1. Introduction
Coxiella (
C.)
burnetii, a Gram-negative obligate intracellular bacterium, is the causative agent of Q fever, a widely distributed zooanthroponosis. After an incubation time of roughly two weeks, Q fever can manifest as an acute, self-limiting flu-like illness with complications such as pneumonia and hepatitis. Chronic disease occurs rarely and presents mainly as endocarditis or vasculitis [
1]. The most common sources for
C. burnetii transmission to humans are domestic ruminants. Humans become infected by aerosols derived from contaminated abortion material, birth products, urine, or feces. Asymptomatic, chronically infected cattle permanently shed
C. burnetii in milk [
2,
3]. Consequently, the consumption of raw milk and dairy food products bears the potential risk of oral human infection.
Different studies could prove that dairy products (raw milk, yogurt, cheese) of unpasteurized milk contained viable
C. burnetii [
4,
5,
6,
7]. Concentrations of
C. burnetii in milk can reach more than 10
6 bacteria in 250 mL [
8]. For a study, volunteers consumed
C. burnetii-contaminated raw milk. None of them developed any symptoms of disease [
9]. Therefore, the oral transmission of
C. burnetii is still controversially discussed. Experiments with mice infected by oral gavage resulted in clinical symptoms, a serological response, and bacterial dissemination [
10]. Furthermore,
C. burnetii directly delivered into the stomach resulted in colonization of the gastrointestinal tract [
11]. Importantly, the oral route of infection is the least efficient one [
11,
12]. Nevertheless, it represents a possible mode of transmission.
In the environment,
C. burnetii is highly resistant to stress factors such as temperature, osmotic pressure, and chemicals. Once inside a host cell, the bacteria replicate in a large lysosome-like parasitophorous vacuole called the
C. burnetii-containing vacuole (CCV). A unique characteristic of the CCV is its acidic environment (pH 4.5 to 5.3).
C. burnetii requires acidic conditions for the activation of their metabolism [
13,
14] and of the type IV secretion system, an essential virulence factor [
15]. The stomach is the first line of defense against bacterial infections from food intake. Gastric acid has a pH of 1 to 3 and contains digestive enzymes, including pepsin, to digest proteins. Different bacterial species like enteric bacteria (e.g.,
Shigella spp.,
E. coli, and
Yersinia enterocolitica) and
Helicobacter pylori have developed strategies to survive the extremely acidic milieu in the stomach [
16,
17] and are therefore regarded as acid-resistant. Acid resistance is defined as the percentage of survival of the bacterial inoculum exposed to pH 2.5 for 2 h. Gorden et al. redefined survival rates for enteric bacteria after acid treatment: (i) bacteria with a survival rate ≥ 10% of the inoculum considered acid-resistant and (ii) bacteria with survival rates less than 0.001% of the inoculum as acid-sensitive [
16]. The aim of this study was to analyze the survival rate of various
C. burnetii strains under extremely acidic conditions simulating the human stomach environment to assess the risk of contracting Q fever by consuming contaminated food. Thus, we treated
C. burnetii with various pH values for different time periods and calculated the recovery rate by determining the colony-forming units on ACCM-2 agar plates. In addition, we included pepsin, stomach medium, and milk in our experiments to determine their influence on
C. burnetii survival.
2. Materials and Methods
Chemicals were purchased from Sigma Aldrich, Darmstadt, Germany unless stated otherwise.
Coxiella burnetii strains. Nine different
C. burnetii strains were selected for the acid survival experiments and are listed in
Table 1. All strains were grown in acidified citrate cysteine medium-2 (ACCM-2) (Sunrise Science Products, San Diego, CA, USA) [
18,
19] for ten days at 37 °C, 2.5% O
2, and 5 % CO
2 prior to storage. Bacteria were harvested and stored in cell-freezing media (ACCM-2 with 10% glycerol) at −40 °C. All experiments with
C. burnetii strains were performed in a Biosafety Level-3 (BSL-3) laboratory with BSL-3 standard operating procedures.
C. burnetii (Nine Mile RSA439 phase II, 1 × 106/mL) was incubated in ACCM-2 medium at 37 °C, 2.5% O2, and 5% CO2 for five days. Bacteria were pelleted for 15 min at 4500× g and 4 °C and resuspended in PBS pH 7.4 to a concentration of 2 × 1010/mL after quantification by optical density at 600 nm (OD600), where an OD600 of 1 equals 1 × 109 C. burnetii per mL. Aliquots were stored for up to six months at −80 °C and used directly for the experiments. Experiments using Nine Mile phase II were performed under BSL-2 conditions.
