Inflammatory Responses to Non-Typeable Haemophilus influenzae Clinical Isolates from Invasive and Non-Invasive Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. H. influenzae Strains and Culture Conditions
2.3. Stimulation with H. influenzae
2.4. ELISA
2.5. Flow Cytometry Analysis
2.6. Statistical Analysis
3. Results
3.1. Stimulation of Differentiated THP-1 Cells with Invasive or Non-Invasive Clinical NTHi Isolates Resulted in an Increase in Cell Surface Expression of ICAM-1 and in TNF-α and IL-1β Release
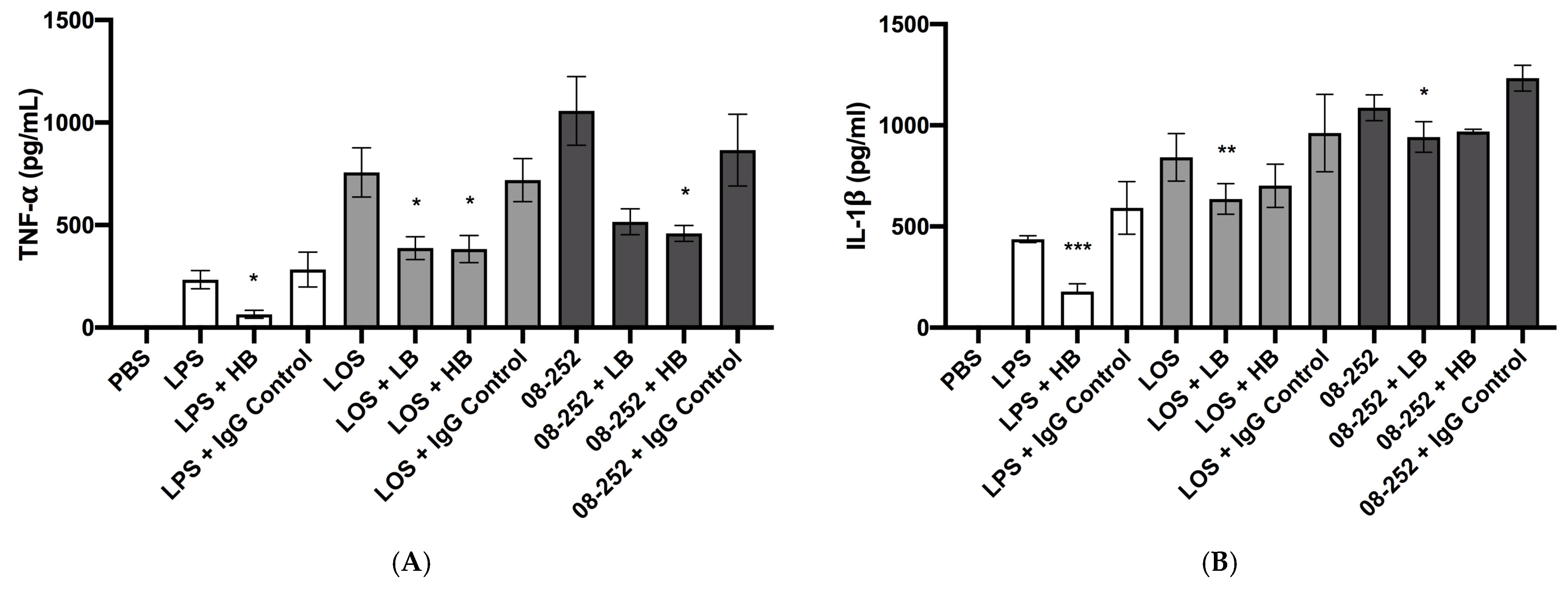
3.2. Immunostimulatory Effect of Hia Partially Depended on LOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, T.F. Haemophilus species (including H. influenzae and Chancroid). In Mandell Gerald L, Bennett J E, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed.; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2010; pp. 2911–2919. [Google Scholar]
- Van Eldere, J.; Slack, M.P.; Ladhani, S.; Cripps, A.W. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. Dis. 2014, 14, 1281–1292. [Google Scholar] [CrossRef]
- Langereis, J.D.; de Jonge, M.I. Invasive Disease Caused by Nontypeable Haemophilus influenzae. Emerg. Infect. Dis. 2015, 21, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; Economopoulou, A.; Dias, J.G.; Bancroft, E.; Ramliden, M.; Celentano, L.P.; European Centre for Disease Prevention and Control Country Experts for Invasive Haemophilus influenzae Disease. Epidemiology of Invasive Haemophilus influenzae Disease, Europe, 2007–2014. Emerg. Infect. Dis. 2017, 23, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Rubis, A.B.; Soeters, H.M.; Reingold, A.; Barnes, M.; Petit, S.; Farley, M.M.; Harrison, L.H.; Como-Sabetti, K.; Khanlian, S.A.; et al. Epidemiology of Invasive Nontypeable Haemophilus influenzae Disease-United States, 2008–2019. Clin. Infect. Dis. 2023, 76, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.; Byce, S.; Tsang, R.S.W.; Jamieson, F.B.; Kus, J.V.; Ulanova, M. Continuing surveillance of invasive Haemophilus influenzae disease in northwestern Ontario emphasizes the importance of serotype A and non-typeable strains as causes of serious disease. Can. J. Microbiol. 2019, 65, 805–813. [Google Scholar] [CrossRef]
- Ulanova, M.; Tsang, R.S.W.; Nix, E.B.; Kelly, L.; Shuel, M.; Lance, B.; Canadian Immunization Research Network Investigators. Epidemiology of invasive Haemophilus influenzae disease in northwestern Ontario: Comparison of invasive and noninvasive H. influenzae clinical isolates. Can. J. Microbiol. 2023, 69, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ulanova, M.; Tsang, R.S.W.; Nix, E.B.; Tan, B.; Huska, B.; Kelly, L.; Shuel, M.; Allarie, J. Carriage of Haemophilus influenzae serotype A in children: Canadian Immunization Research Network (CIRN) study. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2024, 9, 20–31. [Google Scholar] [PubMed]
