Integrated One Health Surveillance of West Nile Virus and Usutu Virus in the Veneto Region, Northeastern Italy, from 2022 to 2023
Abstract
1. Introduction
2. Materials and Methods
2.1. Background
2.2. Entomological Surveillance
2.3. Veterinary Surveillance
2.4. Human Surveillance
3. Statistical Analysis
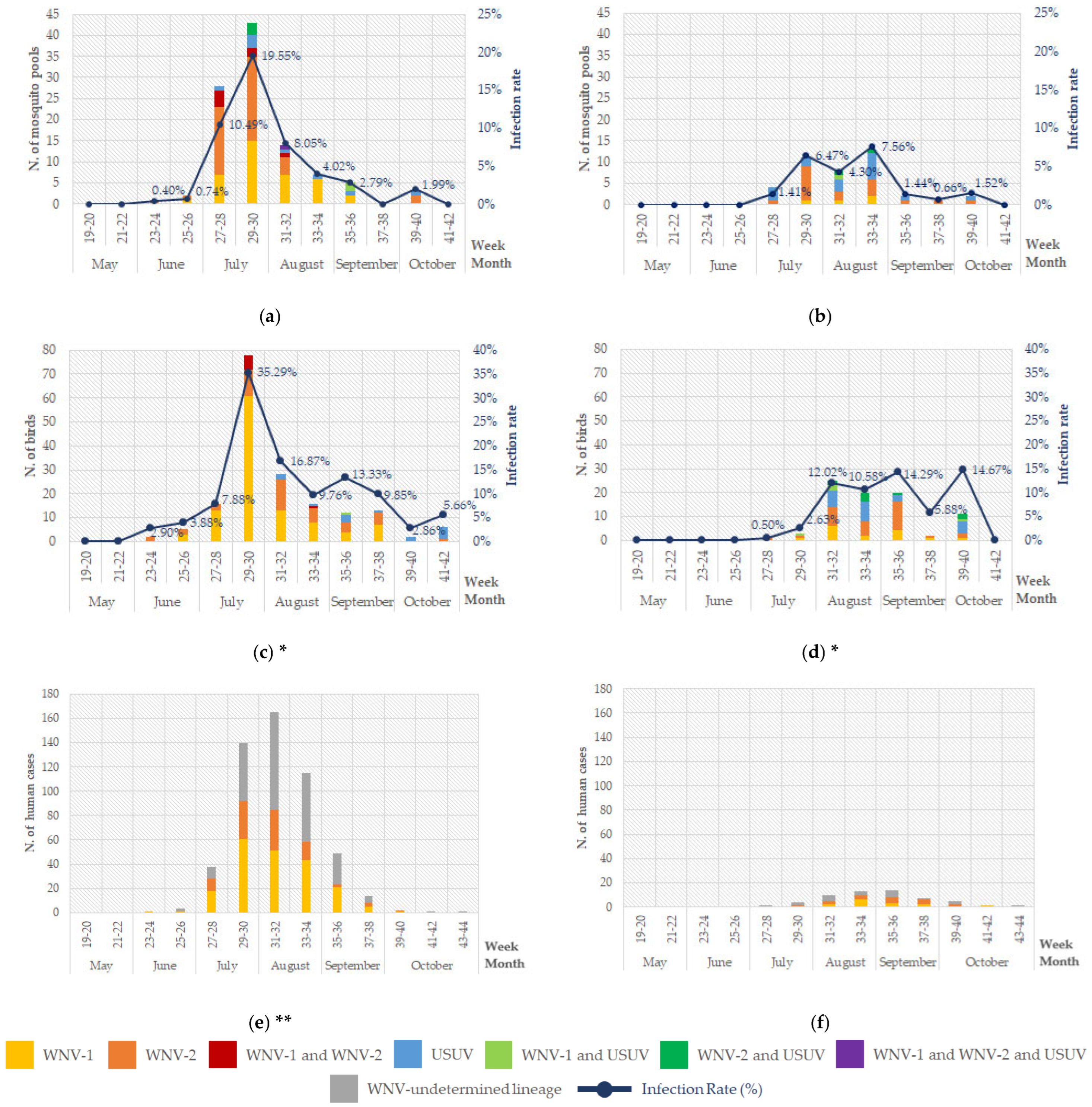
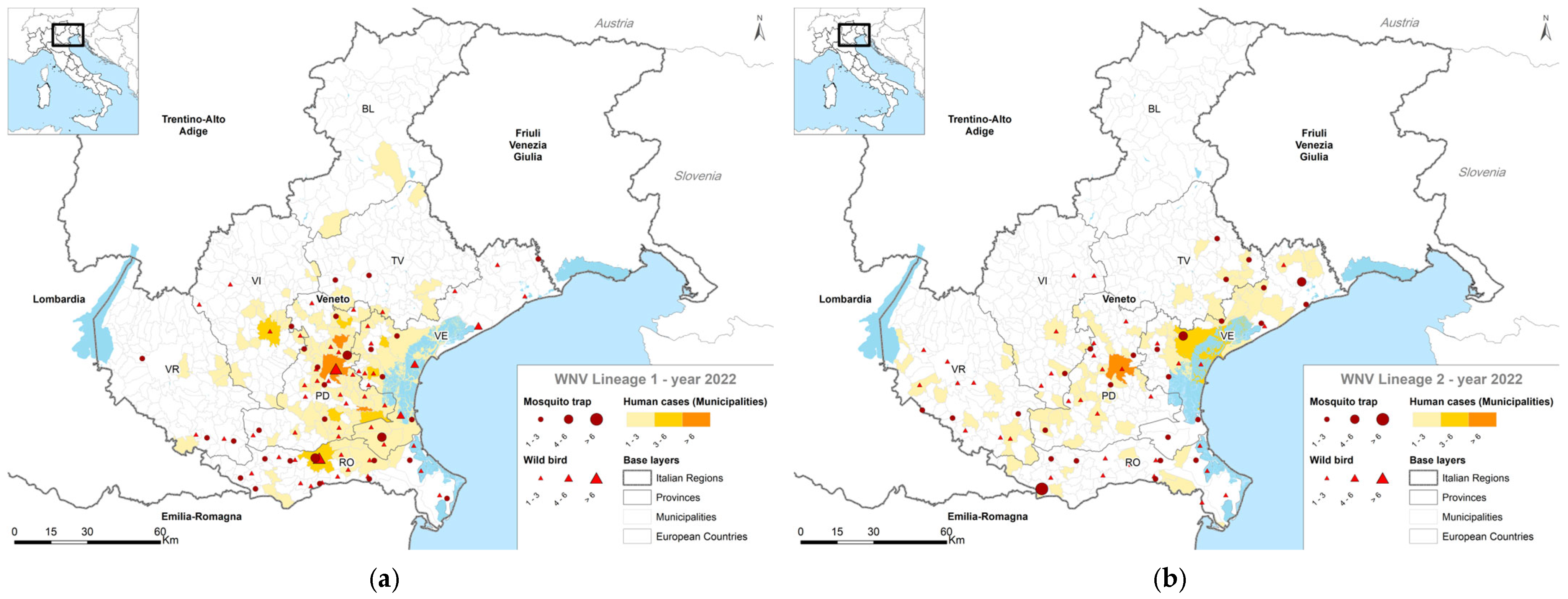
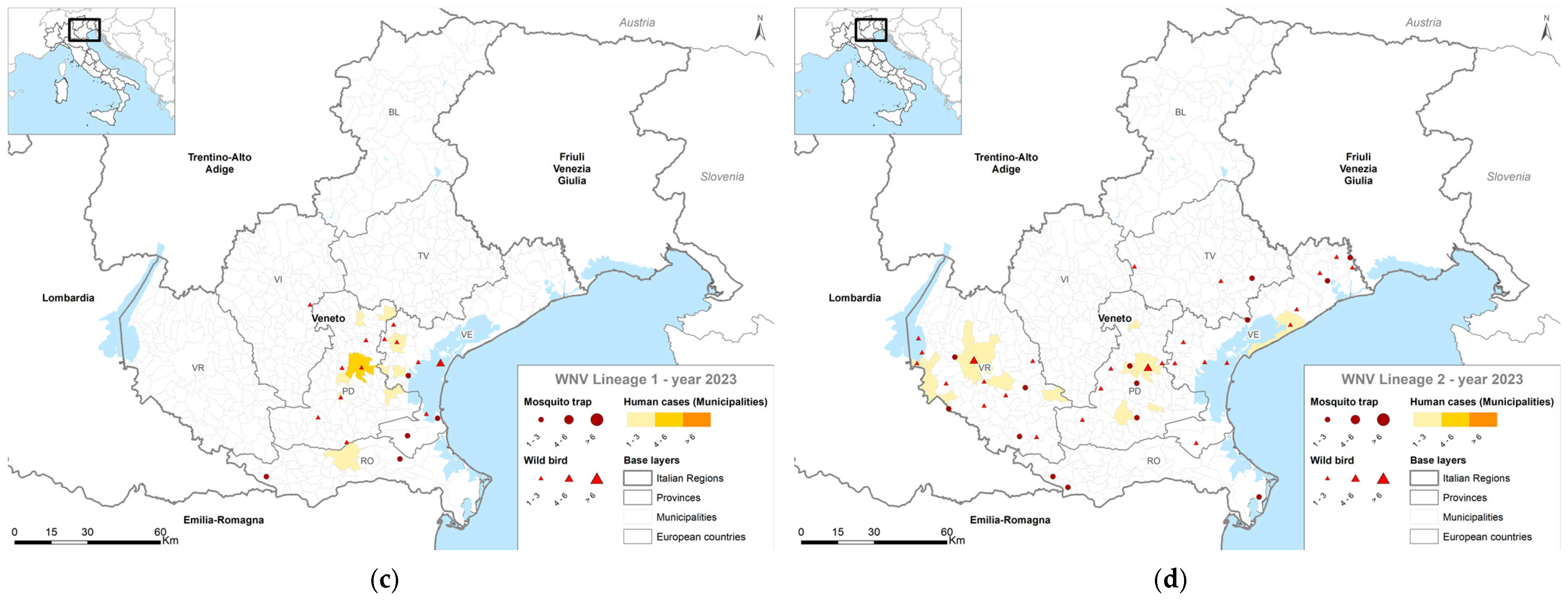
4. Results
4.1. Entomological Surveillance
4.2. Veterinary Surveillance
4.3. Human Surveillance
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calistri, P.; Giovannini, A.; Hubalek, Z.; Ionescu, A.; Monaco, F.; Savini, G.; Lelli, R. Epidemiology of west nile in Europe and in the Mediterranean basin. Open Virol. J. 2010, 4, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Haussig, J.M. West Nile virus keeps on moving up in Europe. Eurosurveillance 2020, 25, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Höper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef]
- Bergmann, F.; Fischer, D.; Fischer, L.; Maisch, H.; Risch, T.; Dreyer, S.; Sadeghi, B.; Geelhaar, D.; Grund, L.; Merz, S.; et al. Vaccination of Zoo Birds against West Nile Virus-A Field Study. Vaccines 2023, 11, 652. [Google Scholar] [CrossRef]
- Vlaskamp, D.R.M.; Thijsen, S.F.T.; Reimerink, J.; Hilkens, P.; Bouvy, W.H.; Bantjes, S.E.; Vlaminckx, B.J.M.; Zaaijer, H.; van den Kerkhof, H.H.T.C.; Raven, S.F.H.; et al. First autochthonous human West Nile virus infections in the Netherlands, July to August 2020. Eurosurveillance 2020, 25, 2001904. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses: ICTV. Family: Flaviviridae. Genus: Orthoflavivirus. Available online: https://ictv.global/report/chapter/flaviviridae/flaviviridae/orthoflavivirus (accessed on 11 February 2025).