2x stomach medium: 4.8 g/L NaCl, 1.56 g/L NaHCO3, 2.2 g/L KCl, 0.22 g/L CaCl2; adjust pH to 2.5 with 5 M HCl and autoclave 20 min at 121 °C; store at 4 °C for up to one month.
Dissolve 13.5 g formula (Holle Bio Anfangsmilch 1 demeter; Holle baby food AG, Riehen, Germany) in 85 mL sterile distilled water (a.d.) and adjust the pH to 2.5 with 5 M HCl, fill up to 100 mL; store aliquots at −20 °C for up to six months.
A pepsin stock solution was prepared at 10 mg/mL in PBS, pH 2.5 or in 2x stomach medium. Aliquots were stored at −20 °C for up to six months.
Determination of acid resistance. Acid resistance evaluation of C. burnetii strains was carried out in PBS with pH values of 2.0 to 4.0 as well as pH 7.4 as control. The pH was adjusted to the desired value with 37% HCl (Carl Roth, Karlsruhe, Germany) and sterile-filtered. Duplicates of each 100 µL pH-conditioned PBS were mixed with 5 µL of 2 × 1010/mL C. burnetii in 1.5 mL reaction tubes. After incubation time at 37 °C and 5% CO2, samples were neutralized with 900 µL PBS (pH 7.4), and the viable bacteria amount was calculated by CFU determination (see below).
Evaluation of proteolytic activity on the survival of C. burnetii. The pepsin stock solution of 10 mg/mL was diluted with PBS (pH 2.5 or 7.4) to 2, 1, and 0.5 mg/mL. Duplicates of each 100 µL dilution were mixed with 5 µL of 2 × 1010/mL C. burnetii in 1.5 mL reaction tubes. After incubation for 2 h or 3 h at 37 °C and 5% CO2, samples were neutralized with 900 µL PBS (pH 7.4), and the viable bacteria were calculated by CFU determination (see below).
Investigation of the role of formula on the survival of C. burnetii in an acidic environment. The pepsin stock solution of 10 mg/mL was diluted with 2x stomach medium, pH 2.5 to 4 mg/mL. Equal volumes of 2× stomach medium with or without pepsin were premixed with a.d., pH 2.5 or with formula. Duplicates of each 100 µL dilution and, as controls, 100 µL PBS with pH 2.5 and pH 7.4 were combined with 5 µL of 2 × 1010/mL C. burnetii in 1.5 mL reaction tubes. After an incubation time of 2 h and 3 h at 37 °C and 5% CO2, samples were neutralized with 900 µL PBS (pH 7.4), and the viable bacteria were calculated by CFU determination (see below).
Determination of colony-forming units (CFU). 2x ACCM-2 medium was prepared as described by the manufacturer and adjusted to pH 4.75 with 6 N NaOH, sterile-filtered, and stored at 4 °C for up to 4 weeks. An equal volume of sterile 0.5% agarose in a.d. was mixed with 2x ACCM-2 medium, and 20 mL was used to pour a 10 cm plate. Serial 10-fold dilutions of the C. burnetii suspension in 1x ACCM-2 medium were prepared in a 96-well dish. Between each dilution step, the bacterial suspension was resuspended thoroughly at least 10 times. The CFU titer was determined by spotting 10 µL of each diluted bacterial suspension on the top of the agarose plate. Three spots were spotted on the plate per dilution and served as technical replicates. ACCM-2 agarose plates were incubated for 9 to 14 d at 37 °C, 2.5% O2, and 5% CO2. Thereafter, colonies were counted, and the concentration of viable bacteria (CFU/mL) was calculated.
Re-infection of buffalo green monkey (BGM) cells. A total of 6 × 10
4 BGM cells were seeded into 24-well culture plates (tissue-culture treated, Greiner bio-one, Frickenhausen, Germany) and cultured for two days until they reached 80% confluence.
C. burnetii were incubated for 2 h in PBS adjusted to different pH values. Cells were infected with the pH-conditioned bacteria for 24 h at 37 °C and 5 % CO
2. Infected cells were washed with pre-warmed PBS (37 °C), and BGM medium (OptiMEM, 5% FCS) was added for further cultivation. After incubation times of 1 and 14 d, the cells were sampled by washing the BGM cell layer three times with pre-warmed PBS and detaching by treatment with 100 µL trypsin/EDTA. After a 10 to 15 min incubation at 37 °C, the trypsin was neutralized with 400 µL PBS. The samples were then stored at −40 °C. For determination of the bacteria concentration, the samples were treated with three freeze (−40 °C) and thaw cycles to release the bacteria from their vacuoles. For PCR detection, 100 µL aliquots of the samples were inactivated by boiling for 20 min at 95 °C. The number of genome equivalents (GEs) was monitored by quantification of the isocitrate dehydrogenase (
icd) gene by quantitative real-time PCR (qPCR) [
20]. A replication factor was calculated by dividing the log10 values of GEs detected at day 14 post-infection by the respective values detected at day 1 post-infection.