- Schweda, E.K.; Richards, J.C.; Hood, D.W.; Moxon, E.R. Expression and structural diversity of the lipopolysaccharide of Haemophilus influenzae: Implication in virulence. Int. J. Med. Microbiol. 2007, 297, 297–306. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Inohara, N.; Nunez, G. Peptidoglycan signaling in innate immunity and inflammatory disease. J. Biol. Chem. 2005, 280, 20177–20180. [Google Scholar] [CrossRef]
- Lorenz, E.; Chemotti, D.C.; Jiang, A.L.; McDougal, L.D. Differential Involvement of Toll-like Receptors 2 and 4 in the Host Response to Acute Respiratory Infections with Wild-type and Mutant Haemophilus Influenzae Strains. Infect. Immun. 2005, 73, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Jalalvand, F.; Thegerström, J.; Riesbeck, K. The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae. Front. Immunol. 2018, 9, 2530. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Jabeen, M.; Bharj, G.; Hinks, T.S.C. Non-typeable Haemophilus influenzae airways infection: The next treatable trait in asthma? Eur. Respir. Rev. 2022, 31, 220008. [Google Scholar] [CrossRef] [PubMed]
- Huska, B.; Ferris, C.; Shahid, Z.; Ulanova, M. The Study of Pro-Inflammatory Molecules Induced by Genetically and Phenotypically Diverse Strains of Haemophilus influenzae Type a in an in vitro Infection Model. Immune Syst. 2024, 1, 14–27. [Google Scholar] [CrossRef]
- Mell, J.C.; Sinha, S.; Balashov, S.; Viadas, C.; Grassa, C.J.; Ehrlich, G.D.; Nislow, C.; Redfield, R.J.; Garmendia, J. Complete Genome Sequence of Haemophilus Influenzae Strain 375 from the Middle Ear of a Pediatric Patient with Otitis Media. Genome Announc. 2014, 2, e01245-14. [Google Scholar] [CrossRef] [PubMed]
- Meats, E.; Feil, E.J.; Stringer, S.; Cody, A.J.; Goldstein, R.; Kroll, J.S.; Popovic, T.; Spratt, B.G. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J. Clin. Microbiol. 2003, 41, 1623–1636. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Nix, E.B.; Gaultier, G.N.; Cox, A.D.; McCready, W.; Ulanova, M. Naturally occurring bactericidal antibodies specific for Haemophilus influenzae lipooligosaccharide are present in healthy adult individuals. Vaccine 2015, 33, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Gaultier, G.N.; Colledanchise, K.N.; Alhazmi, A.; Ulanova, M. The Immunostimulatory Capacity of Nontypeable Haemophilus influenzae Lipooligosaccharide. Pathog. Immun. 2017, 2, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Avadhanula, V.; Rodriguez, C.A.; Ulett, G.C.; Bakaletz, L.O.; Adderson, E.E. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 2006, 74, 830–838. [Google Scholar] [CrossRef]
- Phillips, Z.N.; Brizuela, C.; Jennison, A.V.; Staples, M.; Grimwood, K.; Seib, K.L.; Jennings, M.P.; Atack, J.M. Analysis of Invasive Nontypeable Haemophilus influenzae Isolates Reveals Selection for the Expression State of Particular Phase-Variable Lipooligosaccharide Biosynthetic Genes. Infect. Immun. 2019, 87, e00093-19. [Google Scholar] [CrossRef]
