Extensive T-Cell Profiling Following SARS-CoV-2 mRNA Vaccination in Multiple Sclerosis Patients Treated with DMTs
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
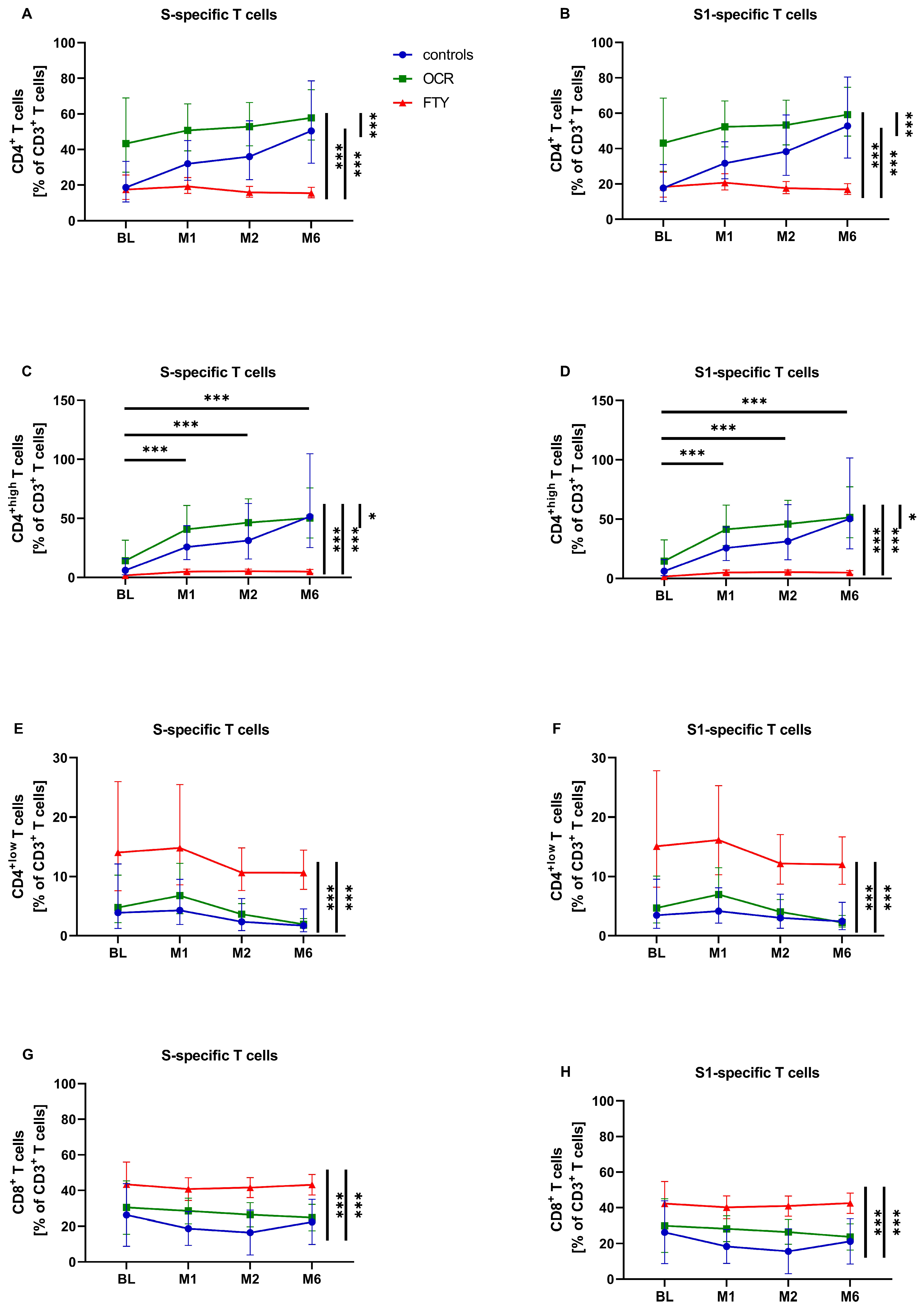
3.2. SARS-CoV-2-Specific T-Cell Response
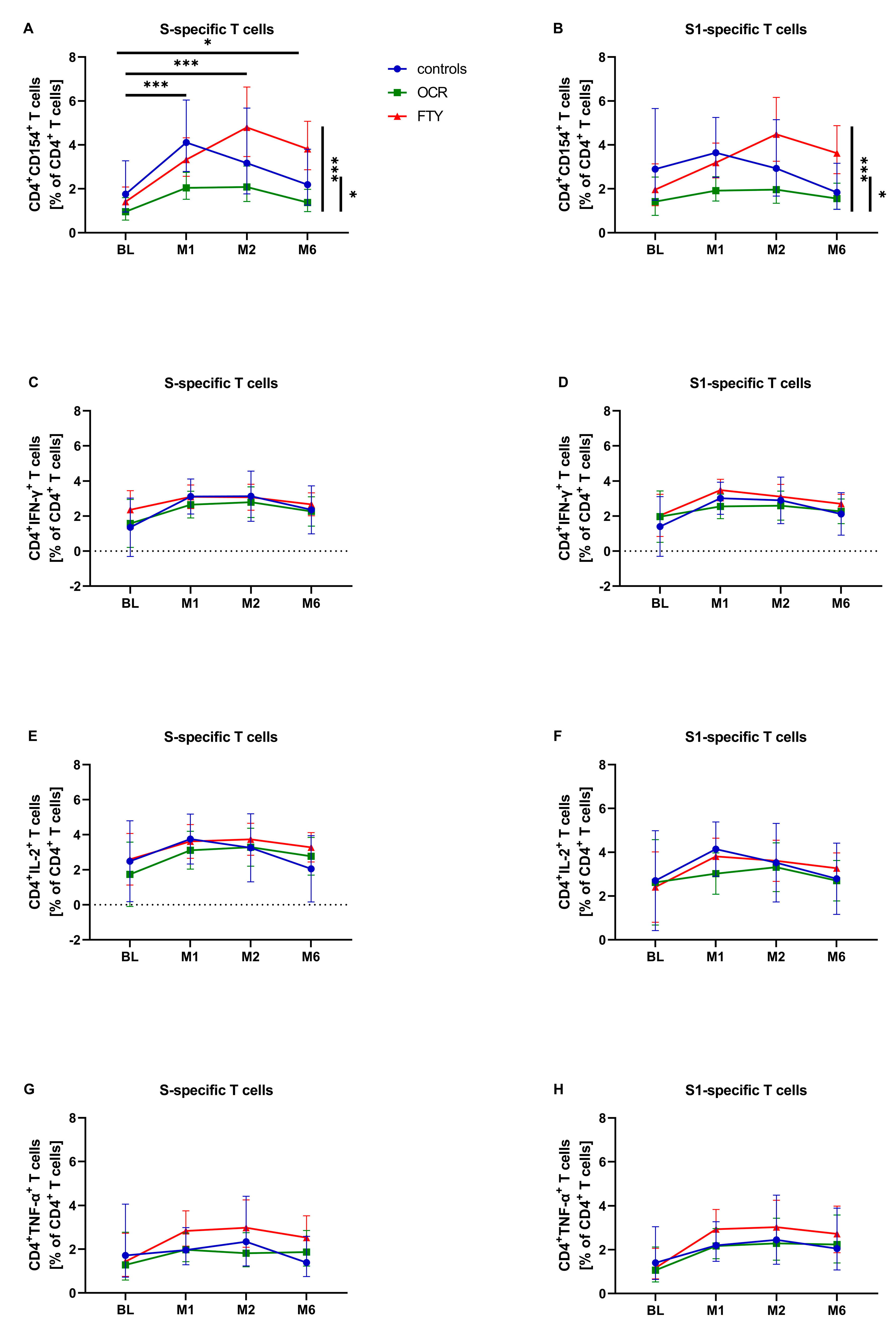
3.3. SARS-CoV-2-Specific T-Cell Response—Activation and Differentiation Markers
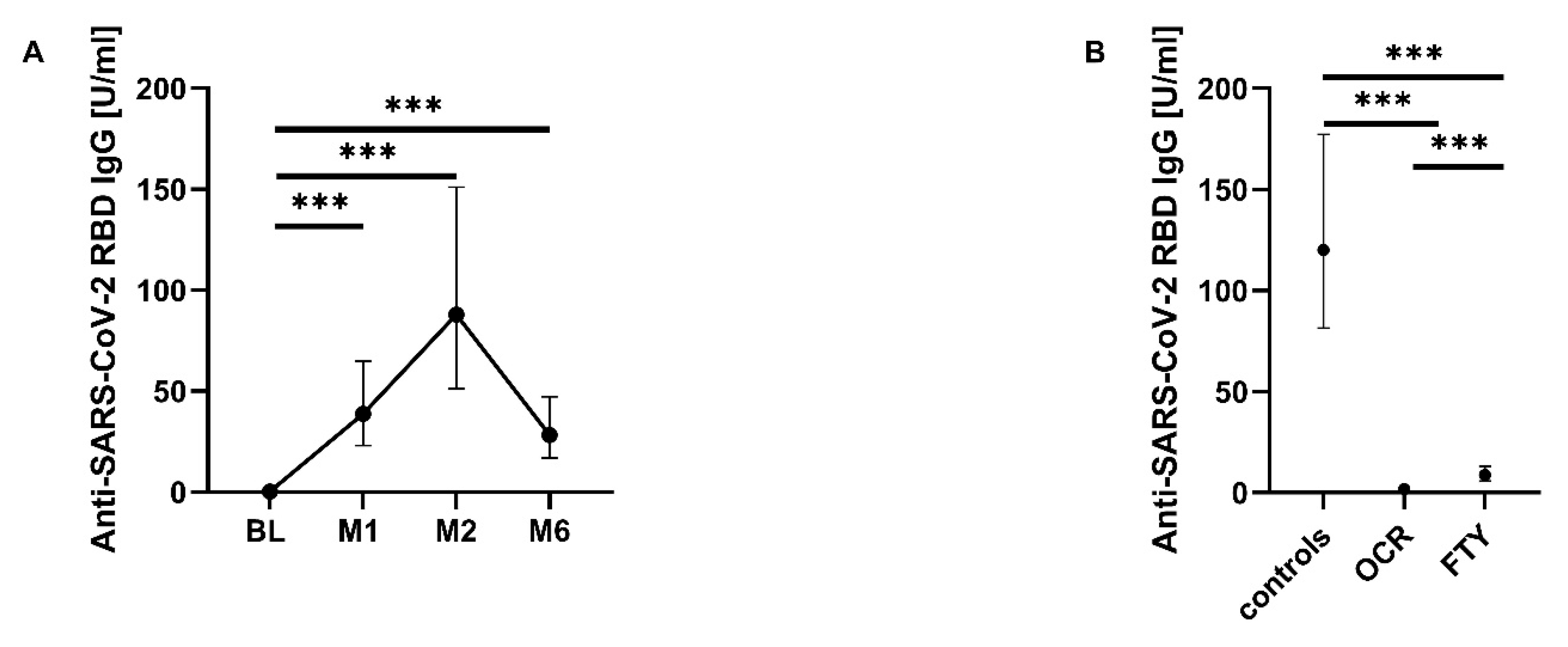
3.4. SARS-CoV-2-Specific B-Cell Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMTs | disease-modifying therapies |
| pwMS | persons with multiple sclerosis |
| mRNA | messenger ribonucleic acid |
| S1PR | sphingosine-1-phosphate receptor |
| COVID-19 | coronavirus disease 19 |
| RBD | receptor-binding domain |
| PCR | polymerase chain reaction |
| BL | baseline |
| PBMCs | peripheral blood mononuclear cells |
| FACS | fluorescence-activated cell sorting |
| S protein | spike protein |
| CD | cluster of differentiation |
| ECLIA | electrochemiluminescence immunoassay |
| IgG | immunoglobulin G |
| BAU | binding antibody units |
| SD | standard deviation |
| GLMM | generalized linear mixed model |
| IQR | interquartile range |
| RRMS | relapsing–remitting multiple sclerosis |
| SPMS | secondary progressive multiple sclerosis |
| PPMS | primary progressive multiple sclerosis |
| EDSS | Expanded Disability Status Scale |
| OCR | ocrelizumab group |
| FTY | fingolimod group |
References
- The Multiple Sclerosis International Federation. Number of People with MS|Atlas of MS. Available online: https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms (accessed on 12 December 2023).
- The Multiple Sclerosis International Federation. Atlas of MS 3rd Edition: PART 1: Mapping Multiple Sclerosis Around the World Key Epidemiology Findings. Available online: https://www.msif.org/wp-content/uploads/2020/10/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (accessed on 12 December 2023).