Immunofluorescence. EA.hy926 cells were seeded on coverslips in a 24-well plate and incubated overnight. Cells were infected with
C. burnetii with MOI 100. At 48 h post-infection, the cells were fixed with 4% paraformaldehyde (Alfa Aesar, Haverhill, MA, USA) in PBS (Biochrom GmbH, Berlin, Germany) for 15 min at room temperature in the dark, permeabilized with ice-cold methanol for 1 min, and quenched with 50 mM NH
4Cl (Roth) in blocking buffer (PBS with 5% goat serum; Thermo Fisher Scientific, Darmstadt, Germany) for 30 min at room temperature. The coverslips were incubated with anti-LAMP2 (H4B4, DSHB) and anti-
C. burnetii antibodies [
21] in blocking buffer, washed 3 times with PBS, and incubated with the secondary Alexa Fluor-labeled antibodies Alexa 488 and Alexa 594 (Dianova, Hamburg, Germany) in blocking buffer. The cells were mounted using ProLong Diamond with DAPI (Thermo Fisher Scientific, Darmstadt, Germany). Analysis was performed using a Carl Zeiss LSM 700 Laser Scanning Confocal Microscope, recording data with a 63x/1.4 oil immersion objective lens and Zen software (Zen 3.0 SR (black) 16.0.1.306) (Carl Zeiss, Oberkochen, Germany).
Statistical analysis. Statistical comparisons were conducted using a t-test with statistic software SPSS (version 24), and a significant difference was established as p ≤ 0.01.
3. Results
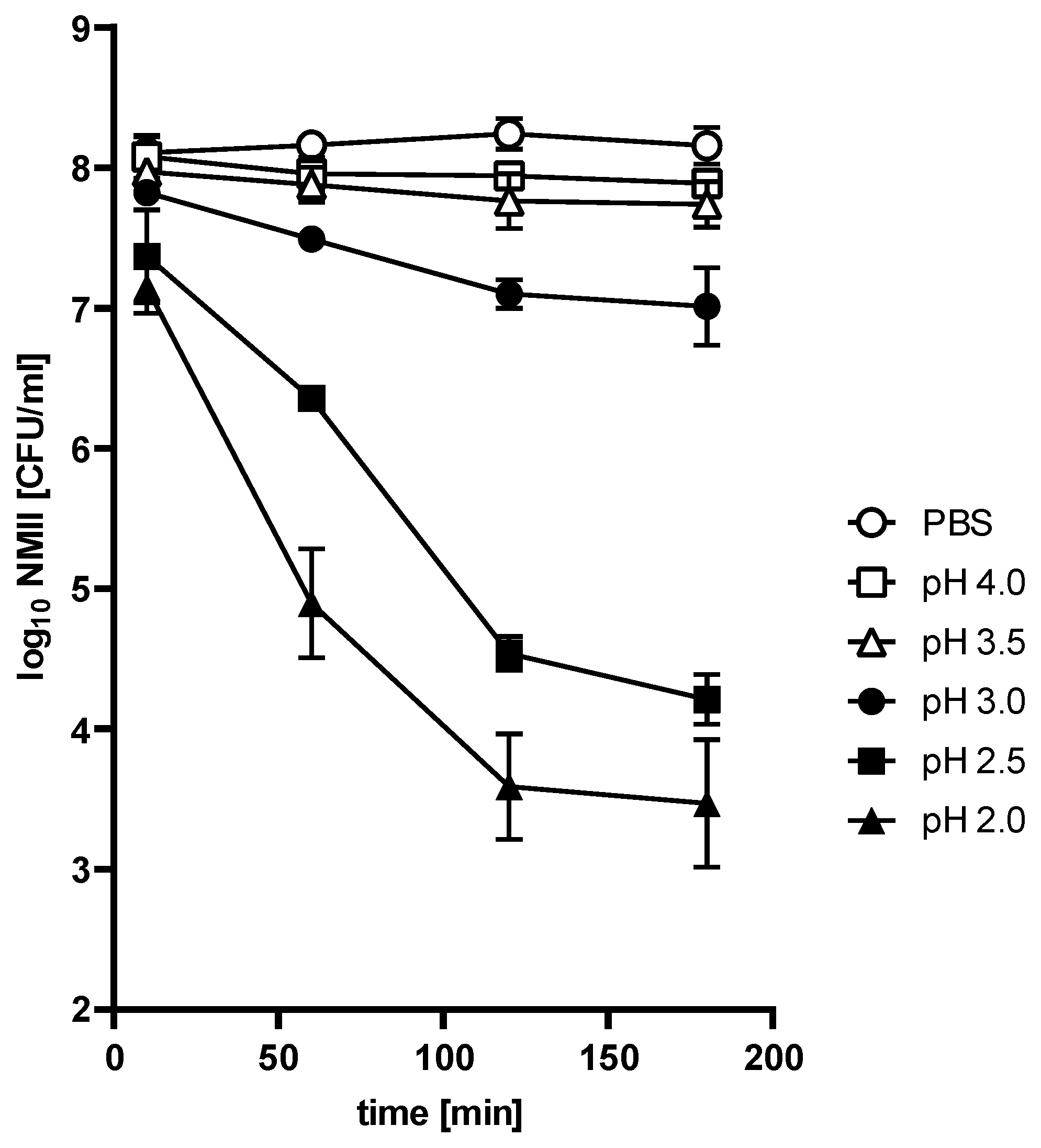
The C. burnetii NMII strain is acid-resistant. To determine whether
C. burnetii would be able to survive the stomach passage, we treated stationary phase
C. burnetii NMII (10 days in ACCM-2) [
22] with different pH values for different time periods. As a control, we incubated NMII in PBS at pH 7.4. Pathogen survival was evaluated by the ability to grow on ACCM-2 plates. As shown in
