An Intensive Care Outbreak Caused by Burkholderia cepacia from Bacterial Filters
Abstract
1. Introduction
2. Materials and Methods
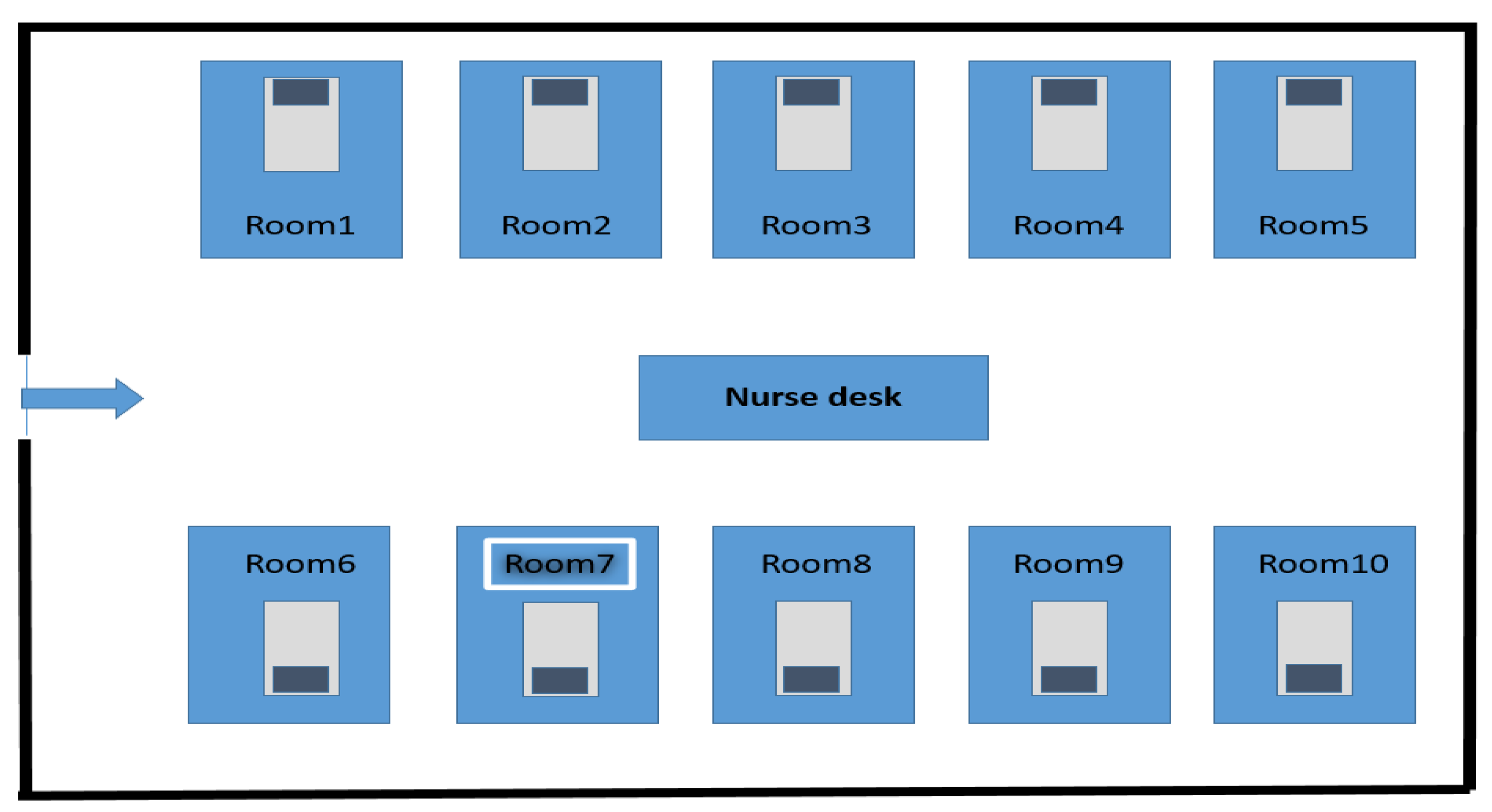
2.1. Hospital Settings
2.2. Environmental Investigation
2.3. Microbiological Analysis and Antibiogram
2.4. Molecular Analysis
2.5. Arbitrarily Primed Polymerase Chain Reaction (AP-PCR)
3. Results
4. Discussion
- Previously, the respiratory circuits were used without change throughout the patient’s hospitalization, but after the outbreak, the respiratory circuits began to be changed when the patients had excessive secretions;
- The respiratory circuits were wiped three times a day;
- It was determined that portable X-ray cassettes were used directly from patient to patient. X-ray cassettes began to be used by putting on protective bags while passing from patient to patient;
- The consultant physician, physiotherapist, dietician, and other personnel who were providing service by visiting all intensive care units at the same time were trained on the use of appropriate gloves, hand hygiene, and infection control measures. Those of these personnel who were not wearing protective gowns were warned to wear them;
- Some intensive care units that did not use bacteria filters constantly were warned about the correct and appropriate use of the filters and their inspections were carried out;
- Dialysis drain covers were open, closed, and disinfection was carried out;
- A detailed room cleaning was carried out after each patient was discharged;
- The humidification rate of humidifiers in the respiratory circuit was reduced to reduce bacterial colonization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP-PCR | Arbitrarily Primed Polymerase Chain Reaction |
| BCC | Burkholderia cepacia complex |
| HAI | Hospital-acquired infections |
| CDC | The Centers for Disease Control and Prevention |
| ICUs | Intensive care units |
| EMB | Eosin methylene blue |
| CLSI | Clinical and Laboratory Standards Institute |
| UPGMA | Unweighted Pairwise Grouping Mathematical Averaging |
| CSF | Cerebrospinal fluid. |
| SAH | Subarachnoid hemorrhage |
| SVD | Serebrovasküler disease |
| MI | Myocardial infarction |
| BFID | Hospital Stay (ICU) before First Isolation (Days) |
| AFID | Hospital Stay (ICU) after First Isolation (Days) |
| IF | Infectious Factor |
| TR | Treatment Response |
| PE | Pulmonary embolism |
| MM | Multiple Myeloma |
References
- Jin, Y.; Zhou, J.; Zhou, J.; Hu, M.; Zhang, Q.; Kong, N.; Hongguang, R.; Long, L.; Yue, J. Genomebased classification of Burkholderia cepacia complex provides new insight into its taxonomic status. Biol. Direct 2020, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.; Dash, L.; Gautam, V.; Shastri, J.; Kumar, S. An outbreak of Burkholderia cepacia complex in the paediatric unit of a tertiary care hospital. Indian J. Med. Microbiol. 2017, 35, 216–220. [Google Scholar] [CrossRef]