- Collins, S.; Litt, D.J.; Flynn, S.; Ramsay, M.E.; Slack, M.P.; Ladhani, S.N. Neonatal invasive Haemophilus influenzae disease in England and Wales: Epidemiology, clinical characteristics, and outcome. Clin. Infect. Dis. 2015, 60, 1786–1792. [Google Scholar] [CrossRef]
- Jalalvand, F.; Riesbeck, K. Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert. Rev. Vaccines 2018, 17, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Langereis, J.D.; de Jonge, M.I. Unraveling Haemophilus Influenzae Virulence Mechanisms Enable Discovery of New Targets for Antimicrobials and Vaccines. Curr. Opin. Infect. Dis. 2020, 33, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Erwin, A.L.; Nelson, K.L.; Mhlanga-Mutangadura, T.; Bonthuis, P.J.; Geelhood, J.L.; Morlin, G.; Unrath, W.C.; Campos, J.; Crook, D.W.; Farley, M.M.; et al. Characterization of genetic and phenotypic diversity of invasive nontypeable Haemophilus influenzae. Infect. Immun. 2005, 73, 5853–5863. [Google Scholar] [CrossRef]
- Langereis, J.D.; Cremers, A.J.H.; Vissers, M.; van Beek, J.; Meis, J.F.; de Jonge, M.I. Nontypeable Haemophilus influenzae Invasive Blood Isolates Are Mainly Phosphorylcholine Negative and Show Decreased Complement-Mediated Killing That Is Associated with Lower Binding of IgM and CRP in Comparison to Colonizing Isolates from the Oropharynx. Infect. Immun. 2019, 87, e00604-18. [Google Scholar] [CrossRef] [PubMed]
- Dudukina, E.; de Smit, L.; Verhagen, G.J.A.; van de Ende, A.; Marimón, J.M.; Bajanca-Lavado, P.; Ardanuy, C.; Marti, S.; de Jonge, M.I.; Langereis, J.D. Antibody Binding and Complement-Mediated Killing of Invasive Haemophilus influenzae Isolates from Spain, Portugal, and the Netherlands. Infect. Immun. 2020, 88, e00454-20. [Google Scholar] [CrossRef] [PubMed]
- Wills, B.M.; Garai, P.; Dickinson, Q.; Meyer, J.G.; Brockman, K.L. Phase variable acetylation of lipooligosaccharide modifies antibody production and opsonophagocytic killing of non-typeable Haemophilus influenzae. iScience 2023, 26, 107785. [Google Scholar] [CrossRef]
- Ahearn, C.P.; Kirkham, C.; Chaves, L.D.; Kong, Y.; Pettigrew, M.M.; Murphy, T.F. Discovery and Contribution of Nontypeable Haemophilus influenzae NTHI1441 to Human Respiratory Epithelial Cell Invasion. Infect. Immun. 2019, 87, e00462-19. [Google Scholar] [CrossRef]
- Brown, M.A.; Morgan, S.B.; Donachie, G.E.; Horton, K.L.; Pavord, I.D.; Arancibia-Cárcamo, C.V.; Hinks, T.S.C. Epithelial immune activation and intracellular invasion by non-typeable Haemophilus influenzae. Front. Cell Infect. Microbiol. 2023, 13, 1141798. [Google Scholar] [CrossRef] [PubMed]