Figure 1, the survival of NMII depended on incubation time and pH value. In comparison to the PBS control, hardly any change in the bacterial recovery rate was observed after treatment at pH 3.5 and 4 over a period of 180 min. However, treatment with pH 2, 2.5, and 3 reduced bacterial viability. The recovery rate of live bacteria decreased exponentially by 0.5 log at pH 3 to 4.3 log at pH 2 after 120 min incubation. There was no further reduction for the remainder of the experiment under all tested conditions. Thus, acid tolerance was observed at all pH values. Importantly, the percent survival rate of NMII after 120 min at pH 2.5 was 0.02%. According to the definition [
16], NMII belongs to the acid-resistant group.
Bacteria might have different physiological properties depending on their growth phase. The experiments presented in
Figure 1 were performed with stationary-phase bacteria. To exclude an influence of the growth phase on acid resistance, we incubated exponentially growing bacteria for 3 h in PBS of different pH values. The growth phase did not seem to affect acid resistance (
Figure 2). Thus, pH 3 only slightly influenced the viability of exponential-phase bacteria. In contrast, pH 2.5 and pH 2 strongly reduced bacterial viability. Similar results were obtained with stationary-phase bacteria (
Figure 1).
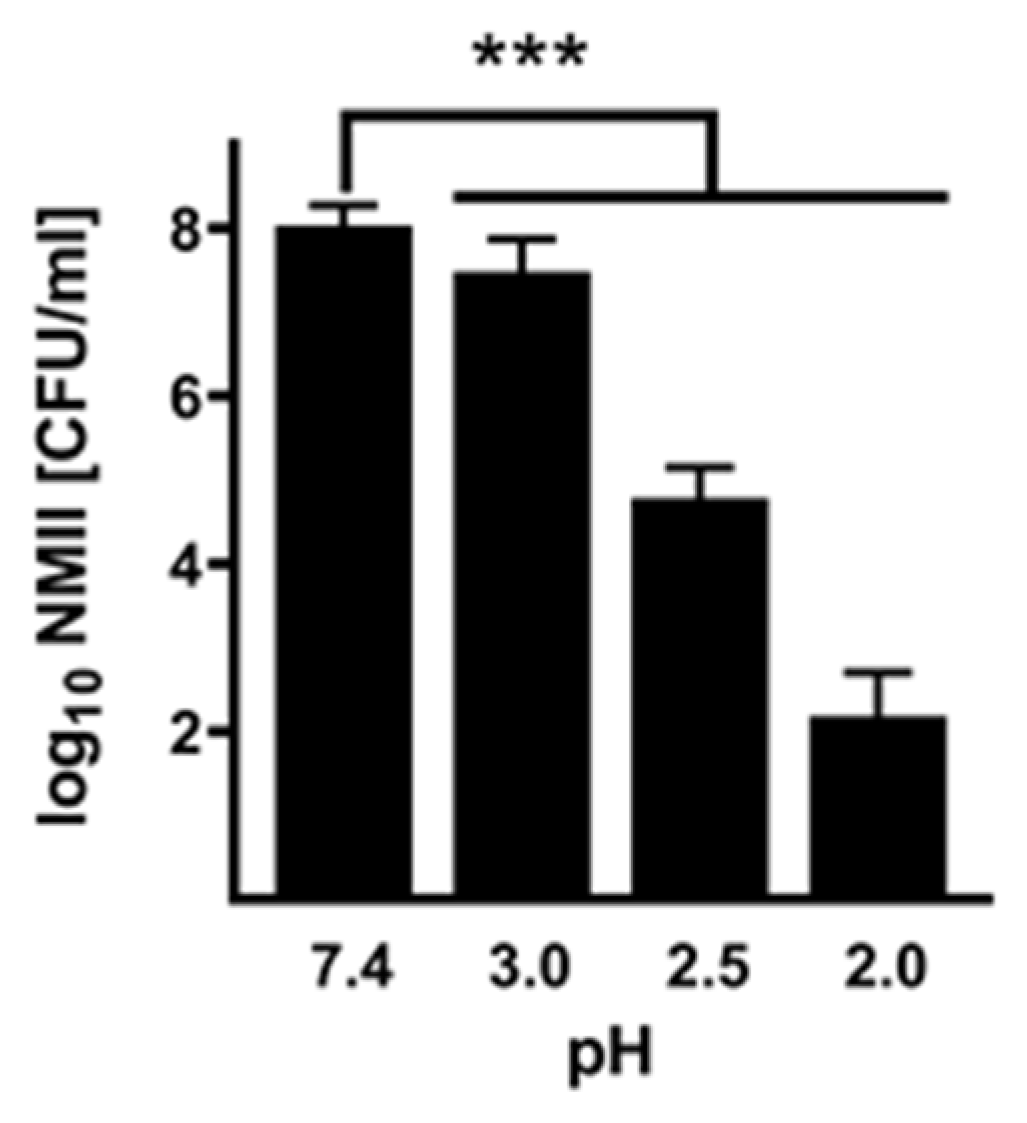
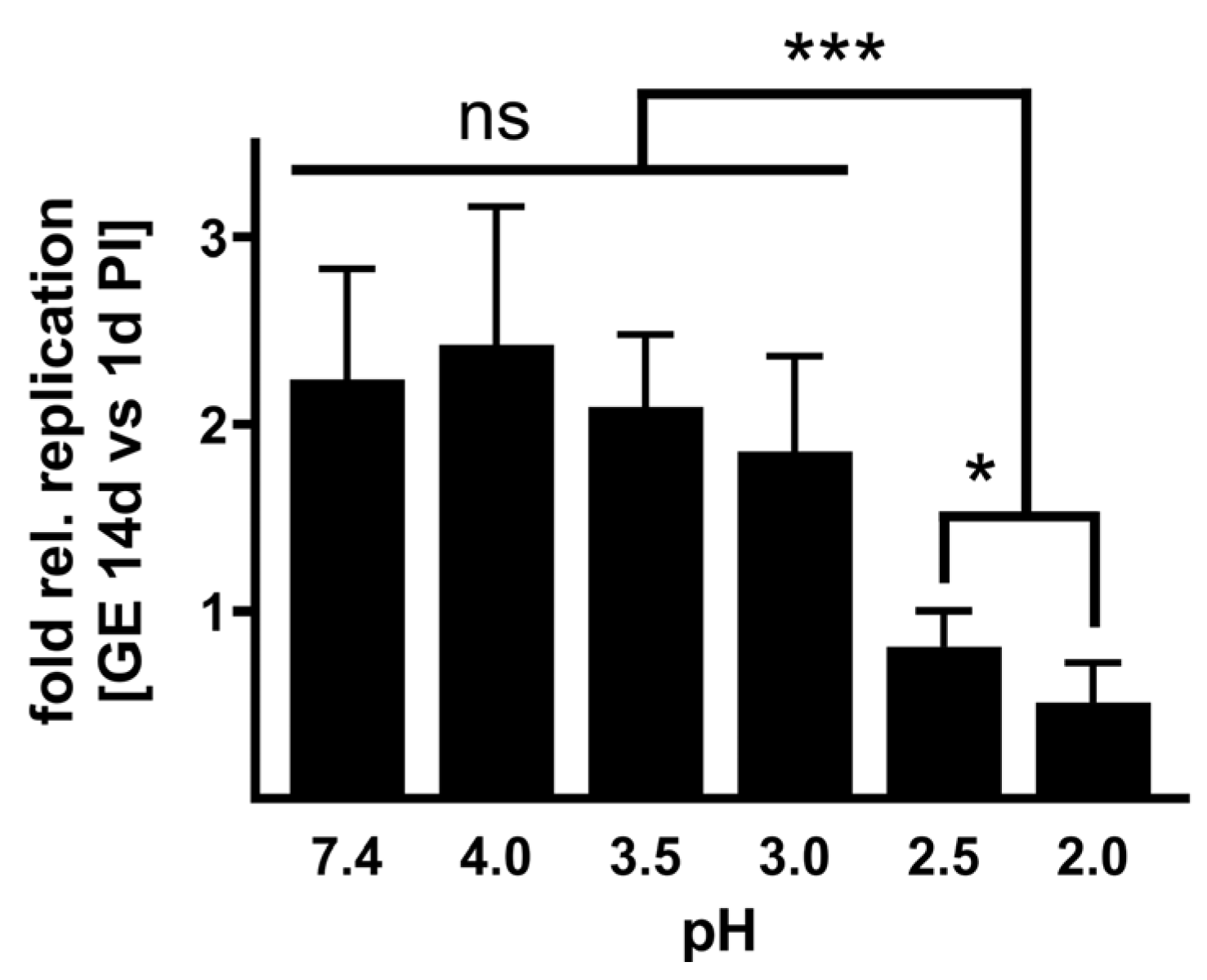
Infectivity of acid-treated NMII. To investigate whether the acid treatment influences the infectivity of
C. burnetii, we infected BGM cells with NMII, which were pre-treated for 2 h with different pH values or PBS as control. After 1 and 14 days, we purified the DNA of the samples and determined the
