Abstract
The main aim of this study was to discuss and compare the threats associated with F. graminearum in wheat production in Poland and in other Central European countries. Wheat is one of the most widely cultivated crops in the world, and pathogens causing Fusarium head blight (FHB) pose the greatest threat to wheat production. Our knowledge of FHB has to be regularly expanded in order to explore the impacts of climate change, new wheat cultivars, and new fungicides on the prevalence of this disease. The pathogen’s resistance to fungicides was analyzed in a global context due to the relative scarcity of studies examining this problem in Central Europe (excluding Germany). This is an interesting research perspective because, despite a relatively large number of Polish studies on FHB, F. graminearum genotypes and the pathogen’s resistance to fungicides remain insufficiently investigated. The hemibiotrophic pathogen Fusarium graminearum causes particularly high losses in wheat cultivation due to its ability to produce mycotoxins that are dangerous to human health (mainly deoxynivalenol, DON), colonize plant residues in soil in the saprotrophic phase, and produce spores that infect the stem base and spikes throughout the growing season. The infection process is highly dynamic, and it is facilitated by DON. The synthesis of DON (trichothecene) is encoded by Tri genes located in four loci. In Poland, the F. graminearum population is mainly composed of the 15ADON genotype, and the spread of FHB cannot effectively be managed with fungicides during epidemic years. Dynamic gene flows in field populations enable the pathogen to rapidly adapt to environmental changes and overcome wheat resistance to FHB. The emergence of fungicide-resistant F. graminearum strains significantly compromises the quality of wheat crops, but the associated mechanisms have not been sufficiently investigated to date. In addition, although some biopreparations are promising and effective in small-scale field trials, very few have been commercialized. Extensive research into pathogen populations, the development of new resistant wheat varieties, and the use of effective fungicides and biopreparations are required to produce wheat grain that is free of mycotoxins.
1. Introduction
Durum wheat (Triticum turgidum L. ssp. durum Desf.) is particularly susceptible to Fusarium pathogens because, compared with the major resistance quantitative trait locus (QTL, Fhb1 gene, which contributes 25 to 41.6% of the resistance to FHB) identified in bread wheat, the effects of the QTL resistant to FHB detected in tetraploid wheat are relatively small (<12%) [1,2]. Next to common wheat, rice, and maize, durum wheat is a major edible grain and a staple food around the world. Durum wheat is cultivated on an estimated 16 million hectares globally, with a total production of 38 million tons per year [3]. Durum wheat is grown on an area of 2 million ha in Turkey and Canada, and more than 1.5 million ha in Algeria, Italy, and India [4]. Research into high-yielding and drought-resistant durum wheat genotypes [5] creates opportunities for introducing this crop into regions where it was not previously grown [4]. At present, durum wheat is cultivated in Northern Europe [6], as well as in Poland, where it occupies a small area of around 2000 ha [7]. Durum wheat grain is mainly processed into semolina for the production of pasta, bread, biscuits, breakfast cereals, groats, dry gluten, and malt [8,9]. The growing popularity of durum wheat around the world can be attributed to the unique characteristics of its grain (high content of carotenoids, protein, and gluten; hard and vitreous endosperm), which are harnessed in the production of pasta with a desirable yellow color and firm texture that are highly appreciated by consumers [6]. Durum wheat cultivated in the Mediterranean Region is the only economically important species of tetraploid wheat. This allotetraploid species (Triticum turgidum L., 2n = 4x = 28, AABB genomes) was derived through intergeneric hybridization and polyploidization of two diploid grass species, T. urartu (AA genome) and a diploid B genome associated with Aegilops speltoides (BB genome) [1].
Durum wheat yields are affected by biotic (pathogens and pests) and abiotic stressors related to environmental conditions and their interactions with genetic traits [10]. Pathogens can decrease global wheat yields by as much as 21.5% [11]. Fusarium head blight (FHB), a ubiquitous disease of wheat caused by more than 20 species and subspecies of the genus Fusarium, including Fusarium graminearum (teleomorph Gibberella zeae Schwabe), poses a particular threat to durum wheat and common wheat [11,12]. Fusarium graminearum is the predominant Fusarium species in many regions of Europe [13], Asia [11,12], and North America [14]. Unsurprisingly, a survey of the most important plant-pathogenic fungi revealed that F. graminearum was one of the top ten pathogens due to its scientific and economic importance [15]. The prevalence of FHB is influenced by environmental factors such as solar radiation, precipitation, temperature, and CO2 levels. The characterization of the linkages between FHB epidemics and environmental changes plays a key role in predictive models and protection methods [16]. Fusarium head blight causes significant losses in wheat production because infected grain is shriveled, small, characterized by low mass and low quality, and often contaminated with mycotoxins that are harmful to humans and animals. The main mycotoxins produced by Fusarium species include trichothecenes, zearalenone (ZEA), moniliformin (MON), fumonisins, beauvericin, enniatins (ENNs), and fusaproliferin [10].
The aim of this review article was to describe the structure of F. graminearum populations, the infection process, mycotoxin production, the presence of mycotoxins in wheat grain, and the emergence of isolates resistant to fungicides in Poland and other Central European countries.
2. Spread and Infection Process of Fusarium graminearum
In the agricultural environment, F. graminearum survives saprotrophically on harvest residues, where ascospores and macroconidia serve as the main disease inocula of FHB. Ascospores (sexual spores) are released into the air by the perithecia and act as the primary inoculum. Perithecia contain numerous tissue types, produced at specific stages of perithecium development. These include (in order of appearance) formation of the perithecium initials (which give rise to the ascogenous hyphae), the outer wall, paraphyses (sterile mycelia that occupy the center of the perithecium until the asci develop), the asci, and the ascospores within the asci [17]. Macroconidia (asexual spores) are produced by phialides in infected plants and can be transported to other plants throughout the growing season [18]. Fusarium graminearum is a highly aggressive pathogen that poses the greatest threat to wheat in the flowering stage [19].
During the infection process, the pathogen produces secondary metabolites, DON and 15ADON, whose concentrations peak in wheat spikes three days after anthesis [20]. Wheat theca are most sensitive to infection, and they are colonized by fungal hyphae that penetrate top and bottom spikelets [20,21]. Fusarium graminearum also infects other parts of wheat spikes, including glumes, lemmas, and palea [21]. This hemibiotrophic pathogen colonizes host tissues as a biotrophic fungus, produces DON, and destroys host cells in the necrotrophic phase. The biotrophic (asymptomatic) phase lasts 24–84 h (Figure 1) [22]. Fusarium graminearum spores germinate 5–12 h after inoculation, and germ tubes are usually short and do not target specific topographical features in host plants [23,24,25]. In the biotrophic phase, hyphae grow on the surface of plant tissues or penetrate intercellular spaces, but plant cells remain viable [25]. In a study by Qiu et al. [25], invasive hyphae with a diameter of 1 µm penetrated wheat tissues 16 h after inoculation with F. graminearum. In infected cells, thick hyphae changed into narrow hyphae in the cell well and penetrated the neighboring cells. Interestingly, the first infected wheat cells remained viable because the hyphae of the pathogen were still sheathed by the host plasma membrane [25]. Fusarium graminearum produced large quantities of deoxynivalenol (DON), triacetylfusarinine C (TAFC), and class 2 effectors in the biotrophic phase, and class 1 effectors in the necrotrophic phase [26]. The first symptoms of the necrotrophic phase in the form of brown spots on glumes were observed after 24–84 h (Figure 2). Hyphal networks and numerous invasive structures were noted on plant surfaces two days after inoculation [23]. Infected spikes were bleached and necrotic, and the peduncle turned black. Symptoms of infection spread from the inoculated apical spikelets to the bottom of the spike. White fungal hyphae were observed on some wheat spikes [22,23] (Figure 2).