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur. J. Neurol. 2018, 25, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Hart, F.M.; Bainbridge, J. Current and Emerging Treatment of Multiple Sclerosis. Am. J. Manag. Care 2016, 22, 159–170. [Google Scholar]
- Wiendl, H.; Korsukewitz, C.; Kieseier, B.C. Multiple Sklerose: Klinik, Diagnostik und Therapie, 2nd ed.; Kohlhammer: Stuttgart, Germany, 2021; ISBN 9783170224964. [Google Scholar]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Sormani, M.P.; Inglese, M.; Schiavetti, I.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Da Rin, G.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine 2021, 72, 103581. [Google Scholar] [CrossRef]
- Yang, L.M.; Costales, C.; Ramanathan, M.; Bulterys, P.L.; Murugesan, K.; Schroers-Martin, J.; Alizadeh, A.A.; Boyd, S.D.; Brown, J.M.; Nadeau, K.C.; et al. Cellular and humoral immune response to SARS-CoV-2 vaccination and booster dose in immunosuppressed patients: An observational cohort study. J. Clin. Virol. 2022, 153, 105217. [Google Scholar] [CrossRef]
- Madelon, N.; Lauper, K.; Breville, G.; Sabater Royo, I.; Goldstein, R.; Andrey, D.O.; Grifoni, A.; Sette, A.; Kaiser, L.; Siegrist, C.A.; et al. Robust T-Cell Responses in Anti-CD20-Treated Patients Following COVID-19 Vaccination: A Prospective Cohort Study. Clin. Infect. Dis. 2022, 75, e1037–e1045. [Google Scholar] [CrossRef]
- Giannoccaro, M.P.; Vacchiano, V.; Leone, M.; Camilli, F.; Zenesini, C.; Panzera, I.; Balboni, A.; Tappatà, M.; Borghi, A.; Salvi, F.; et al. Difference in safety and humoral response to mRNA SARS-CoV-2 vaccines in patients with autoimmune neurological disorders: The ANCOVAX study. J. Neurol. 2022, 269, 4000–4012. [Google Scholar] [CrossRef]