- Martina, B.E.; Barzon, L.; Pijlman, G.P.; de la Fuente, J.; Rizzoli, A.; Wammes, L.J.; Takken, W.; van Rij, R.P.; Papa, A. Human to human transmission of arthropod-borne pathogens. Curr. Opin. Virol. 2017, 22, 13–21. [Google Scholar] [CrossRef]
- Bunning, M.L.; Bowen, R.A.; Cropp, C.B.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; et al. Experimental infection of horses with West Nile virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Mencattelli, G.; Ndione, M.H.D.; Silverj, A.; Diagne, M.M.; Curini, V.; Teodori, L.; Di Domenico, M.; Mbaye, R.; Leone, A.; Marcacci, M.; et al. Spatial and temporal dynamics of West Nile virus between Africa and Europe. Nat. Commun. 2023, 14, 6440. [Google Scholar] [CrossRef]
- Mancuso, E.; Cecere, J.G.; Iapaolo, F.; Di Gennaro, A.; Sacchi, M.; Savini, G.; Spina, F.; Monaco, F. West Nile and Usutu Virus Introduction via Migratory Birds: A Retrospective Analysis in Italy. Viruses 2022, 14, 416. [Google Scholar] [CrossRef]
- Brandolini, M.; De Pascali, A.M.; Zaghi, I.; Dirani, G.; Zannoli, S.; Ingletto, L.; Lavazza, A.; Lelli, D.; Dottori, M.; Calzolari, M.; et al. Advancing West Nile virus monitoring through whole genome sequencing: Insights from a One Health genomic surveillance study in Romagna (Italy). One Health 2024, 19, 100937. [Google Scholar] [CrossRef] [PubMed]
- Koch, R.T.; Erazo, D.; Folly, A.J.; Johnson, N.; Dellicour, S.; Grubaugh, N.D.; Vogels, C.B.F. Genomic epidemiology of West Nile virus in Europe. One Health 2024, 18, 100664. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, F.; Oude Munnink, B.B.; Munger, E.; Sikkema, R.S.; Pappa, S.; Tsioka, K.; Sinigaglia, A.; Dal Molin, E.; Shih, B.B.; et al. West Nile virus spread in Europe: Phylogeographic pattern analysis and key drivers. PLoS Pathog. 2024, 20, e1011880. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Montarsi, F.; Calzolari, M.; Capelli, G.; Dottori, M.; Ravagnan, S.; Lelli, D.; Chiari, M.; Santilli, A.; Quaglia, M.; et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet. Ital. 2017, 53, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The Role of Birds of Prey in West Nile Virus Epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef]
- Williams, R.A.J.; Criollo Valencia, H.A.; López Márquez, I.; González González, F.; Llorente, F.; Jiménez-Clavero, M.Á.; Busquets, N.; Mateo Barrientos, M.; Ortiz-Díez, G.; Santiago, T.A. West Nile Virus Seroprevalence in Wild Birds and Equines in Madrid Province, Spain. Vet. Sci. 2024, 11, 259. [Google Scholar] [CrossRef]
- Agliani, G.; Giglia, G.; Marshall, E.M.; Gröne, A.; Rockx, B.H.G.; van den Brand, J.M.A. Pathological features of West Nile and Usutu virus natural infections in wild and domestic animals and in humans: A comparative review. One Health 2023, 16, 100525. [Google Scholar] [CrossRef]
- Musto, C.; Tamba, M.; Calzolari, M.; Rossi, A.; Grisendi, A.; Marzani, K.; Bonilauri, P.; Delogu, M. Detection of West Nile and Usutu Virus RNA in Autumn Season in Wild Avian Hosts in Northern Italy. Viruses 2023, 15, 1771. [Google Scholar] [CrossRef]
- Sejvar, J.J. Clinical Manifestations and Outcomes of West Nile Virus Infection. Viruses 2014, 6, 606–623. [Google Scholar] [CrossRef]
- Gobbo, F.; Fornasiero, D.; De Marco, M.A.; Zecchin, B.; Mulatti, P.; Delogu, M.; Terregino, C. Active Surveillance for Highly Pathogenic Avian Influenza Viruses in Wintering Waterbirds in Northeast Italy, 2020–2021. Microorganisms 2021, 9, 2188. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; Mrzljak, A.; Ilic, M.; Bogdanic, M.; et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Garigliany, M.; Linden, A.; Gilliau, G.; Levy, E.; Sarlet, M.; Franssen, M.; Benzarti, E.; Derouaux, A.; Francis, F.; Desmecht, D. Usutu virus, Belgium, 2016. Infect. Genet. Evol. 2017, 48, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Giglia, G.; Agliani, G.; Munnink, B.B.O.; Sikkema, R.S.; Mandara, M.T.; Lepri, E.; Kik, M.; Ijzer, J.; Rijks, J.M.; Fast, C.; et al. Pathology and Pathogenesis of Eurasian Blackbirds (Turdus merula) Naturally Infected with Usutu Virus. Viruses 2021, 13, 1481. [Google Scholar] [CrossRef]
