Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement
Abstract
1. Introduction
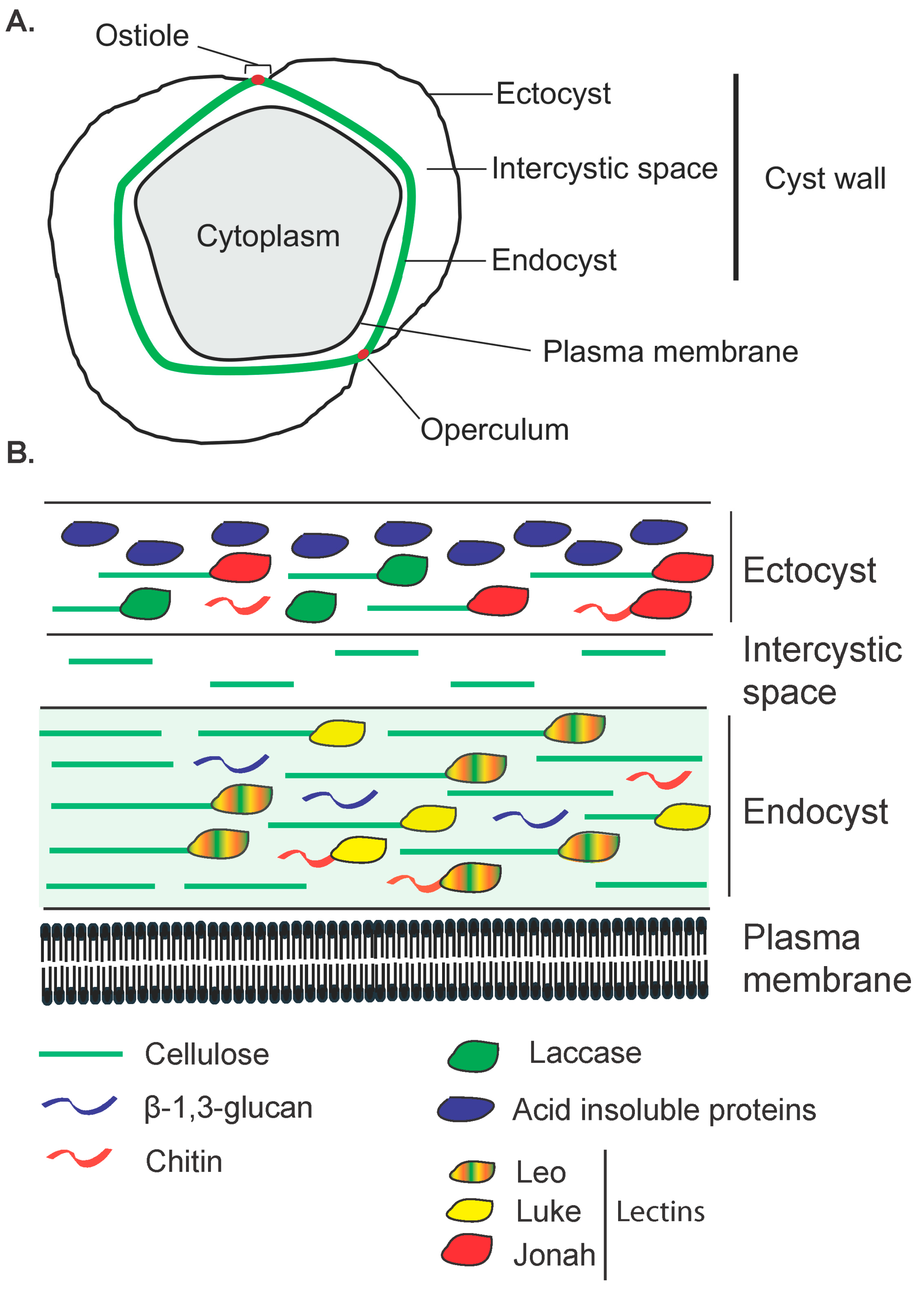
2. Structure of the Cyst Wall
3. Encystment
4. Excystment
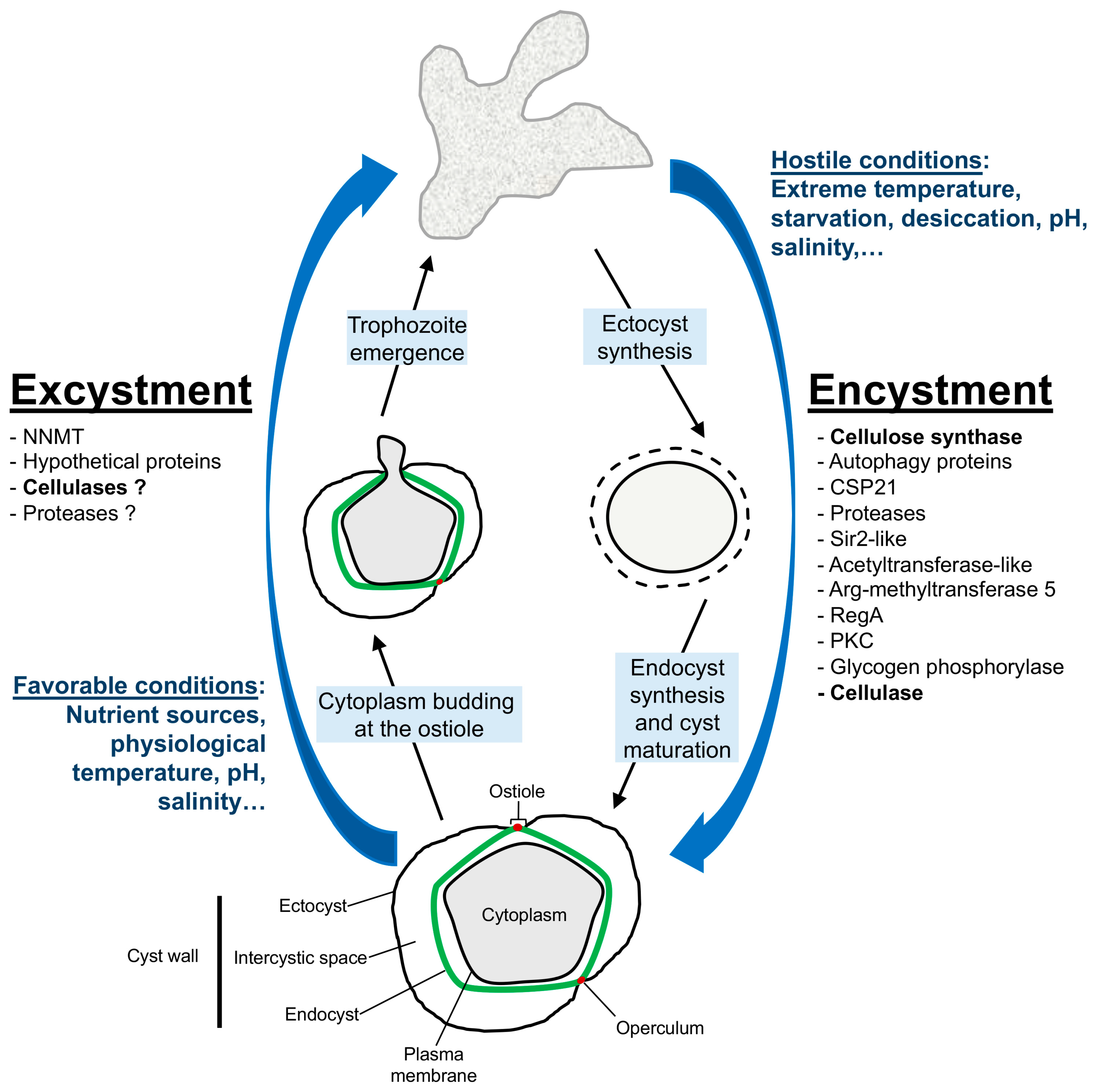
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scheid, P. Free-living amoebae as human parasites and hosts for pathogenic microorganisms. Proceedings 2018, 2, 692. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Delafont, V.; Rodier, M.H.; Héchard, Y. Free-living amoebae and squatters in the wild: Ecological and molecular features. FEMS Microbiol. Rev. 2019, 43, 415–434. [Google Scholar] [CrossRef]
- Abdul-Majid, M.A.; Mahboob, T.; Mong, B.G.; Jaturas, N.; Richard, R.L.; Tian-Chye, T.; Phimphila, A.; Mahaphonh, P.; Aye, K.N.; Aung, W.L.; et al. Pathogenic waterborne free-living amoebae: An update from selected Southeast Asian countries. PLoS ONE 2017, 12, e0169448. [Google Scholar] [CrossRef]
- Bornier, F.; Zas, E.; Potheret, D.; Laaberki, M.H.; Coupat-Goutaland, B.; Charpentier, X. Environmental free-living amoebae can predate on diverse antibiotic-resistant human pathogens. Appl. Environ. Microbiol. 2021, 87, e0074721. [Google Scholar] [CrossRef]
- Shi, Y.; Queller, D.C.; Tian, Y.; Zhang, S.; Yan, Q.; He, Z.; He, Z.; Wu, C.; Wang, C.; Shu, L. The ecology and evolution of amoeba-bacterium interactions. Appl. Environ. Microbiol. 2021, 87, e01866-20. [Google Scholar] [CrossRef]
- Greub, G.; Raoult, D. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 2004, 17, 413–433. [Google Scholar] [CrossRef]
- Salah, I.B.; Ghigo, E.; Drancourt, M. Free-living amoebae, a training field for macrophage resistance of mycobacteria. Clin. Microbiol. Infect. 2009, 15, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Balczun, C.; Scheid, P.L. Free-living amoebae as hosts for and vectors of intracellular microorganisms with public health significance. Viruses 2017, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D. Encystment in Acanthamoeba castellanii: A review. Exp. Parasitol. 2014, 145, S20–S27. [Google Scholar] [CrossRef]
- Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Stimulation of Acanthamoeba castellanii excystment by enzyme treatment and consequences on trophozoite growth. Front. Cell Dev. Biol. 2022, 10, 982897. [Google Scholar] [CrossRef]
- Fouque, E.; Trouilhé, M.C.; Thomas, V.; Hartemann, P.; Rodier, M.H.; Héchard, Y. Cellular, biochemical, and molecular changes during encystment of free-living amoebae. Eukaryot. Cell 2012, 11, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Cirillo, S.L.; Yan, L.; Bermudez, L.E.; Falkow, S.; Tompkins, L.S. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 1999, 67, 4427–4434. [Google Scholar] [CrossRef] [PubMed]