- Woopen, C.; Dunsche, M.; Al Rahbani, G.K.; Dillenseger, A.; Atta, Y.; Haase, R.; Raposo, C.; Pedotti, R.; Ziemssen, T.; Akgün, K. Long-Term Immune Response Profiles to SARS-CoV-2 Vaccination and Infection in People with Multiple Sclerosis on Anti-CD20 Therapy. Vaccines 2023, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Woopen, C.; Dunsche, M.; Haase, R.; Raposo, C.; Pedotti, R.; Akgün, K.; Ziemssen, T. Timing of SARS-CoV-2 Vaccination Matters in People With Multiple Sclerosis on Pulsed Anti-CD20 Treatment. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200031. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012835. [Google Scholar] [CrossRef]
- Matko, S.; Akgün, K.; Tonn, T.; Ziemssen, T.; Odendahl, M. Antigen-shift in varicella-zoster virus-specific T-cell immunity over the course of Fingolimod-treatment in relapse-remitting multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020, 38, 101859. [Google Scholar] [CrossRef]
- Algu, P.; Hameed, N.; DeAngelis, T.; Stern, J.; Harel, A. Post-vaccination SARS-Cov-2 T-cell receptor repertoires in patients with multiple sclerosis and related disorders. Mult. Scler. Relat. Disord. 2023, 79, 104965. [Google Scholar] [CrossRef]
- Iannetta, M.; Landi, D.; Cola, G.; Campogiani, L.; Malagnino, V.; Teti, E.; Coppola, L.; Di Lorenzo, A.; Fraboni, D.; Buccisano, F.; et al. B- and T-Cell Responses After SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Receiving Disease Modifying Therapies: Immunological Patterns and Clinical Implications. Front. Immunol. 2021, 12, 796482. [Google Scholar] [CrossRef]
- Torres, P.; Sancho-Saldaña, A.; Gil Sánchez, A.; Peralta, S.; Solana, M.J.; Bakkioui, S.; González-Mingot, C.; Quibus, L.; Ruiz-Fernández, E.; San Pedro-Murillo, E.; et al. A prospective study of cellular immune response to booster COVID-19 vaccination in multiple sclerosis patients treated with a broad spectrum of disease-modifying therapies. J. Neurol. 2023, 270, 2380–2391. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Braun, J.; Fauchere, F.; Vanshylla, K.; Loyal, L.; Henze, L.; Kruse, B.; Dingeldey, M.; Jürchott, K.; Mangold, M.; et al. SARS-CoV-2 mRNA vaccinations fail to elicit humoral and cellular immune responses in patients with multiple sclerosis receiving fingolimod. J. Neurol. Neurosurg. Psychiatry 2022, 93, 960–971. [Google Scholar] [CrossRef]
- Maglione, A.; Francese, R.; Arduino, I.; Rosso, R.; Matta, M.; Rolla, S.; Lembo, D.; Clerico, M. Long-lasting neutralizing antibodies and T cell response after the third dose of mRNA anti-SARS-CoV-2 vaccine in multiple sclerosis. Front. Immunol. 2023, 14, 1205879. [Google Scholar] [CrossRef]
- Kaufman, M.; Pardo, G.; Rossman, H.; Sweetser, M.T.; Forrestal, F.; Duda, P. Natalizumab treatment shows no clinically meaningful effects on immunization responses in patients with relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2014, 341, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Vågberg, M.; Kumlin, U.; Svenningsson, A. Humoral immune response to influenza vaccine in natalizumab-treated MS patients. Neurol. Res. 2012, 34, 730–733. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.L.; Tuohy, O.; Compston, D.A.S.; Kumararatne, D.S.; Coles, A.J.; Jones, J.L. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology 2013, 81, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Jochum, S.; Kirste, I.; Hortsch, S.; Grunert, V.P.; Legault, H.; Eichenlaub, U.; Kashlan, B.; Pajon, R. Clinical Utility of Elecsys Anti-SARS-CoV-2 S Assay in COVID-19 Vaccination: An Exploratory Analysis of the mRNA-1273 Phase 1 Trial. Front. Immunol. 2022, 12, 798117. [Google Scholar] [CrossRef]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef]
- Kompetenznetz Multiple Sklerose. Fingolimod—MS-Qualitätshandbuch. Available online: https://ms-qualitaetshandbuch.de/wirkstoff/fingolimod/ (accessed on 4 January 2024).