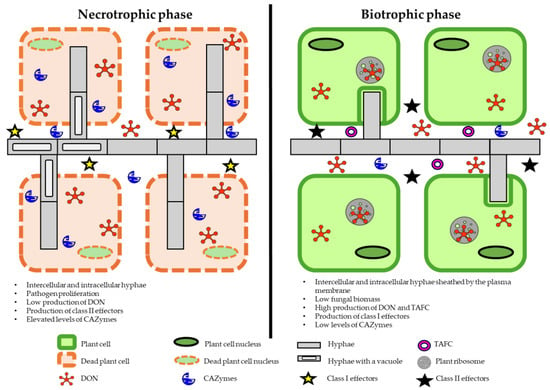
Figure 1.
Spatiotemporal model of wheat infection with Fusarium graminearum. The model presents the transcriptional regulation of the identified and putative virulence strategies in asymptomatic and symptomatic stages of infection in wheat tissue. Carbohydrate-active enzymes (CAZymes) catalyze the synthesis, degradation, and modification of carbohydrates. DON—deoxynivalenol; TAFC—triacetylfusarinine C. Source: own elaboration based on [25,26].

Figure 2.
Symptoms of Fusarium head blight on durum wheat cv. Durasol spikes inoculated with the 15ADON genotype of Fusarium graminearum after 48 (A), 72 (B), and 120 (C) hours after inoculation.
Most of the F. graminearum life cycle is linked with the host plant. This pathogen belongs to the phylum Ascomycota, which is characterized by a long binucleate phase. During this phase, two genetically separate nuclei are coupled inside cells. Fusarium graminearum is also homothallic, which implies that it is capable of producing numerous sexual spores (ascospores) without the involvement of a sexually unique partner. The meiotic cycle of F. graminearum is controlled by mating-type loci (MAT) MAT1-1 and MAT1-2 [27].
3. Fusarium graminearum Species Complex, Genotypes/Chemotypes, and Populations
Due to considerable genetic variability in F. graminearum populations, this pathogenic species is often referred to as the F. graminearum species complex (FGSC). The five most widely researched FGSC species are F. asiaticum (47 studies), F. meridionale (36), F. boothii (20), F. cortaderiae (18), and F. austroamericanum (9), whereas other species have been identified sporadically [28]. The morphological differences between FGSC species do not exist or are too subtle to support accurate identification [29,30,31].
Fusarium graminearum can produce five types of type B trichothecenes, of which three are known to cause gastrointestinal disorders, skin irritation, and neuroendocrine changes [32,33,34]. Selected strains produce DON and its acetylated derivatives 3-acetyl-DON (3ADON genotype) and 15-acetyl-DON (15ADON genotype), as well as nivalenol (NIV) and its acetylated derivative 4-acetyl-NIV (NIV/4ANIV genotype). The 15ADON genotype of F. graminearum is most frequently identified in Canada [14], China [11,12], and the USA [35]. In Poland, research into F. graminearum genotypes revealed an increase in the number of isolates belonging to the 15ADON genotype [13,36]. In 2009, the F. graminearum strains isolated from wheat grain by Wiśniewska et al. [36] belonged exclusively to the 3ADON genotype, whereas in 2019, Duba et al. [37] classified only 46.2% (n = 6) of the isolates to the 3ADON genotypes and the remaining isolates (n = 7) to the 15ADON genotype. In 2016–2017, Bilska et al. [13] confirmed the predominance of the 15ADON genotype but did not detect the NIV genotype in Polish populations of F. graminearum (n = 31). In the work of Kulik et al. [38], 76% (n = 25) of F. graminearum strains were identified as 15ADON, 8% (n = 6) as 3ADON, and only 6% (n = 2) as NIV genotypes. All strains identified in Western Europe have been classified as 15ADON [38]. In Germany, the 15ADON genotype was also predominant in infected wheat spikes, whereas the prevalence of the 3ADON genotype was lower (6.8%, n = 338), and the prevalence of the NIV genotype was lowest (1.2%) [39]. These observations give cause for concern because the 15ADON genotype is much more virulent than NIV [40]. In contrast, Amarasinghe et al. [41] reported that the prevalence of FHB, the percentage of Fusarium-damaged kernels (FDKs), and the DON content of grain were always higher in wheat cultivars inoculated with 3ADON than 15ADON genotypes of F. graminearum. Novel type B trichothecenes, NX-2 and NX-3 (NX), have recently been discovered in F. graminearum, but they have not been identified in Poland to date [38]. In the USA, 2.8% of strains in a collection of 463 F. graminearum isolates were classified as NX-2 [42]. NX trichothecenes play an important role in the initial stage of F. graminearum infection and the spread of FHB [43].
According to research, F. graminearum populations are highly diverse and evolve independently in distinct regions [35,38,42]. Kulik et al. [38] identified two major populations of F. graminearum in Europe: East European (EE) and West European (WE). The WE population consisted of 28 strains from France, Germany, Luxembourg, and the Netherlands, whereas the EE population included 32 strains from Eastern Europe, mostly Poland and Russia. Two separate populations of F. graminearum—endemic (NA1/15ADON) and introduced (NA2/3ADON)—were identified in the USA. In addition, Kelly and Ward [42] assigned a single 3ADON isolate (F328) and the majority (60%) of NX-2 F. graminearum to a third genetic population referred to as NA3. The remaining eight NX-2 isolates had admixed genomes, as evidenced by the fact that they shared most of their ancestry with the NA1 population and some ancestry with the NA2 population. In turn, a collection of 213 strains isolated from 13 German fields separated by a distance of 10–500 km was described as a single population with a high degree of sexual recombination [35]. The cited authors argued that high gene flow in field populations enables the pathogen to rapidly adapt to changes in the environment caused by the introduction of resistant cultivars, the use of fungicides, and a warming climate. According to Talas et al. [39], new haplotypes of F. graminearum can emerge through sexual recombination between the existing haplotypes in the field (such as maize residues) and haplotypes introduced to the field (e.g., with wheat grain).
4. Trichothecene Synthesis—The Key to the Infection Process
Trichothecenes (TCT) are the most ubiquitous mycotoxins produced by FGSC. Trichothecenes with a tricyclic 12,13-epoxytrichothec-9-ene (EPT) core structure are secondary metabolites known as sesquiterpenoids (Figure 3). Type A (TCT-A) and type B (TCT-B) trichothecenes are differentiated based on the substitution at the C-8 position. Type A trichothecenes are characterized by a hydroxyl group, an ester, or no substituent at C-8, whereas TCT-B carry a keto group at this position [44]. Type B trichothecenes are most prevalent and include NIV, DON, and their acetylated derivatives (4ANIV, 3ADON, and 15ADON). Type A trichothecenes produced by FGSC strains are NX toxins (NX-2, NX-3, NX-4, NX-5 and NX-6) that do not have an oxygen substitution at C-8, similarly to TCT-A produced by other Fusarium species, such as 4,15-diacetoxyscirpenol (DAS). Toxins NX-3 and NX-6 have an identical structure to DON and NIV, respectively, excluding the C-8 substituent. NX-2 and NX-4 (acetylated derivatives of NX-3) and NX-5 (acetylated derivative of NX-6) are characterized by structural similarity to 3ADON, 15ADON, and 4ANIV, respectively. Naturally occurring FGSC strains capable of producing NX-4, NX-5, and NX-6 have not been identified to date [45]. However, NX-4 was identified in vitro in rice cultures [46].