C. burnetii genomic equivalents (GEs). The ability to replicate was calculated by a replication factor of
C. burnetii (calculated by dividing log10 values of GE at day 14 by the value detected at day 1 post-infection).
C. burnetii treated with a weakly acidic solution of pH 3 to 4 retained their ability to replicate intracellularly (
Figure 3). In contrast,
C. burnetii treated with strong acidic concentrations of pH 2 to 2.5 showed no or only minor replication. These data stress that a pH of 2 or 2.5 reduces
C. burnetii viability (
Figure 1) and, consequently, the ability to replicate intracellularly (
Figure 3).
Pepsin influences the acid tolerance of C. burnetii. Pepsin is the main digestive enzyme in gastric juice. It is activated from its precursor pepsinogen by the stomach acid. Pepsinogen itself is produced by gastric chief cells in the stomach wall. We analyzed the impact of this enzyme on the acid tolerance of
C. burnetii. Thus, we treated
C. burnetii with different concentrations of pepsin at pH 7.4 or pH 2.5 for 2 h. The addition of pepsin at neutral pH to axenically grown
C. burnetii did not influence the viability of the pathogen (
Figure 4A). In contrast, at pH 2.5, pepsin increased the acid tolerance of
C. burnetii in a dose-dependent manner (
Figure 4A). Comparable results were obtained when we treated
C. burnetii at pH 2.5 and different concentrations of pepsin for 3 h (
Figure 4B). Thus, the presence of pepsin increased the acid tolerance of
C. burnetii.