- Ortolani, C.; Forti, E.; Radin, E.; Cibin, R.; Cossarizza, A. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem. Biophys. Res. Commun. 1993, 191, 601–609. [Google Scholar] [CrossRef]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef]
- Kürtüncü, M.; Yılmaz, V.; Akçay, H.İ.; Türkoğlu, R.; Altunrende, B.; Çınar, S.A.; Ulusoy, C.; Gündüz, T.; İçöz, S.; Kasap, M.; et al. Impact of fingolimod on CD4+ T cell subset and cytokine profile of relapsing remitting multiple sclerosis patients. J. Neuroimmunol. 2019, 337, 577065. [Google Scholar] [CrossRef]
- Kowarik, M.C.; Pellkofer, H.L.; Cepok, S.; Korn, T.; Kümpfel, T.; Buck, D.; Hohlfeld, R.; Berthele, A.; Hemmer, B. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology 2011, 76, 1214–1221. [Google Scholar] [CrossRef]
- Fischer, S.; Proschmann, U.; Akgün, K.; Ziemssen, T. Lymphocyte Counts and Multiple Sclerosis Therapeutics: Between Mechanisms of Action and Treatment-Limiting Side Effects. Cells 2021, 10, 3177. [Google Scholar] [CrossRef] [PubMed]
- Sfikakis, P.P.; Boletis, J.N.; Lionaki, S.; Vigklis, V.; Fragiadaki, K.G.; Iniotaki, A.; Moutsopoulos, H.M. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: An open-label trial. Arthritis Rheum. 2005, 52, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Marsman, C.; Verstegen, N.J.; Streutker, M.; Jorritsma, T.; Boon, L.; ten Brinke, A.; van Ham, S.M. Termination of CD40L co-stimulation promotes human B cell differentiation into antibody-secreting cells. Eur. J. Immunol. 2022, 52, 1662–1675. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Pasculli, P.; Tartaglia, M.; Dominelli, F.; Ciccone, F.; Taglietti, A.; Perri, V.; Malimpensa, L.; Ferrazzano, G.; Iannetta, M.; et al. Evaluation of BAFF, APRIL and CD40L in Ocrelizumab-Treated pwMS and Infectious Risk. Biology 2023, 12, 587. [Google Scholar] [CrossRef]
- Tokunaga, M.; Saito, K.; Kawabata, D.; Imura, Y.; Fujii, T.; Nakayamada, S.; Tsujimura, S.; Nawata, M.; Iwata, S.; Azuma, T.; et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann. Rheum. Dis. 2007, 66, 470–475. [Google Scholar] [CrossRef]
- Sabatino, J.J.; Mittl, K.; Rowles, W.M.; McPolin, K.; Rajan, J.V.; Laurie, M.T.; Zamecnik, C.R.; Dandekar, R.; Alvarenga, B.D.; Loudermilk, R.P.; et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight 2022, 7, e156978. [Google Scholar] [CrossRef]
- Koutsakos, M.; Rowntree, L.C.; Hensen, L.; Chua, B.Y.; van de Sandt, C.E.; Habel, J.R.; Zhang, W.; Jia, X.; Kedzierski, L.; Ashhurst, T.M.; et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2021, 2, 100208. [Google Scholar] [CrossRef]
- Verstegen, N.J.M.; Hagen, R.R.; Kreher, C.; Kuijper, L.H.; van den Dijssel, J.; Ashhurst, T.; Kummer, L.Y.L.; Palomares Cabeza, V.; Steenhuis, M.; Duurland, M.C.; et al. T cell activation markers CD38 and HLA-DR indicative of non-seroconversion in anti-CD20-treated patients with multiple sclerosis following SARS-CoV-2 mRNA vaccination. J. Neurol. Neurosurg. Psychiatry 2024, 95, 855–864. [Google Scholar] [CrossRef]
- Guerrera, G.; Picozza, M.; D’Orso, S.; Placido, R.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Giannessi, F.; Sambucci, M.; et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci. Immunol. 2021, 6, eabl5344. [Google Scholar] [CrossRef]
- Li, L.; Muftuoglu, M.; Liang, S.; Basyal, M.; Lv, J.; Akdogan, M.E.; Chen, K.; Andreeff, M.; Flowers, C.R.; Parmar, S. In-depth analysis of SARS-CoV-2-specific T cells reveals diverse differentiation hierarchies in vaccinated individuals. JCI Insight 2022, 7, e156559. [Google Scholar] [CrossRef]