The synthesis of TCT is encoded by Tri genes located in four loci. The core TRI cluster consists of 12 genes located on chromosome 2, Tri1-Tri16 loci on chromosome 1, Tri101 on chromosome 4, and Tri15 on chromosome 3 [32,34,41,47]. The core TRI cluster on chromosome 2 contains nine genes encoding enzymes that catalyze DON biosynthesis (Tri3-Tri5, Tri7-Tri9, Tri11, and Tri13-14), two genes encoding transcription factors (Tri6 and Tri10), and one gene encoding a protein that participates in the export of mycotoxins (Tri12) [32,34,47,48,49]. Transcription factors containing the Cys2-His2 zinc finger domain are encoded by Tri6 and Tri10 genes and regulate the accumulation of Tri gene transcripts and other genes with diverse functions. Tri10 regulates the expression of Tri6. Furthermore, the biosynthesis of TCT is regulated by long non-coding RNA (RNA5P) that is transcribed from the Tri5 promoter region and suppresses the expression of Tri5 [50].
The biosynthesis of TCT is initiated by the trichodiene synthase enzyme, a virulence factor encoded by the Tri5 gene that catalyzes the cyclization and isomerization of farnesyl pyrophosphate (FPP) to trichodiene [51]. Tri5 mutants show symptoms of infection at the site of inoculation, but the infection does not spread to the neighboring spikelets [52]. The following nine reactions leading to the synthesis of calonectrin are catalyzed by the trichodiene oxygenase enzyme encoded by the Tri4 gene and enzymes encoded by Tri101, Tri11, and Tri3 [32,34]. The quadruple oxidation of trichodiene, which precedes non-enzymatic isomerization and cyclization, leads to the formation of isotrichodermol that undergoes acetylation, hydroxylation, and repeated acetylation to form calonectrin. Calonectrin is a common precursor to DON, NIV, their acetylated derivatives (3-ADON, 15-ADON, 4-ANIV), NX toxins, and their ANX derivatives (NX-2 and NX-4) [14]. In NIV-producing F. graminearum strains, the biosynthesis pathway is maintained by the product of the Tri1 gene, which is transformed to 4-ANIV and then to NIV by enzymes encoded by Tri13, Tri7, and Tri8. Tri7 and Tri13 genes are not active in F. graminearum strains producing DON and its acetylated derivatives, and DON is biosynthesized directly from calonectrin [32,34,41,53]. Calonectrin is transformed into the acetylated derivatives of DON (3ADON and 15ADON) and DON with the involvement of ITD C-15 hydroxylase encoded by the Tri1 gene and esterase encoded by the Tri8 gene [32,41]. In FGSC strains producing TCT-A and TCT-B, polymorphisms were identified in the nucleotide sequence of the Tri1 gene [46,54]. In strains producing TCT-B, the Tri1 enzyme catalyzes oxidation reactions at positions C-7 and C-8 of the EPT group [55]. In FGSC strains producing NX toxins, the NXTri1 enzyme catalyzes the addition of the hydroxyl group to calonectrin only at position C-7 and can lead to the synthesis of the acetylated derivatives of NX toxins [46].
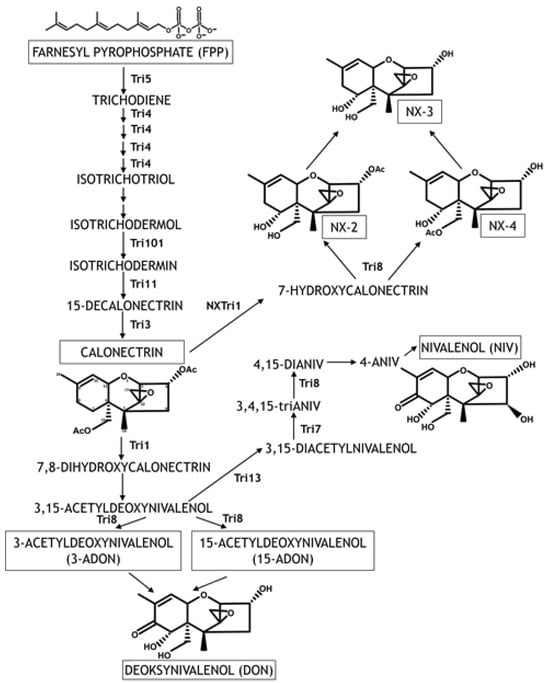
Figure 3.
Trichothecene biosynthesis pathway in FGSC strains (modified according to [45,56,57,58]). The atoms in the EPT ring are numbered for calonectrin as the common precursor to TCT-A and TCT-B. The enzymes catalyzing the reactions are described next to the arrows. Arrows without enzymes denote stages with unknown enzymes/genes or reactions that proceed independently.
5. The Occurrence of FHB and Mycotoxins in the Field
Central Europe is a region with a temperate climate and average precipitation, but in recent years, wheat plants have been subjected to abiotic stresses caused by prolonged dry spells and considerable temperature fluctuations [7,59]. In 2007–2018, F. graminearum was identified on 1–55.1% of common wheat kernels and 2.1–48% of durum wheat kernels in Poland (Table 1). The population size of F. graminearum has increased due to weather conditions during wheat flowering or the sensitivity of wheat cultivars/species to infections caused by Fusarium spp. The spread of F. graminearum can also be attributed to the increase in the area under maize in Poland [13,60,61,62].

Table 1.
Fungi of the genus Fusarium colonizing wheat grain and the mycotoxin content of wheat grain in Poland and several European countries.
Commission Regulation (EU) 2024/1022 of 8 April 2024 amending Regulation (EU) 2023/915 as regards the maximum levels of DON in food decreased the maximum admissible concentration of DON in unprocessed grain to 1000 µg/kg for common wheat and 1500 µg/kg for durum wheat [70]. Research conducted in Poland between 2009 and 2023 demonstrated that all grain samples contained more than one toxin [7,36,59,60,62,64]. Common wheat and durum wheat grain is generally contaminated with DON, MON, and ZEA [7,36,64], but Wachowska et al. [59] identified 11 mycotoxins, including DON, MON, ZEA, several ENNs, NIV, culmorin, and aurofusarin, in durum wheat grain. In Poland, the average content of DON was 30–69,150 µg/kg in common wheat grain and 106–76,700 µg/kg in durum wheat grain (Table 1). In the work of Wiśniewska et al. [36], maximum DON levels were exceeded in all samples. A high concentration of DON in durum wheat grain was also reported by Gorczyca et al. [64], in particular in the Polish cultivar “Komnata” (maximum DON levels were exceeded in 2012/2013 and 2013/2014 seasons). The high content of DON in wheat grain produced in Poland is surprising in the context of the results reported in the Czech Republic, Hungary, and Italy [65,66,69,71]. In most cases, DON concentrations were associated with a higher prevalence of F. graminearum in grain [36,60]. However, Góral et al. [61] and Mesterhazy [71] have argued that elevated DON levels can be attributed to varietal resistance to infection and mycotoxin accumulation. Despite the fact that many cultivars are resistant to Fusarium infections [71,72], fungicides remain one of the main FHB management strategies in many regions of the world where resistant cultivars are not available. In Poland, fungicides are not sufficiently effective in reducing the symptoms of FHB, protecting grain against Fusarium pathogens, and decreasing DON levels in grain. Demethylation inhibitor (DMI) fungicides, in particular tebuconazole, metconazole, and prothioconazole, are most effective in suppressing FHB and decreasing mycotoxin accumulation [7,59,73]. To effectively minimize the spread of FHB, these fungicides should be applied during anthesis. However, according to some reports, the application of triazole and strobilurin fungicides stimulated DON production under field conditions [74]. The associated mechanisms were elucidated in an RT-qPCR analysis by Kulik et al. [75] who observed an increase in the levels of Tri4, Tri5, and Tri11 transcripts in the cultures of all F. graminearum isolates with the addition of sublethal concentrations of propiconazole. Duan et al. [76] also found that QoIs can stimulate DON production and upregulate the expression of Tri5 and Tri6 genes.