- Musto, C.; Tamba, M.; Calzolari, M.; Torri, D.; Marzani, K.; Cerri, J.; Bonilauri, P.; Delogu, M. Usutu virus in blackbirds (Turdus merula) with clinical signs, a case study from northern Italy. Eur. J. Wildl. Res. 2022, 68, 23. [Google Scholar] [CrossRef]
- Manarolla, G.; Bakonyi, T.; Gallazzi, D.; Crosta, L.; Weissenböck, H.; Dorrestein, G.M.; Nowotny, N. Usutu virus in wild birds in northern Italy. Vet. Microbiol. 2010, 141, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Cadar, D.; Simonin, Y. Human Usutu Virus Infections in Europe: A New Risk on Horizon? Viruses 2023, 15, 77. [Google Scholar] [CrossRef]
- Zecchin, B.; Fusaro, A.; Milani, A.; Schivo, A.; Ravagnan, S.; Ormelli, S.; Mavian, C.; Michelutti, A.; Toniolo, F.; Barzon, L.; et al. The central role of Italy in the spatial spread of USUTU virus in Europe. Virus Evol. 2021, 7, veab048. [Google Scholar] [CrossRef]
- Calzolari, M.; Chiapponi, C.; Bonilauri, P.; Lelli, D.; Baioni, L.; Barbieri, I.; Lavazza, A.; Pongolini, S.; Dottori, M.; Moreno, A. Co-circulation of two Usutu virus strains in Northern Italy between 2009 and 2014. Infect. Genet. Evol. 2017, 51, 255–262. [Google Scholar] [CrossRef]
- Angeloni, G.; Bertola, M.; Lazzaro, E.; Morini, M.; Masi, G.; Sinigaglia, A.; Trevisan, M.; Gossner, C.M.; Haussig, J.M.; Bakonyi, T.; et al. Epidemiology, surveillance and diagnosis of Usutu virus infection in the EU/EEA, 2012 to 2021. Eurosurveillance 2023, 28, 2200929. [Google Scholar] [CrossRef]
- Gaibani, P.; Barp, N.; Massari, M.; Negri, E.A.; Rossini, G.; Vocale, C.; Trenti, C.; Gallerani, A.; Cantergiani, S.; Romani, F.; et al. Case report of Usutu virus infection in an immunocompromised patient in Italy, 2022. J. Neurovirol. 2023, 29, 364–366. [Google Scholar] [CrossRef]
- Giglia, G.; Gianfilippo, A.; Mandara, M.T.; de Bruin, E.; Gröne, A.; van den Brand, J.M.A. Usutu virus avian and human infection after more than 25 years of circulation. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 805–807. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. European Food Safety Authority, Surveillance, Prevention and Control of West Nile Virus and Usutu Virus Infections in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-prevention-and-control-west-nile-virus-and-usutu-virus-infections (accessed on 10 January 2025).
- Riccardo, F.; Bolici, F.; Fafangel, M.; Jovanovic, V.; Socan, M.; Klepac, P.; Plavsa, D.; Vasic, M.; Bella, A.; Diana, G.; et al. West Nile virus in Europe: After action reviews of preparedness and response to the 2018 transmission season in Italy, Slovenia, Serbia and Greece. Glob. Health 2020, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Piano Nazionale di Prevenzione, Sorveglianza e Risposta Alle ARBOVIROSI (PNA) 2020–2025 [National Plan for the Prevention, Surveillance and Response to Arboviruses, 2020–2025]; Ministero Della Salute: Rome, Italy, 2019. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2947_allegato.pdf (accessed on 10 January 2025).
- Barzon, L.; Pacenti, M.; Montarsi, F.; Fornasiero, D.; Gobbo, F.; Quaranta, E.; Monne, I.; Fusaro, A.; Volpe, A.; Sinigaglia, A.; et al. Rapid spread of a new West Nile virus lineage 1 associated with increased risk of neuroinvasive disease during a large outbreak in northern Italy, 2022: One Health analysis. J. Travel Med. 2022, 31, taac125. [Google Scholar] [CrossRef] [PubMed]
- Kesavaraju, B.; Kiyoguchi, D.; Dickson, S. Efficacy of gravid traps in trapping Culex pipiens. J. Am. Mosq. Control Assoc. 2011, 27, 320–322. [Google Scholar] [CrossRef]
- Severini, F.; Toma, L.; Di Luca, M.; Romi, R. Le zanzare italiane: Generalità e identificazione degli adulti (Diptera, Culicidae) [Italian mosquitoes: General information and identification of adults (Diptera, Culicidae)]. Fragm. Entomol. 2009, 41, 213–372. [Google Scholar] [CrossRef]
- Ravagnan, S.; Montarsi, F.; Cazzin, S.; Porcellato, E.; Russo, F.; Palei, M.; Monne, I.; Savini, G.; Marangon, S.; Barzon, L.; et al. First report outside Eastern Europe of West Nile virus lineage 2 related to the Volgograd 2007 strain, northeastern Italy, 2014. Parasite Vectors 2015, 8, 418. [Google Scholar] [CrossRef][Green Version]
- Avibase—Il Database Degli Uccelli del Mondo [Avibase—The World Bird Database]. Available online: https://avibase.bsc-eoc.org/avibase.jsp?lang=IT& (accessed on 10 January 2025).