- Mehling, M.; Brinkmann, V.; Antel, J.; Bar-Or, A.; Goebels, N.; Vedrine, C.; Kristofic, C.; Kuhle, J.; Lindberg, R.L.P.; Kappos, L. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008, 71, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, C.; Aiello, A.; Gasperini, C.; Agrati, C.; Castilletti, C.; Ruggieri, S.; Meschi, S.; Matusali, G.; Colavita, F.; Farroni, C.; et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies. Neurology 2022, 98, e541–e554. [Google Scholar] [CrossRef] [PubMed]
- Räuber, S.; Korsen, M.; Huntemann, N.; Rolfes, L.; Müntefering, T.; Dobelmann, V.; Hermann, A.M.; Kölsche, T.; von Wnuck Lipinski, K.; Schroeter, C.B.; et al. Immune response to SARS-CoV-2 vaccination in relation to peripheral immune cell profiles among patients with multiple sclerosis receiving ocrelizumab. J. Neurol. Neurosurg. Psychiatry 2022, 93, 978–985. [Google Scholar] [CrossRef]
- Aiello, A.; Coppola, A.; Ruggieri, S.; Farroni, C.; Altera, A.M.G.; Salmi, A.; Vanini, V.; Cuzzi, G.; Petrone, L.; Meschi, S.; et al. Longitudinal characterisation of B and T-cell immune responses after the booster dose of COVID-19 mRNA-vaccine in people with multiple sclerosis using different disease-modifying therapies. J. Neurol. Neurosurg. Psychiatry 2023, 94, 290–299. [Google Scholar] [CrossRef]
- Suneetha, P.V.; Schlaphoff, V.; Wang, C.; Stegmann, K.A.; Fytili, P.; Sarin, S.K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Effect of peptide pools on effector functions of antigen-specific CD8+ T cells. J. Immunol. Methods 2009, 342, 33–48. [Google Scholar] [CrossRef]
- Kiecker, F.; Streitz, M.; Ay, B.; Cherepnev, G.; Volk, H.-D.; Volkmer-Engert, R.; Kern, F. Analysis of antigen-specific T-cell responses with synthetic peptides—What kind of peptide for which purpose? Hum. Immunol. 2004, 65, 523–536. [Google Scholar] [CrossRef]
| Characteristic | All n = 47 | Controls n = 17 | Ocrelizumab n = 15 | Fingolimod n = 15 | p-Value |
|---|---|---|---|---|---|
| Age, years (mean, SD) | 45.60 ± 11.89 | 48.18 ± 15.17 | 42.53 ± 10.61 | 45.73 ± 8.40 | 0.416 |
| Sex, n (%) Female Male | 35 (74.5) 12 (25.5) | 13 (76.5) 4 (23.5) | 14 (93.3) 1 (6.7) | 8 (53.3) 7 (46.7) | 0.041 |
| Disease course, n (%) RRMS SPMS PPMS | 38 (80.9) 4 (8.5) 5 (10.6) | 12 (70.6) 3 (17.7) 2 (11.8) | 12 (80.0) 0 (0.0) 3 (20.0) | 14 (93.3) 1 (6.7) 0 (0.0) | |
| Disease duration, years (mean, SD) | 13.47 ± 9.97 | 17.76 ± 11.85 | 10.20 ± 9.89 | 11.87 ± 5.66 | 0.054 |
| Treatment duration, years (mean, SD) Alemtuzumab Natalizumab Ocrelizumab Fingolimod | 7.83 ± 4.67 6.40 ± 2.19 | 2.07 ± 0.88 | 7.33 ± 2.92 | ||
| EDSS (mean, SD) (Median) (IQR) | 3.47 ± 1.93 3.00 5.00 | 4.16 ± 2.20 3.75 4.25 | 2.97 ± 1.84 2.50 1.50 | 3.21 ± 1.57 2.75 4.00 | 0.270 |
| Last infusion–first vaccination, days (mean, SD) | 90.73 ± 42.69 | ||||
| BL–first vaccination, days (mean, SD) | 5.72 ± 10.44 | 5.24 ± 7.59 | 4.07 ± 10.87 | 7.93 ± 12.88 | 0.212 |
| First vaccination–M1, days (mean, SD) | 28.49 ± 6.27 | 29.00 ± 9.37 | 27.53 ± 3.09 | 28.87 ± 4.21 | 0.655 |
| First vaccination–M2, days (mean, SD) | 57.45 ± 7.08 | 56.47 ± 8.30 | 60.07 ± 7.49 | 55.93 ± 4.37 | 0.176 |
| First vaccination–M6, days (mean, SD) | 184.19 ± 18.18 | 187.76 ± 19.79 | 176.20 ± 20.20 | 188.13 ± 11.41 | 0.335 |
| All n = 47 | Controls n = 17 | Ocrelizumab n = 15 | Fingolimod n = 15 | ||