6. Fungicide Resistance in Fusarium graminearum and Wheat Biological Control
The use of fungicides is one of the strategies for managing FHB in wheat, including in integrated crop protection systems, because most wheat cultivars, in particular durum wheat cultivars, are susceptible to this disease [77]. Directive 2009/128/EC on the sustainable use of pesticides was the first legal regulation to comprehensively address the use of plant protection products in Europe. As of January 2014, all EU Member States have to abide by the principles of Integrated Pest Management (IMP) as the main crop protection strategy [78]. Fungicides containing only one active ingredient from the DMI group (prothioconazole, metconazole, tebuconazole, bromuconazole), the quinone outside inhibitor (QoI) group (azoxystrobin), and fungicides containing the above compounds in combination with other triazoles, QoIs (fluoxastrobin, trifloxystrobin), and succinate-dehydrogenase inhibitors (SDHI, bixafen, sedaxane, benzovindiflupyr, fluopyram, and boscalid) are recommended in the European Central Zone [79] (Table 2).

Table 2.
Active ingredients in fungicides targeting FHB that have been approved for use in Central Europe.
Demethylation inhibitors suppress the demethylation of the 14-C in lanosterol, a precursor to ergosterol that is responsible for the integrity of fungal cell walls. When the production of sterol 14α-demethylase (an enzyme of the cytochrome P450 superfamily) is inhibited, sterol ergosterol precursors and free fatty acids are accumulated in cells, which disrupts normal fungal growth [81]. However, excessive and prolonged use of selective DMI fungicides has decreased the sensitivity of fungal strains or increased the proportion of fungicide-resistant FHB pathogens [35,82]. Fusarium graminearum harbors three CYP51 genes (CYP51A, CYP51B, and CYP51C) which share 61.65% similarity in the amino acid sequence but have different functions. The CYP51A gene encodes 14α-demethylase and is responsible for intrinsic sensitivity to DMI fungicides [83]. The CYP51B gene encodes an enzyme that is primarily responsible for sterol 14α-demethylation and plays an important role in ascospore formation in F. graminearum. However, the CYP51C gene does not encode a sterol 14α-demethylase but is required for the full virulence of wheat [83].
The resistance or decreased sensitivity of F. graminearum strains to selected triazole fungicides have been well documented in recent years. The efficacy of triazole fungicides decreases significantly after several years of intensive use [84]. Unfortunately, cheap triazole fungicides are the only cost-effective treatments, but FHB infections are practically impossible to eradicate in epidemic years [85]. In F. graminearum, resistance to DMI fungicides is conditioned by three main mechanisms: (1) changes in the amino acid sequence of the CYP51 protein, which decrease the affinity for azoles [35,83], (2) overexpression of CYP51 genes due to changes in the promoter upstream region [86], and (3) overexpression of efflux pumps, including ATP-binding cassette (ABC) transporters and those belonging to the major facilitator superfamily (MSF) of transporters, which can reduce sensitivity by expelling DMI fungicides from fungal cells [87]. In recent years, several F. graminearum strains that were resistant or less sensitive to tebuconazole have been identified in wheat fields in Germany, China, Lithuania, and Serbia [35,45,88,89]. In Germany, the EC50 values of 197 F. graminearum isolates from wheat fields ranged from 5.4 to 62.2 μg mL−1. These values had a normal distribution with a mean of 22.2 μg mL−1 (Table 3) [35]. The isolates did not contain mutations in the promoter regions of three CYP51 genes, but three non-synonymous mutations and one synonymous mutation were identified in the CYP51A gene, five synonymous point mutations were detected in the CYP51B gene, and five non-synonymous mutations and four synonymous mutations were identified in the CYP51C gene [35] (Table 3). In F. graminearum strains isolated in Lithuania, the EC50 values for metconazole were lower than the E50 values for prothioconazole and tebuconazole [89]. In Serbia, F. graminearum strains were also less sensitive to tebuconazole than metconazole [88]. These observations point to an increase in the EC50 values for the inhibition of mycelial growth in Central Europe, particularly in regions where fungicides have been used intensively. The efficacy of fungicides targeting F. graminearum clearly decreased in the past 20 years. For example, F. graminearum strains isolated from fields in the USA between 1981 and 2014 were significantly more sensitive to metconazole (EC50 values for all isolates ranged from 0.0071 to 0.1734 μg/mL, with a mean of 0.0369 μg/mL) and tebuconazole (EC50 values ranged from 0.0301 to 1.7339 μg/mL, with a mean of 0.3052 μg/mL) than European strains [81].

Table 3.
Fungicide sensitivity in Fusarium graminearum isolates obtained from wheat.
Strobilurins (QoIs) inhibit respiration by blocking the cytochrome bc1 enzyme complex (also referred to as complex III). Quinone outside inhibitors are associated with a medium-to-high risk of resistance development in pathogens because point mutations can induce significant changes in the configuration of the target site [80]. In many fungi, resistance to strobilurins is caused by mutations in the cytb gene, including at positions G143A, G137R, and F129L. The substitution of phenylalanine with leucine at position 129 (F129L) or the substitution of glycine with arginine at position 137 (G137R) is associated with moderate resistance. In turn, the substitution of glycine with alanine at position 143 (G143A) is associated with stronger resistance [94,95]. Resistance to QoI fungicides has rarely been studied in F. graminearum, and the results are often contradictory. According to Dubos et al. [96], F. graminearum is naturally resistant to trifloxystrobin and other QoIs. The cited authors analyzed 55 F. graminearum strains for resistance to trifloxystrobin. The examined strains belonged to three known chemotypes, and they were isolated between 1969 and 2009 in Belgium, Canada, Germany, Italy, Luxembourg, and the USA. In the tested strains, the maximum inhibition of fungal growth achieved for trifloxystrobin ranged from 14% to 65%, and it was not significantly affected by the country of origin or chemotype. No significant differences in resistance levels were reported between chemotypes and countries, and equally high resistance was noted in strains that had been isolated before strobilurin was placed on the market in 1996. Therefore, the authors concluded that F. graminearum is naturally resistant to trifloxystrobin and that resistance to QoI is not caused by using strobilurin in agriculture. In contrast, Duan et al. [76] reported that several strobilurin fungicides were highly effective in inhibiting the mycelial growth of F. graminearum strains isolated in China, where the EC50 value for azoxystrobin was estimated at 0.966 μg/mL. In a study evaluating the sensitivity of a large collection of F. graminearum isolates from two regions of Brazil to azoxystrobin and pyraclostrobin, EC50 values were very high (up to 329.7 μg/mL) in several strains, but no point mutations responsible for resistance were detected at any of the target spots in these isolates [91]. The vast majority of the tested isolates were sensitive to the studied fungicides, and pyraclostrobin was more effective than azoxystrobin (the mean EC50 was 0.330 and 28.06 μg/mL, respectively). According to Luan et al. [92], F. graminearum is not highly sensitive to pyraclostrobin (EC50 = 4.175–13.399 μg/mL). The cited authors made the valuable observation that the same isolate can have different EC50 values, depending on the culture medium, incubation time, and the addition of other substances.