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Barzon, L.; Montarsi, F.; Quaranta, E.; Monne, I.; Pacenti, M.; Michelutti, A.; Toniolo, F.; Danesi, P.; Marchetti, G.; Gobbo, F.; et al. Early start of seasonal transmission and co-circulation of West Nile virus lineage 2 and a newly introduced lineage 1 strain, northern Italy, June 2022. Eurosurveillance 2022, 27, 2200548. [Google Scholar] [CrossRef]
- Silverj, A.; Mencattelli, G.; Monaco, F.; Iapaolo, F.; Teodori, L.; Leone, A.; Polci, A.; Curini, V.; Di Domenico, M.; Secondini, B.; et al. Origin and evolution of West Nile virus lineage 1 in Italy. Epidemiol. Infect. 2024, 152, e150. [Google Scholar] [CrossRef]
- Autorino, G.L.; Battisti, A.; Deubel, V.; Ferrari, G.; Forletta, R.; Giovannini, A.; Lelli, R.; Murri, S.; Scicluna, M.T. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg. Infect. Dis. 2002, 8, 1372–1378. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Squarzon, L.; Lavezzo, E.; Cattai, M.; Cusinato, R.; Palù, G. The complex epidemiological scenario of West Nile virus in Italy. Int. J. Environ. Res. Public Health 2013, 10, 4669–4689. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Silverj, A.; Iapaolo, F.; Ippoliti, C.; Teodori, L.; Di Gennaro, A.; Curini, V.; Candeloro, L.; Conte, A.; Polci, A.; et al. Epidemiological and Evolutionary Analysis of West Nile Virus Lineage 2 in Italy. Viruses 2022, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Iapaolo, F.; Monaco, F.; Fusco, G.; de Martinis, C.; Portanti, O.; Di Gennaro, A.; Curini, V.; Polci, A.; Ber-jaoui, S.; et al. West Nile Virus Lineage 1 in Italy: Newly Introduced or a Re-Occurrence of a Previously Circulating Strain? Viruses 2022, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.; Gould, D.; Bowen, R.; Komar, N. Natural and experimental West Nile virus infection in five raptor species. J. Wildl. Dis. 2006, 42, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Magallanes, S.; Llorente, F.; Ruiz-López, M.J.; Puente, J.M.; Ferraguti, M.; Gutiérrez-López, R.; Soriguer, R.; Aguilera-Sepúlveda, P.; Fernández-Delgado, R.; Jímenez-Clavero, M.Á.; et al. Warm winters are associated to more intense West Nile virus circulation in southern Spain. Emerg. Microbes Infect. 2024, 13, 2348510. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef]
- Wang, H.; Abbo, S.R.; Visser, T.M.; Westenberg, M.; Geertsema, C.; Fros, J.J.; Koenraadt, C.J.M.; Pijlman, G.P. Competition between Usutu virus and West Nile virus during simultaneous and sequential infection of Culex pipiens mosquitoes. Emerg. Microbes Infect. 2020, 9, 2642–2652. [Google Scholar] [CrossRef]
- Giesen, C.; Herrador, Z.; Fernandez-Martinez, B.; Figuerola, J.; Gangoso, L.; Vazquez, A.; Gómez-Barroso, D. A systematic review of environmental factors related to WNV circulation in European and Mediterranean countries. One Health 2023, 16, 100478. [Google Scholar] [CrossRef]
- Erazo, D.; Grant, L.; Ghisbain, G.; Marini, G.; Colón-González, F.J.; Wint, W.; Rizzoli, A.; Van Bortel, W.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Contribution of climate change to the spatial expansion of West Nile virus in Europe. Nat. Commun. 2024, 15, 1196. [Google Scholar] [CrossRef]
- Riccardo, F.; Bella, A.; Monaco, F.; Ferraro, F.; Petrone, D.; Mateo-Urdiales, A.; Andrianou, X.D.; Del Manso, M.; Venturi, G.; Fortuna, C.; et al. Rapid increase in neuroinvasive West Nile virus infections in humans, Italy, July 2022. Eurosurveillance 2022, 27, 2200653. [Google Scholar] [CrossRef]
- Scaramozzino, P.; Carvelli, A.; Bruni, G.; Cappiello, G.; Censi, F.; Magliano, A.; Manna, G.; Ricci, I.; Rombolà, P.; Romiti, F.; et al. West Nile and Usutu viruses co-circulation in central Italy: Outcomes of the 2018 integrated surveillance. Parasite Vectors 2021, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging Trends in the Epidemiology of West Nile and Usutu Virus Infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [PubMed]