|---|---|---|---|---|---|
| First-dose vaccine BNT162b2 mRNA-1273 | n (%) n (%) | 45 (95.7) 2 (4.3) | 17 (100.0) | 15 (100.0) | 13 (86.7) 2 (13.3) |
| Second-dose vaccine BNT162b2 mRNA-1273 | n (%) n (%) | 45 (95.7) 2 (4.3) | 17 (100.0) | 15 (100.0) | 13 (86.7) 2 (13.3) |
| Third-dose vaccine BNT162b2 mRNA-1273 | n (%) n (%) | 45 (95.7) 2 (4.3) | 17 (100.0) | 15 (100.0) | 13 (86.7) 2 (13.3) |
| M1 1 vaccination 2 vaccinations | n (%) n (%) | 17 (36.2) 30 (63.8) | 4 (23.5) 13 (76.5) | 8 (53.3) 7 (46.7) | 5 (33.3) 10 (66.7) |
| M2 1 vaccination 2 vaccinations | n (%) n (%) | 1 (2.1) 46 (97.9) | 1 (5.9) 16 (94.1) | 15 (100.0) | 15 (100.0) |
| M6 2 vaccinations 3 vaccinations | n (%) n (%) | 41 (87.2) 6 (12.8) | 17 (100.0) | 12 (80.0) 3 (20.0) | 12 (80.0) 3 (20.0) |
| T-Cell Subsets (%) | Patient Group | S | S1 | ||
|---|---|---|---|---|---|
| IgG + | IgG − | IgG + | IgG − | ||
| CD4+ (% of CD3+) | All | 28.622 CI (22.923, 35.376) | 32.263 CI: (26.721, 38.953) | 28.954 CI: (23.258, 36.046) | 33.994 CI: (28.362, 40.745) |
| Controls | 25.378 CI: (18.798, 34.263) | 41.148 CI: (24.126, 70.300) | 24.855 CI: (18.567, 33.273) | 42.875 CI: (25.716, 71.485) | |
| OCR | 50.464 CI: (36.137, 70.701) | 51.330 CI: (45.299, 58.163) | 50.924 CI: (36.620, 70.816) | 52.413 CI: (46.315, 59.315) | |
| FTY | 18.278 (CI: 14.714, 22.706) | 15.886 CI: (13.764, 18.355) | 19.178 CI: (15.481, 23.759) | 17.481 CI: (15.198, 20.107) | |
| CD4+high (% of CD3+) | All | 11.042 * CI: (7.564, 16.119) | 18.748 * CI: (13.925, 25.241) | 11.219 CI: (7.710, 16.325) | 18.654 CI: (13.891, 25.049) |
| Controls | 13.321 CI: (8.131, 21.825) | 37.877 CI: (16.399, 87.487) | 13.636 CI: (8.364, 22.230) | 36.739 CI: (16.039, 84.158) | |
| OCR | 27.456 CI: (15.749, 47.886) | 42.377 CI: (34.560, 51.962) | 28.239 CI: (16.263, 49.033) | 42.429 CI: (34.585, 52.052) | |
| FTY | 3.681 CI: (2.543, 5.327) | 4.106 CI: (3.251, 5.185) | 3.667 CI: (2.541, 5.290) | 4.164 CI: (3.304, 5.247) | |
| CD4+low (% of CD3+) | All | 4.802 CI: (3.269, 7.053) | 5.544 CI: (3.632, 8.463) | 4.834 CI: (3.331, 7.017) | 6.646 CI: (4.631, 9.537) |
| Controls | 3.461 CI: (1.877, 6.381) | 2.373 CI: (0.685, 8.224) | 3.259 CI: (1.898, 5.598) | 3.143 CI: (1.112, 8.880) | |
| OCR | 2.306 CI: (1.271, 4.187) *** | 6.499 CI: (5.129, 8.234) *** | 2.328 CI: (1.294, 4.188) *** | 7.368 CI: (5.876, 9.239) *** | |
| FTY | 13.870 CI: (9.524, 20.199) | 11.050 CI: (8.427, 14.488) | 14.889 CI: (10.347, 21.426) | 12.676 CI: (9.791, 16.411) | |
| CD8+ (% of CD3+) | All | 32.830 CI: (25.810, 39.850) | 27.732 CI: (22.409, 33.055) | 32.298 CI: (25.271, 39.326) | 27.040 CI: (21.682, 32.397) |
| Controls | 23.661 CI: (14.653, 32.670) | 18.289 CI: (3.387, 33.190) | 23.584 CI: (14.549, 32.618) | 17.214 CI: (2.204, 32.224) | |
| OCR | 29.867 CI: (19.631, 40.103) | 25.290 CI: (21.559, 29.020) | 29.119 CI: (18.869, 39.369) | 25.126 CI: (31.349, 28.904) | |
| FTY | 44.962 CI: (38.099, 51.824) | 39.618 CI: (35.354, 43.882) | 44.192 CI: (37.323, 51.061) | 38.779 CI: (34.520, 43.038) | |
| CD4+CD154+ (% of CD4+) | All | 1.690 ** CI: (1.313, 2.174) | 3.182 ** CI: (2.528, 4.006) | 2.060 CI: (1.558, 2.724) | 2.924 CI: (2.337, 3.658) |
| Controls | 2.125CI: (1.519, 2.973) | 3.329 CI: (1.788, 6.199) | 2.746 CI: (1.932, 3.902) | 2.743 CI: (1.514, 4.970) | |
| OCR | 1.118 CI: (0.721, 1.732) | 2.122 CI: (1.796, 2.508) | 1.389 CI: (0.883, 2.185) | 2.070 CI: (1.741, 2.461) | |