- Molmeret, M.; Horn, M.; Wagner, M.; Santic, M.; Abu-Kwaik, Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 2005, 71, 20–28. [Google Scholar] [CrossRef]
- Rayamajhee, B.; Subedi, D.; Peguda, H.K.; Willcox, M.D.; Henriquez, F.L.; Carnt, N. A systematic review of intracellular microorganisms within Acanthamoeba to understand potential impact for infection. Pathogens 2021, 10, 225. [Google Scholar] [CrossRef]
- Khunkitti, W.; Lloyd, D.; Furr, J.R.; Russell, A.D. Acanthamoeba castellanii: Growth, encystment, excystment and biocide susceptibility. J. Infect. 1998, 36, 43–48. [Google Scholar] [CrossRef]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Russell, A.D.; Furr, J.R.; Lloyd, D. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 2000, 46, 27–34. [Google Scholar] [CrossRef]
- Taravaud, A.; Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Drugs used for the treatment of cerebral and disseminated infections caused by free-living amoebae. Clin. Transl. Sci. 2021, 14, 791–805. [Google Scholar] [CrossRef]
- Garg, D.; Daigavane, S. A comprehensive review on Acanthamoeba keratitis: An overview of epidemiology, risk factors, and therapeutic strategies. Cureus 2024, 16, e67803. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The global epidemiology of clinical diagnosis of Acanthamoeba keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef]
- Ortilles, A.; Belloc, J.; Rubio, E.; Fernandez, M.T.; Benito, M.; Critobal, J.A.; Calvo, B.; Goni, P. In-vitro development of an effective treatment for Acanthamoeba keratitis. Int. J. Antimicrob. Agents 2017, 50, 325–333. [Google Scholar] [CrossRef]
- Bouheraoua, N.; Labbé, A.; Chaumeil, C.; Liang, Q.; Laroche, L.; Borderie, V. Acanthamoeba keratitis. J. Fr. Ophtamol. 2014, 37, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Aqeel, Y.; Khan, N.A. The development of drugs against Acanthamoeba infections. Antimicrob. Agents Chemother. 2016, 60, 6441–6450. [Google Scholar] [CrossRef] [PubMed]
- Franch, A.; Knutsson, K.A.; Pedrotti, E.; Fasolo, A.; Bertuzzi, F.; Birattari, F.; Bonacci, E.; Leon, P.; Papa, V. Treatment of Acanthamoeba keratitis with high dose PHMB (0.08%) monotherapy in clinical practice: A case series. Eur. J. Ophthamol. 2024, 14, 11206721241299470. [Google Scholar] [CrossRef] [PubMed]