- Govan, J.; Balendreau, J.; Vandamme, P. Burkholderia cepacia–Friend and foe. ASM NEWS 2000, 66, 124e5. [Google Scholar]
- Luk, K.S.; Tsang, Y.M.; Ho, A.Y.; To, W.K.; Wong, B.K.; Wong, M.M.; Wong, Y.C. Invasive Burkholderia cepacia complex infections among persons who inject drugs, Hong Kong, China, 2016–2019. Emerg. Infect. Dis. 2022, 28, 323e30. [Google Scholar] [CrossRef] [PubMed]
- Parke, J.L.; Gurian-Sherman, D. Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 2001, 39, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, S.; Ansarı, M.S.; Dhıllo, A.K.; Ishaque, S.; Manzoor, S.; Baqı, S.; Rehman, H.U. Outbreak of Burkholderia Cepaciae Bacteremia in Neonatal Units of a Public Sector Hospital in Karachi, Pakistan. Pak. J. Med. Health Sci. 2023, 17, 466. [Google Scholar] [CrossRef]
- Bilgin, H.; Gelmez Altınkanat, G.; Bayrakdar, F. An outbreak investigation of Burkholderia cepacia infections related with contaminated chlorhexidine mouthwash solution in a tertiary care center in Turkey. Antimicrob. Resist. Infect. Control. 2021, 10, 143. [Google Scholar] [CrossRef]
- Hell, M.; Abel, C.; Albrecht, A.; Wojna, A.; Chmelizek, G.; Kern, J.M.; Maass, M.; Apfalter, P. Burkholderia cepacia e outbreak in obstetric patients due to intrinsic contamination of non-sterile ultrasound gel. BMC Proc. 2011, 5, O75. [Google Scholar] [CrossRef]
- Ghazal, S.S.; Al-Mudaimeegh, K.; Al Fakihi, E.M.; Asery, A.T. Outbreak of Burkholderia cepacia bacteremia in immunocompetent children caused by contaminated nebulized sulbutamol in Saudi Arabia. Am. J. Infect. Control. 2006, 34, 394e8. [Google Scholar] [CrossRef]
- Zurita, J.; Mejia, L.; Zapata STrueba, G.; Vargas, A.C.; Aguirre, S.; Falconi, G. Healthcare-associated respiratory tract infection and colonization in an intensive care unit caused by Burkholderia cepacia isolated in mouthwash. Int. J. Infect. Dis. 2014, 29, 96–99. [Google Scholar] [CrossRef]
- Shaban, R.Z.; Sotomayor-Castillo, C.; Nahidi, S.; Li, C.; Macbeth, D.; Mitchell, B.G.; Russo, P.L. Global burden, point sources, and outbreak management of healthcare-associated Burkholderia cepacia infections: An integrative review. Infect. Control. Hosp. Epidemiol. 2020, 41, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kömeç, Selda and Ceylan, Ayşe Nur and Özalp, Onur and Durmuş, Mehmet Akif and Gunduz, Alper and Yavaş, Cüneyd and Korkusuz, Ramazan, Burkholderia cepacia Outbreak in Intensive Care Units, Türkiye. Available online: https://ssrn.com/abstract=5033636 (accessed on 1 November 2024).
- Cooper, A.S. Oral Hygiene Care to Prevent Ventilator-Associated Pneumonia in Critically Ill Patients. Crit. Care Nurse 2021, 41, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Michetti, C.P.; Fakhry, S.M.; Ferguson, P.L.; Cook, A.; Moore, F.O.; Gross, R. Ventilator-associated pneumonia rates at major trauma centers compared with a national benchmark: A multi-institutional study of the AAST. J. Trauma Acute Care Surg. 2012, 72, 1165–1173. [Google Scholar] [CrossRef]