Research has shown that F. graminearum is becoming increasingly resistant to triazoles in Central Europe, which prompts the search for new fungicides to effectively target FHB and reduce the pathogen’s ability to biosynthesize mycotoxins. Cyclobutrifluram, a novel SDHI fungicide developed by Sygenta in 2013 [97] and approved for use in Argentina in 2022 [98], has been recommended for controlling fungal diseases of wheat in China. This compound has not yet received regulatory approval in Central Europe. According to the Fungicide Resistance Action Committee, the risk for resistance evolution to SDHI fungicides is medium to high [80]. Fungal succinate dehydrogenase (SDH) is required for the electron respiratory chain and the tricarboxylic acid cycle. Fungal SDH consists of four subunits, including flavoprotein (SdhA), iron–sulfur protein (SdhB), and two other integral membrane proteins (SdhC and SdhD) that are localized in the mitochondria [93,99]. Shao et al. [99] identified six strains resistant to pydiflumetofen in a collection of 6468 F. graminearum isolates from various regions of China. All resistant isolates contained point mutation A78V in FgSdhC1 (FgSdhC1A78V). Tong et al. [100] examined the resistance of F. graminearum strains and determined the EC50 value for sedaxane at 0.365 μg/mL (0.188 to 0.784 μg/mL, depending on sedaxane stereoisomers). In a study analyzing the germination of F. graminearum spores, EC50 values ranged from 1.28 to 2.41 μg/mL for boscalid, 2.03 to 2.98 μg/mL for benzovindiflupyr, and 0.52 to 0.69 μg/mL for fluopyram (Table 3) [100].
Risoli et al. [101] concluded that biological control agents (BCAs) are highly effective in controlling FHB in wheat, reducing mycotoxin levels, and protecting crops. They also found that BCAs were more effective in studies that had been published more recently, under controlled conditions, and in highly susceptible wheat cultivars. However, BCAs were generally less effective than conventional agrochemicals, especially in their ability to reduce pathogen abundance, FHB symptoms, and mycotoxin levels. Pythium oligandrum M1 is the only commercial BCA that has been approved for the management of fungal diseases in Central Europe. Its effectiveness has been analyzed by very few studies [102,103]. When applied to suppress the growth of F. culmorum and mycotoxin production during wheat malting, P. oligandrum reduced Fusarium contamination by 20% and DON content by 17%. The tested BCA did not lead to a deterioration in the quality of wheat malts [102]. In the work of Pellan et al. [103], P. oligandrum was also able to settle and colonize the palea or the base of the lemma awn, and it quickly produced a large quantity of characteristic oogonia-containing oospores. This BCA suppressed the development of F. graminearum on wheat spikes, decreased DON levels, and inhibited the formation of perithecia. Integrated pest management (IPM) strategies that combine chemical and biological methods are highly recommended, and government subsidies for biological control contribute to their cost-effectiveness in Poland [79].
7. Conclusions
Fusarium graminearum is a virulent and rapidly spreading hemibiotrophic pathogen of wheat that produces harmful mycotoxins. Dynamic gene flows in field populations enable the pathogen to rapidly adapt to environmental changes, overcome plant resistance, and contribute to the emergence of fungicide resistance. The spread of fungicide-resistant and/or -tolerant F. graminearum strains appears to be limited in Central Europe, but it may compromise the quality of wheat crops. The mechanisms responsible for fungicide resistance in F. graminearum have not yet been fully elucidated. However, low doses of strobilurin and triazole fungicides have been found to alter metabolic processes in fungal cells by enhancing the release and biosynthesis of mycotoxins, which gives serious cause for concern. These observations warrant further research to improve molecular methods for detecting fungicide-resistant strains and strains with a modified ability to produce mycotoxins and to introduce resistant wheat varieties whose microbiome effectively suppresses the development of F. graminearum infections.
Author Contributions
Conceptualization, U.W.; software, W.E.P. and U.W.; validation, W.E.P.; formal analysis, W.G., W.E.P. and U.W.; investigation, W.G.; data curation, W.G., W.E.P. and U.W.; writing—original draft preparation, W.G., W.E.P. and U.W.; writing—review and editing, W.G. and U.W.; visualization, W.G. and W.E.P.; project administration, U.W.; funding acquisition, U.W. All authors have read and agreed to the published version of the manuscript.
Funding
Funded by the Minister of Science under “the Regional Initiative of Excellence Program”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns.
Acknowledgments
The authors express their gratitude to A. Poprawska for language editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kubaláková, M.; Kovárová, P.; Suchánková, P.; Cíhalíková, J.; Bartos, J.; Lucretti, S.; Watanabe, N.; Kianian, S.F.; Dolezel, J. Chromosome sorting in tetraploid wheat and its potential for genome analysis. Genetics 2005, 170, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium head blight in durum wheat: Recent status, breeding directions, and future research prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Statistical Database. 2023. Available online: http://www.fao.org/faostat/en/#data (accessed on 22 July 2024).
- Tidiane, S.A.; Chiari, T.; Legesse, W.; Seid-Ahmed, K.; Ortiz, R.; van Ginkel, M.; Bassi, F.M. Durum wheat (Triticum durum Desf.): Origin, cultivation and potential expansion in Sub-Saharan Africa. Agronomy 2019, 9, 263. [Google Scholar] [CrossRef]
- Galal, A.A.; Safhi, F.A.; El-Hity, M.A.; Kamara, M.M.; Gamal El-Din, E.M.; Rehan, M.; Farid, M.; Behiry, S.I.; El-Soda, M.; Mansour, E. Molecular genetic diversity of local and exotic durum wheat genotypes and their combining ability for agronomic traits under water deficit and well-watered conditions. Life 2023, 13, 2293. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, D.; Roumenina, E.; Dimitrov, P.; Gikov, A.; Bozhanova, V.; Dragov, R.; Jelev, G.; Taneva, K. Preharvest durum wheat yield, protein content, and protein yield estimation using unmanned aerial vehicle imagery and pléiades satellite data in field breeding experiments. Remote Sens. 2024, 16, 559. [Google Scholar] [CrossRef]
- Wachowska, U.; Stuper-Szablewska, K.; Perkowski, J. Yeasts isolated from wheat grain can suppress fusarium head blight and decrease trichothecene concentrations in bread wheat and durum wheat grain. Pol. J. Environ. Stud. 2020, 29, 4345–4360. [Google Scholar] [CrossRef] [PubMed]
- Rachoń, L.; Szumiło, G.; Stankowski, S. Comparison of selected technological value indicators of common wheat (Triticum aestivum spp. vulgare), durum wheat (Triticum durum) and spelt wheat (Triticum aestivum spp. spelta). Fragm. Agron. 2011, 28, 52–59. [Google Scholar]