- European Commission Grants Marketing Authorization for Acanthamoeba Keratitis Treatment. Available online: https://www.optometrytimes.com/view/european-commission-grants-marketing-authorization-for-acanthamoeba-keratitis-treatment (accessed on 27 January 2025).
- Fouque, E.; Héchard, Y.; Hartemann, P.; Humeau, P.; Trouilhé, M.C. Sensitivity of Vermamoeba (Hartmanella) vermiformis cysts to conventional disinfectants and protease. J. Water Health 2015, 13, 302–310. [Google Scholar] [CrossRef]
- Dupuy, M.; Berne, F.; Herbelin, P.; Binet, M.; Berthelot, N.; Rodier, M.H.; Soreau, S.; Héchard, Y. Sensitivity of free-living amoeba trophozoites and cysts to water disinfectants. Int. J. Hyg. Environ. Health 2014, 217, 335–339. [Google Scholar] [CrossRef]
- Cervero-Arago, S.; Rodriguez-Martinez, S.; Canals, O.; Salvado, H.; Araujo, R.M. Effect of thermal treatment on free-living amoeba inactivation. J. Appl. Microbiol. 2014, 116, 728–736. [Google Scholar] [CrossRef]
- Krishna-Murti, C.R.; Shukla, O.P. Differentiation of pathogenic amoebae: Encystation and excystation of Acanthamoeba culbertsoni—A model. J. Biosci. 1984, 6, 475–489. [Google Scholar] [CrossRef]
- Weisman, R.A. Differentiation in Acanthamoeba castellanii. Annu. Rev. Microbiol. 1976, 30, 189–219. [Google Scholar] [CrossRef]
- Griffiths, A.J.; Hughes, D.E. Starvation and encystment of a soil amoeba Hartmanella castellanii. J. Protozool. 1968, 15, 673–677. [Google Scholar] [CrossRef]
- Rodriguez-Zaragoza, S. Ecology of free-living amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef]
- Sriram, R.; Schoff, M.; Booton, G.; Fuerst, P.; Visvesvara, G.S. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J. Clin. Microbiol. 2008, 46, 4045–4048. [Google Scholar] [CrossRef] [PubMed]
- Jahangeer, M.; Mahmood, Z.; Munir, N.; Waraich, U.E.A.; Mahmood-Tahir, I.; Akram, M.; Ali-Shah, S.M.; Zulfqar, A.; Zainab, R. Naegleria fowleri: Sources of infection, pathophysiology, diagnosis, and management; a review. Clin. Exp. Pharmacol. Physiol. 2020, 47, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Coulon, C.; Collignon, A.; McDonnell, G.; Thomas, V. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J. Clin. Microbiol. 2010, 48, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Aksozek, A.; McClellan, K.; Howard, K.; Niederkorn, J.Y.; Alizadeh, H. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J. Parasitol. 2002, 88, 621–623. [Google Scholar] [CrossRef]