| FTY | 2.031 CI: (1.583, 2.605) | 4.562 CI: (3.728, 5.582) | 2.292 CI: (1.740, 3.019) | 4.404 CI: (3.592, 5.399) | |
| CD4+IFN-γ+ (% of CD4+) | All | 2.202 CI: (1.539, 2.865) | 2.882 CI: (2.303, 3.460) | 2.282 CI: (1.593, 2.972) | 2.751 CI: (2.220, 3.281) |
| Controls | 1.851 CI: (0.969, 2.734) | 3.137 CI: (1.544, 4.730) | 1.886 CI: (1.007, 2.766) | 2.839 CI: (1.378, 4.300) | |
| OCR | 2.263 CI: (1.275, 3.451) | 2.288 CI: (1.877, 2.698) | 2.556 CI: (1.518, 3.595) | 2.143 CI: (1.753, 2.532) | |
| FTY | 2.390 CI: (1.740, 3.041) | 3.220 CI: (2.735, 3.705) | 2.404 CI: (1.728, 3.080) | 3.270 CI: (2.824, 3.715) | |
| CD4+IL-2+ (% of CD4+) | All | 2.882 CI: (1.991, 3.773) | 3.068 CI: (2.265, 3.872) | 3.037 CI: (2.119, 3.956) | 3.284 CI: (2.569, 4.000) |
| Controls | 3.029 CI: (1.806, 4.252) | 2.745 CI: (0.489, 5.002) | 3.034 CI: (1.855, 4.213) | 3.546 CI: (1.572, 5.521) | |
| OCR | 2.638 CI: (1.201, 4.075) | 2.819 CI: (2.277, 3.361) | 3.151 CI: (1.769, 4.533) | 2.683 CI: (2.164, 3.203) | |
| FTY | 2.978 CI: (2.107, 3.850) | 3.641 CI: (3.006, 4.276) | 2.927 CI: (2.026, 3.828) | 3.623 CI: (3.030, 4.217) | |
| CD4+TNF-α+ (% of CD4+) | All | 1.815 CI: (1.262, 2.609) | 2.083 CI: (1.613, 2.690) | 1.800 CI: (1.294, 2.505) | 2.311 CI: (1.798, 2.969) |
| Controls | 2.146 CI: (1.381, 3.335) | 1.545 CI: (0.783, 3.049) | 1.964 CI: (1.310, 2.944) | 2.001 CI: (1.040, 3.849) | |
| OCR | 1.484 CI: (0.861, 2.560) | 1.980 CI: (1.621, 2.419) | 1.723 CI: (1.021, 2.909) | 1.995 CI: (1.635, 2.434) | |
| FTY | 1.875 CI: (1.313, 2.679) | 2.955 CI: (2.336, 3.737) | 1.725 CI: (1.245, 2.390) | 3.091 CI: (2.440, 3.914) | |
| BL | M1 | M2 | M6 | ||
|---|---|---|---|---|---|
| All seronegative seropositive | n (%) n (%) | 45 (97.8) 1 (2.2) | 22 (46.8) 25 (53.2) | 18 (38.3) 29 (61.7) | 18 (38.3) 29 (61.7) |
| Controls seronegative seropositive | n (%) n (%) | 17 (100.0) | 3 (17.6) 14 (82.4) | 17 (100.0) | 17 (100.0) |
| Ocrelizumab seronegative seropositive | n (%) n (%) | 15 (100.0) | 11 (73.3) 4 (26.7) | 12 (80.0) 3 (20.0) | 13 (86.7) 2 (13.3) |
| Fingolimod seronegative seropositive | n (%) n (%) | 13 (92.9) 1 (7.1) | 8 (53.3) 7 (46.7) | 6 (40.0) 9 (60.0) | 5 (33.3) 10 (66.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solchenberger, H.; Odendahl, M.; Schriefer, D.; Proschmann, U.; Rahbani, G.K.a.; Ziemssen, T.; Akgün, K. Extensive T-Cell Profiling Following SARS-CoV-2 mRNA Vaccination in Multiple Sclerosis Patients Treated with DMTs. Pathogens 2025, 14, 235. https://doi.org/10.3390/pathogens14030235
Solchenberger H, Odendahl M, Schriefer D, Proschmann U, Rahbani GKa, Ziemssen T, Akgün K. Extensive T-Cell Profiling Following SARS-CoV-2 mRNA Vaccination in Multiple Sclerosis Patients Treated with DMTs. Pathogens. 2025; 14(3):235. https://doi.org/10.3390/pathogens14030235
Chicago/Turabian StyleSolchenberger, Hannah, Marcus Odendahl, Dirk Schriefer, Undine Proschmann, Georges Katoul al Rahbani, Tjalf Ziemssen, and Katja Akgün. 2025. "Extensive T-Cell Profiling Following SARS-CoV-2 mRNA Vaccination in Multiple Sclerosis Patients Treated with DMTs" Pathogens 14, no. 3: 235. https://doi.org/10.3390/pathogens14030235
APA StyleSolchenberger, H., Odendahl, M., Schriefer, D., Proschmann, U., Rahbani, G. K. a., Ziemssen, T., & Akgün, K. (2025). Extensive T-Cell Profiling Following SARS-CoV-2 mRNA Vaccination in Multiple Sclerosis Patients Treated with DMTs. Pathogens, 14(3), 235. https://doi.org/10.3390/pathogens14030235