- Defilippo, F.; Dottori, M.; Lelli, D.; Chiari, M.; Cereda, D.; Farioli, M.; Chianese, R.; Cerioli, M.P.; Faccin, F.; Canziani, S.; et al. Assessment of the Costs Related to West Nile Virus Monitoring in Lombardy Region (Italy) between 2014 and 2018. Int. J. Environ. Res. Public Health 2022, 19, 5541. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.; Calzolari, M.; Mattivi, A.; Tamba, M.; Angelini, P.; Bonilauri, P.; Albieri, A.; Cagarelli, R.; Carrieri, M.; Dottori, M.; et al. The experience of West Nile virus integrated surveillance system in the Emilia-Romagna region: Five years of implementation, Italy, 2009 to 2013. Eurosurveillance 2014, 19, 20953. [Google Scholar] [CrossRef]
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Eurosurveillance 2010, 15, 19547. [Google Scholar] [CrossRef]
- Simonin, Y. Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges. Viruses 2024, 16, 599. [Google Scholar] [CrossRef]


| Genus | Species | 2022 N (%) | 2023 N (%) |
|---|---|---|---|
| Aedes | Ae. albopictus ** | 4161 (4.46) | 4784 (3.58) |
| Ae. cantans | 19 (0.02) | 1 (0.00) | |
| Ae. caspius ** | 28,094 (30.14) | 11,478 (8.59) | |
| Ae. detritus | 2 (0.00) | 14 (0.01) | |
| Ae. geniculatus | 2 (0.00) | 5 (0.00) | |
| Ae. koreicus | 32 (0.03) | 11 (0.01) | |
| Ae. vexans | 482 (0.52) | 1307 (0.98) | |
| Anopheles | An. claviger/petragnani | 4 (0.00) | 5 (0.00) |
| An. maculipennis s.l. | 654 (0.70) | 244 (0.18) | |
| An. plumbeus | 8 (0.01) | 9 (0.01) | |
| Coquillettidia | Cq. richiardii | 39 (0.04) | 2 (0.00) |
| Culex | Cx. hortensis | 0 | 2 (0.00) |
| Cx. modestus * | 13 (0.01) | 40 (0.03) | |
| Cx. pipiens * | 54,937 (58.94) | 110,800 (82.90) | |
| Cx. spp. * | 2 (0.00) | 0 | |
| Culiseta | Cs. annulata | 6 (0.01) | 32 (0.02) |
| Cs. spp. | 1 (0.00) | 0 | |
| Cs. longiareolata | 0 | 1 (0.00) | |
| Not identified | 4757 (5.10) | 4913 (3.68) | |
| Total | 93,213 | 133,648 | |
| Viruses | Province | Total in the Veneto Region (Tot = 2123) Pos (%) | |||||
|---|---|---|---|---|---|---|---|
| Padua (Tot = 269) Pos (%) | Rovigo (Tot = 559) Pos (%) | Treviso (Tot = 207) Pos (%) | Venice (Tot = 572) Pos (%) | Verona (Tot = 336) Pos (%) | Vicenza (Tot = 180) Pos (%) | ||
| WNV-1 | 12 (4.46) | 13 (2.33) | 1 (0.48) | 8 (1.40) | 3 (0.89) | 1 (0.56) | 38 (1.79) |
| WNV-2 | 1 (0.37) | 13 (2.33) | 4 (1.93) | 18 (3.15) | 4 (1.19) | 4 (2.22) | 44 (2.07) |
| WNV-1 and WNV-2 | 1 (0.37) | 3 (0.54) | 0 | 3 (0.52) | 0 | 0 | 7 (0.33) |
| USUV | 1 (0.37) | 1 (0.18) | 0 | 1 (0.17) | 3 (0.89) | 2 (1.11) | 8 (0.38) |
| WNV-1 and USUV | 1 (0.37) | 0 | 1 (0.48) | 0 | 0 | 0 | 2 (0.09) |
| WNV-2 and USUV | 0 | 0 | 1 (0.48) | 1 (0.17) | 1 (0.30) | 0 | 3 (0.14) |
| WNV-1, WNV-2 and USUV | 1 (0.37) | 0 | 0 | 0 | 0 | 0 | 1 (0.05) |
| Total | 17 (6.32) | 30 (5.37) | 7 (3.38) | 31 (5.42) | 11 (3.27) | 7 (3.89) | 103 (4.85) |
| Viruses | Province | Total in the Veneto Region (Tot = 2301) Pos (%) | |||||
|---|---|---|---|---|---|---|---|
| Padua (Tot = 366) Pos (%) | Rovigo (Tot = 550) Pos (%) | Treviso (Tot = 258) Pos (%) | Venice (Tot = 557) Pos (%) | Verona (Tot = 375) Pos (%) | Vicenza (Tot = 195) Pos (%) | ||
| WNV-1 | 0 | 2 (0.36) | 0 | 2 (0.36) | 0 | 0 | 4 (0.17) |
| WNV-2 | 5 (1.37) | 2 (0.36) | 1 (0.39) | 5 (0.90) | 5 (1.33) | 0 | 18 (0.78) |
| WNV-1 and WNV-2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| USUV | 2 (0.55) | 1 (0.18) | 1 (0.39) | 6 (1.08) | 3 (0.80) | 3 (1.54) | 16 (0.70) |
| WNV-1 and USUV | 0 | 0 | 0 | 1 (0.18) | 0 | 0 | 1 (0.04) |
| WNV-2 and USUV | 0 | 1 (0.18) | 0 | 0 | 1 (0.27) | 0 | 2 (0.09) |
| WNV-1, WNV-2 and USUV | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 7 (1.91) | 6 (1.09) | 2 (0.78) | 14 (2.51) | 9 (2.40) | 3 (1.54) | 41 (1.78) |
| Order | 2022 N (%) | 2023 N (%) |
|---|---|---|
| Accipitriformes | 44 (2.01) | 33 (1.82) |
| Anseriformes | 54 (2.46) | 44 (2.43) |
| Apodiformes | 100 (4.56) | 64 (3.53) |
| Bucerotiformes | 1 (0.05) | 1 (0.06) |
| Caprimulgiformes | 1 (0.05) | 0 |
| Charadriiformes | 161 (7.34) | 181 (9.99) |
| Columbiformes | 401 (18.29) | 441 (24.34) |
| Coraciiformes | 3 (0.14) | 8 (0.44) |
| Falconiformes | 93 (4.24) | 50 (2.76) |