- Özcan, P.E.; Esen, F. Nosocomial pneumonia in intensive care. Curr. Med. J. 2002, 7, 37–40. [Google Scholar]
- Özdemir, L.; Özdemir, B.; Gegin, S.; Aksu, E.A. Rare Pathogenic Lower Respiratory Tract Factors and Antibiotic Sensitivity Situa-tions in Respiratory Intensive Care. Hitit Med. J. 2024, 6, 200–207. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance defnition of health care-associated infection and criteria for specifc types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI supplement M100 [Internet]; Clinical and Laboratory Standards Institute: Wayne, AP, USA, 2017; Available online: https://clsi.org/media/1469/m100s27_sample.pdf (accessed on 29 August 2023).
- Durmaz, R.; Ayan, M. Acinetobacter baumannii izolatlarının moleküler epidemiyolojisinde “Arbitrarily Primed” PZR ve “Pulsed-Field Gel” elektroforezi. In Uygulamalı Moleküler Mikrobiyoloji, 2nd ed.; Durmaz, R., Ed.; Nobel Tıp Kitabevleri: Ankara, Turkey, 2001; pp. 219–228. [Google Scholar]
- Durmaz, R. Why are bacterial typing studies necessary? XXXIII. In Congress Book; Turkish Microbiology Congress: Istanbul, Turkey, 2008; pp. 27–30. [Google Scholar]
- Li, W.; Raoult, D.; Fournier, P.E. Bacterial strain typing in the genomic era. FEMS Microbiol. Rev. 2009, 33, 892–916. [Google Scholar] [CrossRef]
- Bolcato, V.; Bassetti, M.; Basile, G.; Bianco Prevot, L.; Speziale, G.; Tremoli, E.; Maffessanti, F.; Tronconi, L.P. The State-of-the-Art of Mycobacterium chimaera Infections and the Causal Link with Health Settings: A Systematic Review. Healthcare 2024, 12, 1788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakoullis, L.; Economidou, S.; Mehrotra, P.; Panos, G.; Karampitsakos, T.; Stratakos, G.; Tzouvelekis, A.; Sampsonas, F. Bronkoskopi ile ilişkili salgınlar ve yalancı salgınlar: Sistematik bir derleme. Enfeksiyon Kontrolü Ve Hastan. Epidemiyolojisi 2024, 45, 509–519. [Google Scholar]
- Mazzotta, M.; Salaris, S.; Pascale, M.R.; Girolamini, L.; Cristino, S. Legionella spp. İnsan Yapımı Su Kaynaklarında: Emilia-Romagna Bölgesinde İzolat Dağılımı ve Filogenetik Karakterizasyon. Pathogens 2021, 10, 552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bharara, T.; Chakravarti, A.; Sharma, M.; Agarwal, P. Investigation of Burkholderia cepacia complex bacteremia outbreak in a neonatal intensive care unit: A case series. J. Med. Case Rep. 2020, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.S.K.; Chau, S.K.-Y.; Chan, W.-S.; Chen, J.H.-K.; Wong, B.K.-C.; Fung, K.S.-C. An outbreak of Burkholderia cepacia complex exit site infection among peritoneal dialysis patients caused by contaminated spray dressing. Infect. Prev. Pract. 2024, 6, 100359. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Document M100-S25 Performance Standards for Antimicrobial Susceptibility Testing. 2020, Volume 40, pp. 50–51. Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 10 December 2024).

| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Denaturation | 94 °C | 5 min | 2 cycles |
| Primer annealing | 40 °C | 5 min | |
| Primer extension | 72 °C | 5 min | |
| Denaturation | 94 °C | 1 min | 40 cycles |
| Primer annealing | 40 °C | 1 min | |
| Primer extension | 72 °C | 2 min |
| Admission | Patients Diagnosis | BFID | AFID | Isolate Site | İF | TR |
|---|---|---|---|---|---|---|
| 1 | MM, Pneumonia | 15 | 8 | Tracheal aspirate | NO | - |
| 2 | Cardiac arrest | 150 | 40 | Tracheal aspirate | YES | YES |
| 3 | Cardiac arrest | 103 | 123 | Tracheal aspirate | YES | YES |
| 4 | Ischemic SVH | 23 | 37 | Tracheal aspirate | NO | - |
| 5 | COPD | 49 | 55 | Tracheal aspirate | NO | - |
| 6 | Parkinson | 5 | 3 | Tracheal aspirate | NO | - |
| 7 | COPD | 64 | 5 | Tracheal aspirate | YES | YES |
| 8 | PE | 55 | 15 | Tracheal aspirate | YES | YES |
| 9 | Traffic accident | 6 | 4 | Tracheal aspirate | YES | YES |
| 10 | Traffic accident | 118 | 74 | Tracheal aspirate | YES | YES |
| 11 | SAH | 26 | 8 | Tracheal aspirate | YES | YES |
| 12 | Traffic accident | 3 | 7 | Tracheal aspirate | YES | YES |
| 13 | SVD | 3 | 6 | Tracheal aspirate | NO | - |
| 14 | MI | 4 | 15 | Tracheal aspirate | YES | YES |
| 15 | Arrest | 159 | 6 | Tracheal aspirate | YES | YES |
| 16 | PE | 10 | 60 | Tracheal aspirate | NO | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aytaç, Ö.; Tanrıverdi, E.S.; Gündağ, Ö.; Şenol, F.F.; Karlıdağ, G.E.; Otlu, B. An Intensive Care Outbreak Caused by Burkholderia cepacia from Bacterial Filters. Pathogens 2025, 14, 266. https://doi.org/10.3390/pathogens14030266
Aytaç Ö, Tanrıverdi ES, Gündağ Ö, Şenol FF, Karlıdağ GE, Otlu B. An Intensive Care Outbreak Caused by Burkholderia cepacia from Bacterial Filters. Pathogens. 2025; 14(3):266. https://doi.org/10.3390/pathogens14030266
Chicago/Turabian StyleAytaç, Özlem, Elif Seren Tanrıverdi, Ömür Gündağ, Feray Ferda Şenol, Gülden Eser Karlıdağ, and Barış Otlu. 2025. "An Intensive Care Outbreak Caused by Burkholderia cepacia from Bacterial Filters" Pathogens 14, no. 3: 266. https://doi.org/10.3390/pathogens14030266
APA StyleAytaç, Ö., Tanrıverdi, E. S., Gündağ, Ö., Şenol, F. F., Karlıdağ, G. E., & Otlu, B. (2025). An Intensive Care Outbreak Caused by Burkholderia cepacia from Bacterial Filters. Pathogens, 14(3), 266. https://doi.org/10.3390/pathogens14030266