Formula in combination with pepsin boosts acid tolerance and infectivity of C. burnetii. Bacterial survival upon passage through the stomach is influenced not only by the pH and the presence of pepsin but also by food and stomach medium, which contains several ions. To mimic conditions in the human stomach, we used an artificial stomach medium [
23]. To test the influence of milk and its components on
C. burnetii acid tolerance, we used baby formula. First, we confirmed that pepsin at a concentration of 2 mg/mL increases
C. burnetii viability not only in PBS (
Figure 4A,B) but also in stomach media (
Figure 5). Next, we aimed to analyze whether the addition of formula influences the acid tolerance of
C. burnetii. However, the addition of formula to the medium increases the pH value, which consequently would allow the increased survival of
C. burnetii (
Figure 1 and
Figure 2). Thus, we adjusted the pH value after the addition of the formula back to pH 2.5. We did not observe an increase in acidic tolerance if
C. burnetii was incubated in stomach medium containing formula. Importantly, in the presence of stomach medium, pepsin, and formula, we observed an increase in survival of nearly 4 logs (
Figure 5). These data suggest that pepsin might digest the formula into different products (amino acids), which might affect the acid tolerance of
C. burnetii.
Importantly, the treatment of
C. burnetii in stomach medium with pepsin and formula at pH 2.5 allowed the pathogen to infect and establish a replicative CCV in endothelial cells, similar to bacteria incubated in PBS at pH 7.4 (
Figure 6). In contrast, the incubation of
C. burnetii in PBS at pH 2.5 or in stomach medium +/− pepsin at pH 2.5 prevented the ability of
C. burnetii to replicate intracellularly (
Figure 6). Taken together, the composition of the stomach content seems to be decisive for the ability of