- Wyzińska, M.; Sułek, A. Cultivation, importance and use of hard wheat. In Analysis, Production and Application of Substances and Components of Plant Origin; Maciąg, M., Maciąg, K., Eds.; Wydawnictwo Naukowe TYGIEL: Lublin, Poland, 2018; pp. 240–248. [Google Scholar]
- Alisaac, E.; Mahlein, A.-K. Fusarium head blight on wheat: Biology, modern detection and diagnosis and integrated disease management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Ji, L.; Li, Q.; Wang, Y.; Burgess, L.W.; Sun, M.; Cao, K.; Kong, L. Monitoring of Fusarium species and trichothecene genotypes associated with Fusarium head blight on wheat in Hebei province, China. Toxins 2019, 11, 243. [Google Scholar] [CrossRef]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in phenylpropanoid and trichothecene production by Fusarium culmorum and F. graminearum sensu stricto via exposure to flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Crippin, T.; Renaud, J.B.; Sumarah, M.W.; Miller, J.D. Comparing genotype and chemotype of Fusarium graminearum from cereals in Ontario, Canada. PLoS ONE 2019, 14, e0216735. [Google Scholar] [CrossRef] [PubMed]
- Summerell, B.A. Resolving Fusarium: Current status of the genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Zhu, Y.; Xi, J.; Yao, Y.; Xu, H.; Tang, C.; Wu, L. Characterizing the dynamic linkages between environmental changes and wheat Fusarium head blight epidemics. Ecol. Inform. 2024, 80, 102524. [Google Scholar] [CrossRef]
- Cavinder, B.; Sikhakolli, U.; Fellows, K.M.; Trail, F. Sexual development and ascospore discharge in Fusarium graminearum. J. Vis. Exp. 2012, 29, 3895. [Google Scholar] [CrossRef]
- Trail, F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef]
- Yoshida, M. Studies on the control of Fusarium head blight of barley and wheat and mycotoxin levels in grains based on time of infection and toxin accumulation. J. Gen. Plant Pathol. 2012, 78, 425–426. [Google Scholar] [CrossRef]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef]
- Siou, D.; Gélisse, S.; Laval, V.; Repinçay, C.; Canalès, R.; Suffert, F.; Lannou, C. Effect of wheat spike infection and mycotoxin accumulation. Plant Pathol. 2014, 63, 390–399. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A. Infection process and wheat response to Fusarium head blight caused by Fusarium graminearum. Eur. J. Plant Pathol. 2019, 153, 489–502. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Buttar, Z.A.; Cheng, M.; Wei, P.; Zhang, Z.; Lv, C.; Zhu, C.; Ali, N.F.; Kang, G.; Wang, D.; Zhang, K. Update on the basic understanding of Fusarium graminearum virulence factors in common wheat research. Plants 2024, 13, 1159. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhao, X.; Fang, W.; Wu, H.; Abubakar, Y.S.; Lu, G.D.; Wang, Z.; Zheng, W. Spatiotemporal nature of Fusarium graminearum-wheat coleoptile interactions. Phytopathol. Res. 2019, 1, 26. [Google Scholar] [CrossRef]
- Brown, N.A.; Evans, J.; Mead, A.; Hammond-Kosack, K.E. A spatial temporal analysis of the Fusarium graminearum transcriptome during symptomless and symptomatic wheat infection. Mol. Plant Pathol. 2017, 18, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cho, E.J.; Lee, S.; Lee, Y.S.; Yun, S.H. Functional analyses of individual mating-type transcripts at MAT loci in Fusarium graminearum and Fusarium asiaticum. FEMS Microbiol. Lett. 2012, 337, 89–96. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium graminearum species complex: A bibliographic analysis and web-accessible database for global mapping of species and trichothecene toxin chemotypes. Phytopathology 2022, 112, 741–751. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Aberra, D.; Kistler, H.C.; Aoki, T.; Orwig, N.; Kimura, M.; Bjørnstad, Å.; Klemsdal, S.S. Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal. Genet. Biol. 2008, 45, 1514–1522. [Google Scholar] [CrossRef]
- Starkey, D.E.; Ward, T.J.; Aoki, T.; Gale, L.R.; Kistler, H.C.; Geiser, D.M.; Suga, H.; Tóth, B.; Varga, J.; O’donnell, K. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal. Genet. Biol. 2007, 44, 1191–1204. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Gagkaeva, T.; Ward, T.J.; Aoki, T.; Kistler, H.C.; O’Donnell, K. A novel Asian clade within the Fusarium graminearum species complex includes a newly discovered cereal head blight pathogen from the Russian Far East. Mycologia 2009, 101, 841–852. [Google Scholar] [CrossRef]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Mycotoxins: Toxicology. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C., Tortorello, M.L., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 1539–1547. [Google Scholar] [CrossRef]
- Merhej, J.; Richard-Forget, F.; Barreau, C. Regulation of trichothecene biosynthesis in Fusarium: Recent advances and new insights. Appl. Microbiol. Biotechnol. 2011, 91, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Talas, F.; McDonald, B.A. Genome-wide analysis of Fusarium graminearum field populations reveals hotspots of recombination. BMC Genom. 2015, 16, 996. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, H.; Stępień, Ł.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium species infecting wheat heads in Poland. Cent. Eur. J. Biol. 2014, 9, 163–172. [Google Scholar] [CrossRef]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U. Trichothecene genotypes analysis of Fusarium isolates from di-, tetra- and hexaploid wheat. Agronomy 2019, 9, 698. [Google Scholar] [CrossRef]
- Kulik, T.; Molcan, T.; Bilska, K.; Beyer, M.; Pasquali, M.; van Diepeningen, A.; Myszczynski, K. Two distinct Fusarium graminearum populations colonized European wheat in the past two decades. PLoS ONE 2023, 18, e0296302. [Google Scholar] [CrossRef]
- Talas, F.; Parzies, H.K.; Miedaner, T. Diversity in genetic structure and chemotype composition of Fusarium graminearum sensu stricto populations causing wheat head blight in individual fields in Germany. Eur. J. Plant Pathol. 2011, 131, 39–48. [Google Scholar] [CrossRef]
- Spolti, P.; Barros, N.C.; Gomes, L.B.; Dos Santos, J.; Del Ponte, E.M. Phenotypic and pathogenic traits of two species of the Fusarium graminearum complex possessing either 15-ADON or NIV genotype. Eur. J. Plant Pathol. 2012, 133, 621–629. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Fernando, W.G.D. Comparative analysis of deoxynivalenol biosynthesis related gene expression among different chemotypes of Fusarium graminearum in spring wheat. Front. Microbiol. 2016, 7, 1229. [Google Scholar] [CrossRef]
- Kelly, A.C.; Ward, T.J. Population genomics of Fusarium graminearum reveals signatures of divergent evolution within a major cereal pathogen. PLoS ONE 2018, 13, e0194616. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Tiley, H.; Gutiérrez, S.; Yulfo-Soto, G.; Vaughan, M.M.; Ward, T.J. NX trichothecenes are required for Fusarium graminearum infection of wheat. Mol. Plant Microbe. Interact. 2023, 36, 294–304. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Wang, H.; Yang, X.; Zhang, C.; Zhao, Z.; Wang, J. NX toxins: New threat posed by Fusarium graminearum species complex. Trends Food Sci. Technol. 2022, 119, 79–191. [Google Scholar] [CrossRef]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Gale, L.R.; Bryant, J.; Calvo, S.; Giese, H.; Katan, T.; O Donnell, K.; Suga, H.; Taga, M.; Usgaard, T.R.; Ward, T.J.; et al. Chromosome complement of the fungal plant pathogen Fusarium graminearum based on genetic and physical mapping and cytological observations. Genetics 2005, 171, 985–1001. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A review. Int. J. Microbiol. 2020, 23, 89–96. [Google Scholar] [CrossRef]