- Thomas, V.; Loret, J.F.; Jousset, M.; Greub, G. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 2008, 10, 2728–2745. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Jamerson, M.; Kaneshiro, E.S. Free-living amoebae, Legionella and Mycobacterium in tap water supplied by a municipal drinking water utility in the USA. J. Water Health 2010, 8, 71–82. [Google Scholar] [CrossRef]
- Taravaud, A.; Ali, M.; Lafosse, B.; Nicolas, V.; Féliers, C.; Thibert, S.; Lévi, Y.; Loiseau, P.M.; Pomel, S. Enrichment of free-living amoebae in biofilms developed at upper water levels in drinking water storage towers: An inter- and intra-seasonal study. Sci. Total Environ. 2018, 633, 157–166. [Google Scholar] [CrossRef]
- Fechtali-Moute, Z.; Pomel, S. Influence of the age of free-living amoeba cysts on their vertical distribution in a water column. Microorganisms 2024, 12, 474. [Google Scholar] [CrossRef]
- Garajova, M.; Mrva, M.; Vaskonicova, N.; Martinka, M.; Melicherova, J.; Valigurova, A. Cellulose fibrils formation and organisation of cytoskeleton during encystment are essential for Acanthamoeba cyst wall architecture. Sci. Rep. 2019, 9, 4466. [Google Scholar] [CrossRef]
- Upadhyay, J.M.; Crow, S.; Cox, A. The cyst wall composition of Harmanella glebae. Proc. Exp. Biol. Med. 1984, 175, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Locard-Paulet, M.; Noël, C.; Duchateau, M.; Giai Gianetto, Q.; Moumen, B.; Rattei, T.; Héchard, Y.; Jensen, L.J.; Matondo, M.; et al. A time-resolved multi-omics atlas of Acanthamoeba castellanii encystment. Nat. Commun. 2022, 13, 4104. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, Y.; Siddiqui, R.; Khan, N.A. Silencing of xylose isomerase and cellulose synthase by siRNA inhibits encystation in Acanthamoeba castellanii. Parasitol. Res. 2013, 112, 1221–1227. [Google Scholar] [CrossRef]
- Du, Q.; Schaap, P. The social amoeba Polysphondylium pallidum loses encystation and sporulation, but can still erect fruiting bodies in the absence of cellulose. Protist 2014, 165, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, J.; Bushkin, G.G.; Chatterjee, A.; Robbins, P.W. Strategies to discover the structural components of cyst and oocyst wals. Eukaryot. Cell 2013, 12, 1578–1587. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, S.; Maurer-Alcala, X.X.; Yan, Y. How ciliated protists survive by cysts: Come key points during encystment and excystment. Front. Microbiol. 2022, 13, 785502. [Google Scholar] [CrossRef]
- Aguilar-Diaz, H.; Carrero, J.C.; Argüello-Garcia, R.; Laclette, J.P.; Morales-Montor, J. Cyst and encystment in protozoan parasites: Optimal targets for new life-cycle interrupting strategies. Trends Parasitol. 2011, 27, 450–458. [Google Scholar] [CrossRef]