C. burnetii to survive the stomach passage as an infective agent.
Different C. burnetii strains show different acid tolerance. So far, we used the model strain NMII to investigate the acid tolerance of
C. burnetii. However, different
C. burnetii isolates seem to have different activity profiles and physiological properties, as demonstrated by the ability to grow in axenic media [
24] or by containing different subsets of virulence factors [
25]. Therefore, we analyzed the acid tolerance of
C. burnetii strains from different sources. Importantly, the response to the acid treatment was strain-specific (
Figure 7). Indeed, acid treatment divided the
C. burnetii strains into two groups: (I) acid-tolerant strains (survival rate between 0.001–10%) and (II) acid-sensitive strains (survival rate < 0.001%). The acid-tolerant strains, such as the human strains 1349, NL-Limburg, and Scurry, as well as the sheep strain 1348, were decreased in their viability by 1 to 3 log level at pH 2.5 with a survival rate between 0.01% (Scurry) and 2.86% (1349). Treatment with pH 2 reduced the live bacteria concentration of the acid-tolerant strains clearly and resulted in a survival rate lower than 500 bacteria. However, acid-sensitive strains, such as the human strains Henzerling and D-Jena, as well as the sheep strain Z3055/91 and model strain NMI, did not survive treatment with pH 2.5. Taken together, the acid tolerance of
C. burnetii is strain-specific.
4. Discussion
Chronically infected ruminants, especially cattle, permanently shed
C. burnetii in milk [
26]. Nevertheless, consuming unpasteurized dairy products is considered to pose a low risk for Q fever infection in humans even though there is evidence that most bulk tank milk samples contain viable
C. burnetii and that human consumption of
C. burnetii-contaminated dairy products leads to a higher risk for seroconversion [
27]. There are two possible ways of human infection by contaminated milk products: (i) By regurgitation of gastric content and aspiration into the respiratory tract. Several pulmonary syndromes may occur after aspiration, including aspiration pneumonia, which is defined as inhalation of oropharyngeal secretions that are colonized by pathogenic bacteria [
28]. Especially, infants and elderly persons possess a higher risk. Indeed, milk aspiration might cause death in infants [
29]; and (ii) by infection via the gastric route.
One early border of gastric host defense is the very low pH value of the stomach. The amount of pathogens that survive the gastric environment is influenced by stomach acidity. In this study, we analyzed the survival rate of
C. burnetii under the extreme acidic condition of the human stomach. To determine the acid resistance of
C. burnetii, we treated the reference strain NMII with pH values between 2.0 and 4.0 for different periods of time. The survival of NMII was dependent on incubation time and pH value (
Figure 1 and
Figure 2). With increasing treatment time, the recovery rate of NMII decreased for highly acidic conditions (pH < 3.0). In contrast, less acidic conditions (pH > 3.5) hardly affected bacterial vitality (recovery rate after 120 min: 35% of the inoculum). Under extremely acidic conditions (120 min, pH 2.5), NMII only survives with a recovery rate of 0.02%. According to the definition of Gorden and colleagues [
16], NMII is classified as an acid-resistant bacterium. In contrast, gastrointestinal tract pathogens like
Shigella spp. and
E. coli survive under the same conditions with clearly higher rates (50 to 55%) [
16]. These differences might be caused by the different main infection route of gastrointestinal bacteria versus respiratory pathogens, like
C. burnetii, and, thus, different infection and adaptation strategies.
Several bacteria have evolved resistance mechanisms against the lethal pH value ≤ 2.5 by acid tolerance response (e.g., gas system, biofilm formation, proton pumps) to sense and adapt to an acidified environment [
30]. Bacteria causing foodborne diseases need approximately 90 to 120 min after exposure to the triggering pH to mount a fully active acid response [
31]. In our study, NMII develops a pH value-independent acid tolerance response between 120 and 180 min post-exposure (
Figure 1). The mechanism responsible for the acid tolerance response in
C. burnetii is currently unknown. However, this tolerance is not only important for the adaptation to the gastric environment because
C. burnetii localizes in an acidic, lysosomal-like compartment within its host cells [
32,
33]. This obligate intracellular pathogen not only survives this harsh environment—it even requires it for intracellular replication [
18]. Key to the ability to replicate is the presence of the type IVB secretion system (T4SS) [
34], which is activated by the low pH within the CCV [
15].