- Proctor, R.H.; McCormick, S.P.; Alexander, N.J.; Desjardins, A.E. Evidence that a secondary metabolic biosynthetic gene cluster has grown by gene relocation during evolution of the filamentous fungus Fusarium. Mol. Microbiol. 2009, 74, 1128–1142. [Google Scholar] [CrossRef]
- Huang, P.; Yu, X.; Liu, H.; Ding, M.; Wang, Z.; Xu, J.-R.; Jiang, C. Regulation of TRI5 expression and deoxynivalenol biosynthesis by a long non-coding RNA in Fusarium graminearum. Nat. Commun. 2024, 15, 1216. [Google Scholar] [CrossRef]
- Hohn, T.M.; Beremand, P.D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene 1989, 79, 131–138. [Google Scholar] [CrossRef]
- Bai, G.H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J.; Proctor, R.H. Gang, D., Ed.; Trichothecene triangle: Toxins, genes, and plant disease. In Phytochemicals, Plant Growth, and the Environment; Recent Advances in Phytochemistry; Springer: New York, NY, USA, 2013; Volume 42. [Google Scholar] [CrossRef]
- Liang, J.M.; Xayamongkhon, H.; Broz, K.; Dong, Y.; McCormick, S.P.; Abramova, S.; Ward, T.J.; Ma, Z.H.; Kistler, H.C. Temporal dynamics and population genetic structure of Fusarium graminearum in the upper Midwestern United States. Fungal Genet. Biol. 2014, 73, 83–92. [Google Scholar] [CrossRef]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; O’Donnell, K.; Ward, T.J.; Fujimura, M.; Hamamoto, H.; Shibata, T.; Yamaguchi, I. The trichothecene biosynthesis gene cluster of Fusarium graminearum F15 contains a limited number of essential pathway genes and expressed non-essential genes. FEBS Lett. 2003, 539, 105–110. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J. Fusarium Tri8 encodes a trichothecene C-3 esterase. Appl. Environ. Microbiol. 2002, 68, 2959–2964. [Google Scholar] [CrossRef]
- Villafana, R.T.; Ramdass, A.C.; Rampersad, S.N. Selection of Fusarium trichothecene toxin genes for molecular detection depends on TRI gene cluster organization and gene function. Toxins 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, U.; Sulyok, M.; Wiwart, M.; Suchowilska, E.; Giedrojć, W.; Gontarz, D.; Kandler, W.; Krska, R. Secondary metabolites of pathogenic fungi in Triticum durum grain protected with Debaryomyces hansenii in two different locations in Poland. Agronomy 2023, 13, 721. [Google Scholar] [CrossRef]
- Chełkowski, J.; Gromadzka, K.; Stępień, Ł.; Lenc, L.; Kostecki, M.; Berthiller, F. Fusarium species, zearalenone and deoxynivalenol content in preharvest scabby wheat heads from Poland. World Mycotoxin J. 2012, 5, 133–141. [Google Scholar] [CrossRef]
- Góral, T.; Łukanowski, A.; Małuszyńska, E.; Stuper-Szablewska, K.; Buśko, M.; Perkowski, J. Performance of winter wheat cultivars grown organically and conventionally with focus on Fusarium head blight and Fusarium trichothecene toxins. Microorganisms 2019, 7, 439. [Google Scholar] [CrossRef]
- Lenc, L.; Czecholiński, G.; Wyczling, D.; Turów, T.; Kaźmierczak, A. Fusarium head blight (FHB) and Fusarium spp. on grain of spring wheat cultivars grown in Poland. J. Plant Prot. Res. 2015, 55, 266–277. [Google Scholar] [CrossRef]
- Sadowski, C.; Lenc, L.; Kuś, J. Fusarium head blight and Fusarium spp. on grain of winter wheat, a mixture of cultivars and spelt grown in organic system. J. Res. App. Agric. Eng. 2010, 55, 79–83. [Google Scholar]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Sci. Nat. 2018, 105, 2. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef]
- Polišenská, I.; Jirsa, O.; Salava, J.; Sedláčková, I.; Frydrych, J. Fusarium mycotoxin content and Fusarium species presence in Czech organic and conventional wheat. World Mycotoxin J. 2021, 14, 201–211. [Google Scholar] [CrossRef]
- Suproniene, S.; Sakalauskas, S.; Stumbriene, K.; Zvirdauskiene, R.; Svegzda, P. Variances in trichothecene chemotype distribution in Lithuanian wheat grain and within pure culture Fusarium graminearum isolated from the same grain samples. Eur. J. Plant Pathol. 2016, 144, 371–381. [Google Scholar] [CrossRef]
- Sakalauskas, S.; Stumbriene, K.; Suproniene, S.; Svegzda, P. Changes in Fusarium link species composition from Lithuanian wheat grain in years 2005–2007 to 2011–2013. Rural Sustain. Res. 2014, 32, 45–50. [Google Scholar] [CrossRef]
- Tima, H.; Brückner, A.; Mohácsi-Farkas, C.; Kiskó, G. Fusarium mycotoxins in cereals harvested from Hungarian fields. Food Addit. Contam. Part B Surveill. 2016, 9, 127–131. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food. Available online: https://eur-lex.europa.eu/eli/reg/2024/1022/oj/eng (accessed on 12 October 2024).
- Mesterhazy, A. What Is Fusarium Head Blight (FHB) Resistance and what are its food safety risks in wheat? Problems and solutions—A review. Toxins 2024, 16, 31. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Simsek, S.; Brûlé-Babel, A.; Fernando, W.D. Analysis of deoxynivalenol and deoxynivalenol-3-glucosides content in Canadian spring wheat cultivars inoculated with Fusarium graminearum. Food Addit. Contamin. Part A 2016, 33, 1254–1264. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Lemańczyk, G.; Twarużek, M.; Błajet-Kosicka, A.; Kazek, M.; Grajewski, J. Fusarium head blight incidence and detection of Fusarium toxins in wheat in relation to agronomic factors. Eur. J. Plant Pathol. 2017, 149, 515–531. [Google Scholar] [CrossRef]
- Baturo-Cieśniewska, A.; Lukanowski, A.; Kolenda, M. Effect of fungicide application on wheat head blight, occurrence of Fusarium spp. and mycotoxin production. Plant Breed. Seed Sci. 2011, 63, 29–38. [Google Scholar] [CrossRef]
- Kulik, T.; Łojko, M.; Jestoi, M.; Perkowski, J. Sublethal concentrations of azoles induce tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012, 335, 58–67. [Google Scholar] [CrossRef]
- Duan, Y.; Lu, F.; Zhou, Z.; Zhao, H.; Zhang, J.; Mao, Y.; Li, M.; Wang, J.; Zhou, M. Quinone outside inhibitors affect DON biosynthesis, mitochondrial structure and toxisome formation in Fusarium graminearum. J. Hazard. Mater. 2020, 398, 122908. [Google Scholar] [CrossRef]
- Klocke, B.; Sommerfeldt, N.; Wagner, C.; Schwarz, J.; Baumecker, M.; Ellmer, F.; Jacobi, A.; Matschiner, K.; Petersen, J.; Wehling, P.; et al. Disease threshold-based fungicide applications: Potential of multi-disease resistance in winter wheat cultivars in Germany. Eur. J. Plant Pathol. 2023, 165, 363–383. [Google Scholar] [CrossRef]
- Parliament European Commission. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off J Eur Union. 2009, L309, 71–86. [Google Scholar]
- Ministry of Agriculture and Rural Development. Republic of Poland. 2024. Available online: https://www.gov.pl/web/piorin/wyszukiwarka-sor-mrirw (accessed on 1 December 2024).