- Magistrado-Coxen, P.; Aqeel, Y.; Lopez, A.; Haserick, J.R.; Urbanowicz, B.R.; Costello, C.E.; Samuelson, J. The most abundant cyst wall proteins of Acanthamoeba castellanii are lectins that bind cellulose and localize to distinct structures in developing and mature cyst walls. PLoS Neglect. Trop. Dis. 2019, 13, e0007352. [Google Scholar] [CrossRef]
- Kanakapura-Sundararaj, B.; Goyal, M.; Samuelson, J. Cellulose binding and the timing of expression influence protein targeting to the double-layered cyst wall of Acanthamoeba. mSphere 2024, 9, e0046624. [Google Scholar] [CrossRef]
- Samba-Louaka, A. Encystment of free-living amoebae, so many blind spots to cover. Parasitologia 2023, 3, 53–58. [Google Scholar] [CrossRef]
- Chavez-Munguia, B.; Salazar-Villatoro, L.; Lagunes-Guillen, A.; Omana-Molina, M.; Espinosa-Cantellano, M.; Martinez-Palomo, A. Acanthamoeba castellanii cysts: New ultrastructural findings. Parasitol. Res. 2013, 112, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Bowers, B.; Korn, E.D. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J. Cell Biol. 1969, 41, 786–805. [Google Scholar] [CrossRef]
- Chavez-Munguia, B.; Omana-Molina, M.; Gonzalez-Lazaro, M.; Gonzalez-Robles, A.; Bonilla, P.; Martinez-Palomo, A. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J. Eukaryot. Microbiol. 2005, 52, 153–158. [Google Scholar] [CrossRef]
- Lemgruber, L.; Lupetti, P.; de Souza, W.; Vommaro, R.C.; da Rocha-Azevedo, B. The fine structure of the Acanthamoeba polyphaga cyst wall. FEMS Microbiol. Lett. 2010, 305, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.; Jarroll, E.L.; Khan, N.A. Carbohydrate analysis of Acanthamoeba castellanii. Exp. Parasitol. 2009, 122, 338–343. [Google Scholar] [CrossRef]
- Dudley, R.; Alsam, S.; Khan, N.A. Cellulose biosynthesis pathway is a potential target in the improved treatment of Acanthamoeba keratitis. Appl. Microbiol. Biotechnol. 2007, 75, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Chung, D.I.; Hong, Y.C.; Kong, H.H. Differentially expressed genes of Acanthamoeba castellanii during encystation. Korean. J. Parasitol. 2007, 45, 283–285. [Google Scholar] [CrossRef]
- Bouyer, S.; Rodier, M.H.; Guillot, A.; Héchard, Y. Acanthamoeba castellanii: Proteins involved in actin dynamics, glycolysis, and proteolysis are regulated during encystation. Exp. Parasitol. 2009, 123, 90–94. [Google Scholar] [CrossRef]
- Moon, E.K.; Chung, D.I.; Hong, Y.C.; Kong, H.H. Autophagy protein 8 mediating autophagosome in encysting Acanthamoeba. Mol. Biochem. Parasitol. 2009, 168, 43–48. [Google Scholar] [CrossRef]