Furthermore, the acid treatment did not affect the ability of
C. burnetii to invade eukaryotic cells (
Figure 3 and
Figure 6). However, it might be possible that passage through the acidic stomach might limit the ability of
C. burnetii to replicate intracellularly (
Figure 3 and
Figure 6). In the future, it will be interesting to investigate the invasion and replication of
C. burnetii in human colon cells after acid treatment to assess the risk for intestinal entry of
C. burnetii in humans. In cattle, intestinal epithelial cells permit the invasion of
C. burnetii and serve as a possible entry site for dissemination in the body but not as host cells for bacterial replication [
35].
Different clinical outcomes of Q fever in humans and animals have been associated with distinct virulence in genomic groups of
C. burnetii [
36,
37]. In this study, we investigated the acid stress reaction of different
C. burnetii strains (
Figure 7). The recovery rate of
C. burnetii showed considerable differences between the strains. They could be divided into two groups: acid-resistant (1349, NL-Limburg, Scurry, 1348) and acid-sensitive strains (Henzerling, D-Jena, Z3055/91, NMI). The acid response of the strains was independent of the strain’s original host (human, sheep, tick) and the genotype.
C. burnetii strains isolated from humans (Henzerling, Hz, Scurry, 1349, NL-Limburg), sheep (Z3055/91, 1348), or ticks (NM) reacted differently to pH 2.5 treatment. The Dutch outbreak strain NL-Limburg survived the acid treatment (pH 2.5, 120 min) with a recovery rate of 0.12 %. NL-Limburg belongs to the same genotype (MST33) as the sheep strain Z3055/91, and a genome comparison indicated a high agreement with the genome of strain Henzerling (MST18) [
38]. In our study, both Z3055/91 and Henzerling did not survive the acid treatment. The genome comparison found mutations in genes coding for ankyrin repeat domain proteins, membrane proteins, and proteins involved in translation and transcription [
39]. These mutations could influence the acid stress response of NL-Limburg. Furthermore, the acid response of
C. burnetii showed differences with respect to the LPS phase type. Virulent NMI, with a full-length LPS, did not survive a pH 2.5 treatment for 120 min. In contrast, avirulent NMII, which only lacks LPS O-polysaccharide chains [
40], survived the treatment. These findings are contrary to the decreasing acid resistance of enterobacteria that lack LPS O antigens [
41,
42]. In the future, the strain-specific mechanism(s) responsible for the acid response under extremely acidic conditions should be identified and characterized to assess the risk for the oral transmission of
C. burnetii strains.
The hypothesis that the consumption of
C. burnetii-contaminated dairy products can lead to foodborne Q fever in humans is controversial. Our data suggest that the composition of the stomach content might be decisive for
C. burnetii acid tolerance and, thus, its ability to survive passage through the stomach in a physiological state that retains pathogen infectivity. Thus, the presence of pepsin and formula allows the survival of
C. burnetii even at pH 2.5, which, consequently, sustains the infectivity and ability of the bacteria to replicate intracellularly (
Figure 4,
Figure 5 and
Figure 6). Especially, the combination of pepsin and formula alters the acid tolerance of
C. burnetii, suggesting that individual milk components liberated by pepsin might be the trigger (
Figure 5 and
Figure 6). Milk and milk components have already been described for several bacteria as being protective during the stomach passage. Thus, the survival of
Bacillus cereus strains was improved by milk products [
43], and the viability of
Bifidobacteria was increased by milk components during a simulated stomach passage [
44]. In general, food is a protective factor for bacteria during passage through the stomach, as it increases the stomach pH. However, in addition to the buffering function of food, milk components might provide further signals, allowing the bacteria to adapt to the low pH within the stomach.
One risk for oral transmission is the fluctuating acid concentration in the human stomach. The pH value of adult gastric juice increased depending on the food composition, from pH 2.0 to pH 5, and returned to a normal value within an hour [
45]. In contrast, the pH value of newborn infants increased from pH 3.5 to pH 6.4 after feeding and dropped to a normal value after 210 min [
46]. The results of the study suggest that depending on bacterial strain, pH value, stomach content, and time,
C. burnetii can survive passage through the stomach. As a consequence, persons with impaired acid production by functional dyspepsia and infants seem to have an enhanced risk for
C. burnetii survival after passing the stomach. The Q fever guidelines (RKI, CDC) recommend that (I)
C. burnetii-infected women should not feed their newborns by breastfeeding and (II) persons at particular risk should avoid the consumption of unpasteurized milk products. In the future, further studies are needed to determine the risk of food transmission for
C. burnetii and the role of the digestive infection route in the manifestation of Q fever in humans.