| Galliformes | 27 (1.23) | 11 (0.61) |
| Gaviiformes | 0 | 1 (0.06) |
| Gruiformes | 11 (0.50) | 12 (0.66) |
| Passeriformes | 942 (42.95) | 701 (38.69) |
| Pelecaniformes | 62 (2.83) | 47 (2.59) |
| Phoenicopteriformes | 2 (0.09) | 0 |
| Piciformes | 53 (2.42) | 21 (1.16) |
| Podicipediformes | 3 (0.14) | 0 |
| Psittaciformes | 0 | 7 (0.39) |
| Strigiformes | 182 (8.30) | 124 (6.84) |
| Not identified | 53 (2.42) | 66 (3.64) |
| Total | 2193 | 1812 |
| Order | Common Name | Scientific Name | MP | WNV-1 Pos/N (%) | WNV-2 Pos/N (%) | WNV-1 and WNV-2 Pos/N (%) | USUV Pos/N (%) |
|---|---|---|---|---|---|---|---|
| Accipitriformes | Eurasian Sparrowhawk | Accipiter nisus | M † | 1/27 (3.70) | 0/27 | 0/27 | 0/27 |
| Anseriformes | Mallard | Anas platyrhynchos | M | 1/17 (5.88) | 0/17 | 0/17 | 1/17 (5.88) |
| Apodiformes | Common Swift | Apus apus | M | 5/100 (5.00) | 0/100 | 0/100 | 0/100 |
| Charadriiformes | European Herring Gull | Larus argentatus | M † | 2/105 (1.90) | 0/105 | 0/105 | 0/105 |
| Seagull | Larus spp. | M † | 5/32 (15.63) | 3/32 (9.38) | 0/32 | 0/32 | |
| Columbiformes | Common Wood Pigeon | Columba palumbus | M † | 6/99 (6.06) | 3/99 (3.03) | 1/99 (1.01) | 2/99 (2.02) +1 * |
| European Turtle Dove | Streptopelia spp. | M | 3/180 (1.67) | 4/180 (2.22) | 0/180 | 0/180 | |
| Rock Dove | Columba livia | R | 1/122 (0.82) | 0/122 | 0/122 | 0/122 | |
| Falconiformes | Common Kestrel | Falco tinnunculus | M † | 4/88 (4.55) | 4/88 (4.55) | 1/88 (1.14) | 0/88 |
| Galliformes | Pheasant | Phasianus colchicus | R | 1/12 (8.33) | 0/12 | 0/12 | 0/12 |
| Passeriformes | Barn Swallow | Hirundo rustica | M | 1/33 (3.03) | 1/33 (3.03) | 0/33 | 0/33 |
| Carrion Crow | Corvus corone | R | 14/133 (10.53) | 6/133 (4.51) | 1/133 (0.75) | 0/133 | |
| Common Blackbird | Turdus merula | M † | 12/250 (4.80) | 3/250 (1.20) | 0/250 | 9/250 (3.60) | |
| Domestic Canary | Serinus canaria domestica | R | 1/7 (14.29) | 1/7 (14.29) | 0/7 | 0/7 | |
| Eurasian Jay | Garrulus glandarius | M † | 4/34 (11.76) | 1/34 (2.94) | 1/34 (2.94) | 0/34 | |
| Eurasian Magpie | Pica pica | R | 8/286 (2.80) | 8/286 (2.80) | 0/286 | 1/286 (0.35) | |
| European Goldfinch | Carduelis carduelis | M † | 1/10 (10.00) | 0/10 | 0/10 | 0/10 | |
| Rook | Corvus spp. | M † | 1/2 (50.00) | 0/2 | 0/2 | 0/2 | |
| Sparrow | Passer spp. | R | 4/35 (11.43) | 2/35 (5.71) | 0/35 | 1/35 (2.86) | |
| Pelecaniformes | Great Cormorant | Phalacrocorax carbo | R | 1/5 (20.00) | 1/5 (20.00) | 0/5 | 0/5 |
| Grey Heron | Ardea cinerea | M † | 1/16 (6.25) | 0/16 | 0/16 | 0/16 | |
| Little Egret | Egretta garzetta | M | 1/12 (8.33) | 0/12 | 0/12 | 0/12 | |
| Little Bittern | Ixobrychus minutus | M | 2/5 (40.00) | 0/5 | 0/5 | 0/5 | |
| Purple Heron | Ardea purpurea | M | 0/3 | 1/3 (33.33) | 0/3 | 0/3 | |
| Western Cattle Egret | Bubulcus ibis | M † | 2/18 (11.11) | 1/18 (5.56) | 0/18 | 1/18 (5.56) | |
| Piciformes | European Green Woodpecker | Picus viridis | R | 2/13 (15.38) | 0/13 | 0/13 | 0/13 |
| Woodpecker | n.a. | R | 1/34 (2.94) | 0/34 | 0/34 | 0/34 | |
| Strigiformes | Eurasian Scops Owl | Otus scops | R | 2/28 (7.14) | 5/28 (17.86) | 0/28 | 0/28 |
| Little Owl | Athene noctua | R | 21/120 (17.50) | 2/120 (1.67) | 3/120 (2.50) | 0/120 | |
| Long-Eared Owl | Asio otus | M † | 1/19 (5.26) | 1/19 (5.26) | 0/19 | 0/19 | |
| Tawny Owl | Strix aluco | R | 0/4 | 0/4 | 0/4 | 1/4 (25.00) | |
| Western Barn Owl | Tyto alba | R | 2/11 (18.18) | 0/11 | 0/11 | 0/11 | |
| Infection Rate (Tot = 2193) | 111 (5.06) | 47 (2.14) | 7 (0.32) | 16 (0.73) +1 * | |||
| Order | Common Name | Scientific Name | MP | WNV-1 Pos/N (%) | WNV-2 Pos/N (%) | USUV Pos/N (%) |
|---|---|---|---|---|---|---|
| Anseriformes | Gadwall | Mareca strepera | M | 0/2 | 0/2 | 0/2 +1 * |
| Mallard | Anas platyrhynchos | M | 0/22 | 1/22 (4.55) | 1/22 (4.55) | |
| Charadriiformes | European Herring Gull | Larus argentatus | M † | 1/106 (0.94) | 0/106 | 0/106 |
| Seagull | Larus spp. | M † | 0/20 | 1/20 (5.00) | 0/20 | |