- Moon, E.K.; Chung, D.I.; Hong, Y.; Kong, H.H. Atg3-mediated lipidation of Atg8 is involved in encystation of Acanthamoeba. Korean J. Parasitol. 2011, 49, 103–108. [Google Scholar] [CrossRef]
- Moon, E.K.; Hong, Y.; Chung, D.I.; Kong, H.H. Identification of Atg8 isoform in encysting Acanthamoeba. Korean J. Parasitol. 2013, 51, 497–502. [Google Scholar] [CrossRef]
- Song, S.M.; Han, B.I.; Moon, E.K.; Lee, Y.R.; Yu, H.S.; Jha, B.K.; Sylvatrie-Danne, D.B.; Kong, H.H.; Chung, D.I.; Hong, Y. Autophagy protein 16-mediated autophagy is required for the encystation of Acanthamoeba castellanii. Mol. Biochem. Parasitol. 2012, 183, 158–165. [Google Scholar] [CrossRef]
- Samba-Louaka, A.; Labruyere, E.; Matondo, M.; Locard-Paulet, M.; Olivo-Marin, J.C.; Guillen, N. Encystation and stress responses under the control of ubiquitin-like proteins in pathogenic amoebae. Microorganisms 2023, 11, 2670. [Google Scholar] [CrossRef] [PubMed]
- Hirukawa, Y.; Nakato, H.; Izumi, S.; Tsuruhara, T.; Tomino, S. Structure and expression of a cyst specific protein of Acanthamoeba castellanii. Biochim. Biophys. Acta 1998, 1398, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Chung, D.I.; Hong, Y.C.; Kong, H.H. Characterization of a serine proteinase mediating encystation of Acanthamoeba. Eukaryot. Cell 2008, 7, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Köhsler, M.; Marchetti-Deschmann, M.; Deutsch, A.; Allmaier, G.; Duchêne, M.; Walochnik, J. Major role for cysteine proteases during early phase of Acanthamoeba castellanii encystment. Eukaryot. Cell 2010, 9, 611–618. [Google Scholar] [CrossRef]
- Moon, E.K.; Hong, Y.; Chung, D.I.; Kong, H.H. Cysteine protease involving in autophagosomal degradation of mitochondria during encystation of Acanthamoeba. Mol. Biochem. Parasitol. 2012, 185, 121–126. [Google Scholar] [CrossRef]
- Lee, Y.R.; Na, B.K.; Moon, E.K.; Song, S.M.; Joo, S.Y.; Kong, H.H.; Goo, Y.K.; Chung, D.I.; Hong, Y. Essential role for an M17 leucine aminopeptidase in encystation of Acanthamoeba castellanii. PLoS ONE 2015, 10, e0129884. [Google Scholar] [CrossRef]
- Joo, S.Y.; Aung, J.M.; Shin, M.; Moon, E.K.; Goo, Y.K.; Chung, D.I.; Hong, Y. The role of the Acanthamoeba castellanii Sir2-like protein in the growth and encystation of Acanthamoeba. Parasit. Vectors 2020, 13, 368. [Google Scholar] [CrossRef]
- Rolland, S.; Mengue, L.; Noël, C.; Crapart, S.; Mercier, A.; Aucher, W.; Héchard, Y.; Samba-Louaka, A. Encystment induces down-regulation of acetyltransferase-like gene in Acanthamoeba castellanii. Pathogens 2020, 9, 321. [Google Scholar] [CrossRef]