- FRAC. Fungicide Resistance Action Committee. 2024. Available online: https://www.frac.info (accessed on 10 October 2024).
- Anderson, N.R.; Freije, A.N.; Bergstrom, G.C.; Bradley, C.A.; Cowger, C.; Faske, T.; Hollier, C.; Kleczewski, N.; Padgett, G.B.; Paul, P.; et al. Sensitivity of Fusarium graminearum to metconazole and tebuconazole fungicides before and after widespread use in wheat in the United States. Plant Health Prog. 2020, 21, 85–90. [Google Scholar] [CrossRef]
- Miao, J.; Li, Y.; Hu, S.; Li, G.; Gao, X.; Dai, T.; Liu, X. Resistance risk, resistance mechanism and the effect on DON production of a new SDHI fungicide cyclobutrifluram in Fusarium graminearum. Pestic. Biochem. Physiol. 2024, 199, 105795. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Du, J.; Chi, M.; Sun, X.; Liang, W.; Huang, J.; Li, B. The Y137H mutation in the cytochrome P450 FgCYP51B protein confers reduced sensitivity to tebuconazole in Fusarium graminearum. Pest Manag. Sci. 2018, 74, 1472–1477. [Google Scholar] [CrossRef]
- Rekanović, E.; Mihajlović, M.; Potočnik, I. In vitro sensitivity of Fusarium graminearum (Schwabe) to difenoconazole, prothioconazole and thiophanate-mythyl. Pestic. Fitomed. 2010, 25, 325–333. [Google Scholar] [CrossRef]
- Duffeck, M.R.; dos Santos Alves, K.; Machado, F.J.; Esker, P.D.; Del Ponte, E.M. Modeling yield losses and fungicide profitability for managing Fusarium head blight in Brazilian spring wheat. Phytopathology 2020, 110, 370–378. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Shao, J.; Yin, Y.; Ma, Z. Gene transcription profiling of Fusarium graminearum treated with an azole fungicide tebuconazole. Appl. Microbiol. Biotechnol. 2020, 85, 1105–1114. [Google Scholar] [CrossRef]
- Qi, P.F.; Zhang, Y.Z.; Liu, C.H.; Zhu, J.; Chen, Q.; Guo, Z.R.; Wang, Y.; Xu, B.J.; Zheng, T.; Jiang, Y.F.; et al. Fusarium graminearum ATP-Binding cassette transporter gene FgABCC9 is required for its transportation of salicylic acid, fungicide resistance, mycelial growth and pathogenicity towards wheat. Int. J. Mol. Sci. 2018, 19, 2351. [Google Scholar] [CrossRef]
- Ivić, D.; Sever, Z.; Kuzmanovska, B. In vitro Sensitivity of Fusarium graminearum, f. avenaceum and F. verticillioides to carbendazim, tebuconazole, flutriafol, metconazole and prochloraz. Pestic. Fitomed. 2011, 26, 35–42. [Google Scholar] [CrossRef]
- Matelionienė, N.; Žvirdauskienė, R.; Kadžienė, G.; Zavtrikovienė, E.; Supronienė, S. In Vitro sensitivity test of Fusarium species from weeds and non-gramineous plants to triazole fungicides. Pathogens 2024, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.J.; Silva, C.N.; Paiva, G.F.; Feksa, H.R.; Tessmann, D.J.; Mizubuti, E.S.G.; Del Ponte, E.M. Sensitivity to tebuconazole and carbendazim in Fusarium graminearum species complex populations causing wheat head blight in southern Brazil. Trop. Plant Pathol. 2024, 49, 157–167. [Google Scholar] [CrossRef]
- Andrade, S.M.; Augusti, G.R.; Paiva, G.F.; Feksa, H.R.; Tessmann, D.J.; Machado, F.J.; Mizubuti, E.S.G.; Del Ponte, E.M. Phenotypic and molecular characterization of the resistance to azoxystrobin and pyraclostrobin in Fusarium graminearum populations from Brazil. Plant Pathol. 2022, 71, 1152–1163. [Google Scholar] [CrossRef]
- Luan, S.; Chen, Y.; Wang, X.; Yan, D.; Xu, J.; Cui, H.; Huang, Q. Synergy of cystamine and pyraclostrobin against Fusarium graminearum involves membrane permeability mitigation and autophagy enhancement. Pestic. Biochem. Physiol. 2022, 188, 105287. [Google Scholar] [CrossRef]
- Xu, C.; Li, M.; Zhou, Z.; Li, J.; Chen, D.; Duan, Y.; Zhou, M. Impact of five succinate dehydrogenase inhibitors on don biosynthesis of Fusarium asiaticum, causing Fusarium head blight in wheat. Toxins 2019, 11, 272. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Field resistance to QoI fungicides in Podosphaera fusca is not supported by typical mutations in the mitochondrial cytochrome b gene. Pest. Manag. Sci. 2008, 64, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Qiu, H.; Gao, X.; Li, Y.; Zheng, X.; Cai, Y.; Sheng, G.; Shen, Y.; Wang, J.; Zhou, M.; et al. Resistance risk and molecular mechanism of tomato wilt pathogen Fusarium oxysporum f. sp. lycopersici to pyraclostrobin. J. Agric. Food Chem. 2024, 72, 3998–4007. [Google Scholar] [CrossRef]
- Dubos, T.; Pasquali, M.; Pogoda, F.; Hoffmann, L.; Beyer, M. Evidence for natural resistance towards trifloxystrobin in Fusarium graminearum. Eur. J. Plant Pathol. 2022, 130, 239–248. [Google Scholar] [CrossRef]
- Tsukamoto, M.; Nakamura, T.; Kimura, H.; Nakayama, H. Synthesis and application of trifluoromethyl pyridines as a key structural motif in active agrochemical and pharmaceutical ingredients. J. Pestic. Sci. 2021, 46, 125–142. [Google Scholar] [CrossRef]
- Syngenta. 2024. Available online: https://www.syngenta.com/ (accessed on 14 October 2024).
- Shao, W.; Wang, J.; Wang, H.; Wen, Z.; Liu, C.; Zhang, Y.; Zhao, Y.; Ma, Z. Fusarium graminearum FgSdhC1 point mutation A78V confers resistance to the succinate dehydrogenase inhibitor pydiflumetofen. Pest Manag. Sci. 2022, 78, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Chu, Y.; Wen, H.; Li, B.; Dong, X.; Sun, M.; Meng, D.; Wang, M.; Gao, T.; Duan, J. Stereoselective bioactivity, toxicity and degradation of novel fungicide sedaxane with four enantiomers under rice-wheat rotation mode. Ecotoxic. Environ. Saf. 2022, 241, 113784. [Google Scholar] [CrossRef] [PubMed]
- Risoli, S.; Cotrozzi, L.; Pisuttu, C.; Nali, C. Biocontrol agents of fusarium head blight in wheat: A meta-analytic approach to elucidate their strengths and weaknesses. Phytopathology 2024, 114, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.A.; Pernica, M.; Yap, J.; Belakova, S.; Vaculova, K.; Branyik, T. Biocontrol effect of Pythium oligandrum on artificial Fusarium culmorum infection during malting of wheat. J. Cereal Sci. 2021, 100, 103258. [Google Scholar] [CrossRef]
- Pellan, L.; Dieye, C.A.T.; Durand, N.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Biocontrol agents reduce progression and mycotoxin production of Fusarium graminearum in spikelets and straws of wheat. Toxins 2021, 13, 597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).