| Columbiformes | Common Wood Pigeon | Columba palumbus | M † | 4/144 (2.78) | 6/144 (4.17) | 5/144 (3.47) +1 * +8 ** |
| European Turtle Dove | Streptopelia spp. | M | 3/163 (1.84) | 3/163 (1.84) | 3/163 (1.84) +1 ** | |
| Rock Dove | Columba livia | R | 0/134 | 2/134 (1.49) | 1/134 (0.75) | |
| Falconiformes | Common Kestrel | Falco tinnunculus | M † | 0/44 | 1/44 (2.27) | 0/44 |
| Passeriformes | Barn Swallow | Hirundo rustica | M | 0/19 | 1/19 (5.26) | 0/19 |
| Carrion Crow | Corvus corone | R | 2/131 (1.53) | 2/131 (1.53) | 0/131 | |
| Common Blackbird | Turdus merula | M † | 0/138 | 0/138 | 10/138 (7.25) +1 * | |
| Common House Martin | Delichon urbicum | M | 0/15 | 2/15 (13.33) | 0/15 | |
| Eurasian Jay | Garrulus glandarius | M † | 3/36 (8.33) | 2/36 (5.56) | 0/36 | |
| Eurasian Magpie | Pica pica | R | 1/247 (0.40) | 4/247 (1.62) | 0/247 | |
| European Greenfinch | Chloris chloris | M † | 0/3 | 1/3 (33.33) | 0/3 | |
| Hooded Crow | Corvus cornix | R | 1/2 (50.00) | 0/2 | 0/2 | |
| House Sparrow | Passer domesticus | R | 0/2 | 1/2(50.00) | 0/2 | |
| Sparrow | Passer spp. | R | 0/9 | 0/9 | 1/9 (11.11) | |
| Pelecaniformes | Black-Crowned Night-Heron | Nycticorax nycticorax | M | 0/2 | 0/2 | 1/2 (50.00) |
| Grey Heron | Ardea cinerea | M † | 0/15 | 0/15 | 0/15 +1 * | |
| Little Egret | Egretta garzetta | M | 0/6 | 1/6 (16.67) | 0/6 | |
| Purple Heron | Ardea purpurea | M | 0/1 | 0/1 | 1/1 (100) | |
| Western Cattle Egret | Bubulcus ibis | M † | 0/10 | 1/10 (10.00) | 0/10 | |
| Psittaciformes | Parrot | n.a. | R | 1/7 (14.29) | 0/7 | 0/7 |
| Strigiformes | Eurasian Scops Owl | Otus scops | R | 0/22 | 1/22 (4.55) | 1/22 (4.55) |
| Little Owl | Athene noctua | R | 0/85 | 2/85 (2.35) | 0/85 | |
| Infection Rate (Tot = 1812) | 16 (0.89) | 32 (1.79) | 24 (1.34) +4 * +9 ** | |||
| Year | Province | WNV * | WNV-1 | WNV-2 | Total (%) |
|---|---|---|---|---|---|
| 2022 | Belluno ** | 0 | 2 | 0 | 2 (0.38) |
| Padua | 114 | 126 | 38 | 278 (52.35) | |
| Rovigo | 11 | 17 | 8 | 36 (6.78) | |
| Treviso | 23 | 7 | 9 | 39 (7.34) | |
| Venice | 44 | 26 | 21 | 91 (17.14) | |
| Verona | 19 | 5 | 14 | 38 (7.16) | |
| Vicenza | 21 | 20 | 6 | 47 (8.85) | |
| Total (%) | 232 (43.69) | 203 (39.23) | 96 (18.08) | 531 | |
| 2023 | Padua | 9 | 9 | 6 | 24 (42.86) |
| Rovigo | 1 | 1 | 0 | 2 (3.57) | |
| Treviso | 1 | 0 | 0 | 1 (1.79) | |
| Venice | 2 | 4 | 2 | 8 (14.29) | |
| Verona | 6 | 0 | 11 | 17 (30.36) | |
| Vicenza | 4 | 0 | 0 | 4 (7.14) | |
| Total (%) | 23 (41.07) | 14 (25.00) | 19 (33.93) | 56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbo, F.; Chiarello, G.; Sgubin, S.; Toniolo, F.; Gradoni, F.; Danca, L.I.; Carlin, S.; Capello, K.; De Conti, G.; Bortolami, A.; et al. Integrated One Health Surveillance of West Nile Virus and Usutu Virus in the Veneto Region, Northeastern Italy, from 2022 to 2023. Pathogens 2025, 14, 227. https://doi.org/10.3390/pathogens14030227
Gobbo F, Chiarello G, Sgubin S, Toniolo F, Gradoni F, Danca LI, Carlin S, Capello K, De Conti G, Bortolami A, et al. Integrated One Health Surveillance of West Nile Virus and Usutu Virus in the Veneto Region, Northeastern Italy, from 2022 to 2023. Pathogens. 2025; 14(3):227. https://doi.org/10.3390/pathogens14030227
Chicago/Turabian StyleGobbo, Federica, Giulia Chiarello, Sofia Sgubin, Federica Toniolo, Francesco Gradoni, Lidia Iustina Danca, Sara Carlin, Katia Capello, Giacomo De Conti, Alessio Bortolami, and et al. 2025. "Integrated One Health Surveillance of West Nile Virus and Usutu Virus in the Veneto Region, Northeastern Italy, from 2022 to 2023" Pathogens 14, no. 3: 227. https://doi.org/10.3390/pathogens14030227
APA StyleGobbo, F., Chiarello, G., Sgubin, S., Toniolo, F., Gradoni, F., Danca, L. I., Carlin, S., Capello, K., De Conti, G., Bortolami, A., Varotto, M., Favero, L., Brichese, M., Russo, F., Mutinelli, F., Vogiatzis, S., Pacenti, M., Barzon, L., & Montarsi, F. (2025). Integrated One Health Surveillance of West Nile Virus and Usutu Virus in the Veneto Region, Northeastern Italy, from 2022 to 2023. Pathogens, 14(3), 227. https://doi.org/10.3390/pathogens14030227









