleHan, Jeong Su, Hyeong Ho Kim, Jae-Sik Jeon, Yoo Na Chung, and Jae Kyung Kim. 2025. "Retrospective Epidemiological Analysis of Influenza A Infections in a Single Hospital in Korea (2007–2024): Age, Sex, and Seasonal Patterns" Pathogens 14, no. 3: 282. https://doi.org/10.3390/pathogens14030282
APA StyleHan, J. S., Kim, H. H., Jeon, J.-S., Chung, Y. N., & Kim, J. K. (2025). Retrospective Epidemiological Analysis of Influenza A Infections in a Single Hospital in Korea (2007–2024): Age, Sex, and Seasonal Patterns. Pathogens, 14(3), 282. https://doi.org/10.3390/pathogens14030282