- Moon, E.K.; Hong, Y.; Chung, D.I.; Goo, Y.K.; Kong, H.H. Identification of protein arginine methyltransferase 5 as a regulator for encystation of Acanthamoeba. Korean J. Parasitol. 2016, 54, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Schilde, C.; Birgersson, E.; Chen, Z.H.; McElroy, S.; Schaap, P. The cyclic AMP phosphodiesterase RegA critically regulated encystation in social and pathogenic amoebas. Cell. Signal. 2014, 26, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Chung, D.I.; Hong, Y.; Kong, H.H. Protein kinase C signaling molecules regulate encystation of Acanthamoeba. Exp. Parasitol. 2012, 132, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Kliescikova, J.; Martinez-Carretero, E.; de Pablos, L.M.; Profotova, B.; Nohynkova, E.; Osuna, A.; Valladares, B. Glycogen phosphorylase in Acanthamoeba spp.: Determining the role of the enzyme during the encystment process using RNA interference. Eukaryot. Cell 2008, 7, 509–517. [Google Scholar] [CrossRef]
- Moon, E.K.; Kong, H.H. Short-cut pathway to synthesize cellulose of encysting Acanthamoeba. Korean J. Parasitol. 2012, 50, 361–364. [Google Scholar] [CrossRef]
- Stewart, J.R.; Weisman, R.A. A chemical and autoradiographic study of cellulose synthesis during the encystment of Acanthamoeba castellanii. Arch. Biochem. Biophys. 1974, 161, 488–498. [Google Scholar] [CrossRef]
- Griffiths, A.J.; Hughes, D.E. The physiology of encystment of Hartmanella castellanii. J. Protozool. 1969, 16, 93–99. [Google Scholar] [CrossRef]
- Moon, E.K.; Hong, Y.; Chung, D.I.; Goo, Y.K.; Kong, H.H. Down regulation of cellulose synthase inhibits the formation of endocysts in Acanthamoeba. Korean J. Parasitol. 2014, 52, 131–135. [Google Scholar] [CrossRef]
- Derda, M.; Winiecka-Krusnell, J.; Linder, M.B.; Linder, E. Labeled Trichoderma reesei cellulase as a marker for Acanthamoeba cyst wall cellulose in infected tissues. Appl. Environ. Microbiol. 2009, 75, 6827–6830. [Google Scholar] [CrossRef]
- Deichmann, U.; Jantzen, H. The cellulase enzyme system during growth and development of Acanthamoeba castellanii. Arch. Microbiol. 1977, 113, 309–313. [Google Scholar] [CrossRef]
- Mattar, F.E.; Byers, T.J. Morphological changes and the requirements for macromolecule synthesis during excystment of Acanthamoeba castellanii. J. Cell. Biol. 1971, 49, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.P.; Griffiths, A.J. Excystment of the amoeba Hartmanella castellanii. J. Gen. Microbiol. 1971, 66, 247–249. [Google Scholar] [CrossRef][Green Version]
- Kaushal, D.C.; Shukla, O.P. Excystment of axenically prepared cysts of Hartmanella culbertsoni. J. Gen. Microbiol. 1977, 98, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.A.; Thompson, J.E. A scanning electron microscopic study of the excystment process of Acanthamoeba castellanii. Exp. Cell Res. 1972, 73, 415–421. [Google Scholar] [CrossRef]
- Kim, M.J.; Jo, H.J.; Quan, F.S.; Chu, K.B.; Kong, H.H.; Moon, E.K. Identification of essential genes for Acanthamoeba castellanii excystation during encystation and excystation. Parasites Hosts Dis. 2024, 62, 399–407. [Google Scholar] [CrossRef]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Cellulose degradation: A therapeutic strategy in the improved treatment of Acanthamoeba infections. Parasit. Vectors 2015, 8, 23. [Google Scholar] [CrossRef]
- Baig, A.M.; Khan, N.A.; Katyara, P.; Lalani, S.; Baig, R.; Nadeem, M.; Akbar, N.; Nazim, F.; Khaleeq, A. “Targeting the feast of a sleeping beast”: Nutrient and mineral dependencies of encysted Acanthamoeba castellanii. Chem. Biol. Drug Des. 2021, 97, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lazuana, T.; Astuty, H.; Sari, I.P. Effect of cellulase enzyme treatment on cyst wall degradation of Acanthamoeba sp. J. Parasitol. Res. 2019, 2019, 8915314. [Google Scholar] [CrossRef]
- Linder, M.; Winiecka-Krusnell, J.; Linder, E. Use of recombinant cellulose-binding domains of Trichoderma reesei cellulase as a selective immunocytochemical marker for cellulose in protozoa. Appl. Environ. Microbiol. 2002, 68, 2503–2508. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choaji, M.; Samba-Louaka, A.; Fechtali-Moute, Z.; Aucher, W.; Pomel, S. Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement. Pathogens 2025, 14, 268. https://doi.org/10.3390/pathogens14030268
Choaji M, Samba-Louaka A, Fechtali-Moute Z, Aucher W, Pomel S. Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement. Pathogens. 2025; 14(3):268. https://doi.org/10.3390/pathogens14030268
Chicago/Turabian StyleChoaji, Mathew, Ascel Samba-Louaka, Zineb Fechtali-Moute, Willy Aucher, and Sébastien Pomel. 2025. "Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement" Pathogens 14, no. 3: 268. https://doi.org/10.3390/pathogens14030268
APA StyleChoaji, M., Samba-Louaka, A., Fechtali-Moute, Z., Aucher, W., & Pomel, S. (2025). Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement. Pathogens, 14(3), 268. https://doi.org/10.3390/pathogens14030268