- Clementi, C.F.; Murphy, T.F. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Front. Cell Infect. Microbiol. 2011, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Ackland, J.; Heinson, A.I.; Cleary, D.W.; Christodoulides, M.; Wilkinson, T.M.A.; Staples, K.J. Dual RNASeq Reveals NTHi-Macrophage Transcriptomic Changes During Intracellular Persistence. Front. Cell Infect. Microbiol. 2021, 11, 723481. [Google Scholar] [CrossRef] [PubMed]
- Saliu, F.; Rizzo, G.; Bragonzi, A.; Cariani, L.; Cirillo, D.M.; Colombo, C.; Daccò, V.; Girelli, D.; Rizzetto, S.; Sipione, B.; et al. Chronic infection by nontypeable Haemophilus influenzae fuels airway inflammation. ERJ Open Res. 2021, 7, 00614–2020. [Google Scholar] [CrossRef]
- Phillips, Z.N.; Garai, P.; Tram, G.; Martin, G.; Van Den Bergh, A.; Husna, A.U.; Staples, M.; Grimwood, K.; Jennison, A.V.; Guillon, P.; et al. Characterization of the Phase-Variable Autotransporter Lav Reveals a Role in Host Cell Adherence and Biofilm Formation in Nontypeable Haemophilus influenzae. Infect. Immun. 2022, 90, e0056521. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A Master Regulator of Cellular Responses in Inflammation, Injury Resolution, and Tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.A.; Bakaletz, L.O. Intercellular Adhesion Molecule 1 Serves as a Primary Cognate Receptor for the Type IV Pilus of Nontypeable Haemophilus Influenzae. Cell Microbiol. 2016, 18, 1043–1055. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional Control of the Inflammatory Response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y.J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007, 76, 447–480. [Google Scholar] [CrossRef]
- Lévêque, M.; Simonin-Le Jeune, K.; Jouneau, S.; Moulis, S.; Desrues, B.; Belleguic, C.; Brinchault, G.; Le Trionnaire, S.; Gangneux, J.P.; Dimanche-Boitrel, M.T.; et al. Soluble CD14 Acts as a DAMP in Human Macrophages: Origin and Involvement in Inflammatory Cytokine/Chemokine Production. FASEB J. 2017, 31, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Dentener, M.A.; Bazil, V.; Von Asmuth, E.J.; Ceska, M.; Buurman, W.A. Involvement of CD14 in Lipopolysaccharide-Induced Tumor Necrosis Factor-Alpha, IL-6 and IL-8 Release by Human Monocytes and Alveolar Macrophages. J. Immunol. 1993, 150, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and Reacting to Microbes Through the Inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Vanaja, S.K.; Waggoner, L.; Sokolovska, A.; Becker, C.; Stuart, L.M.; Leong, J.M.; Fitzgerald, K.A. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell 2012, 150, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Rotta Detto Loria, J.; Rohmann, K.; Droemann, D.; Kujath, P.; Rupp, J.; Goldmann, T.; Dalhoff, K. Nontypeable Haemophilus Influenzae Infection Upregulates the NLRP3 Inflammasome and Leads to Caspase-1-Dependent Secretion of Interleukin-1β-A Possible Pathway of Exacerbations in COPD. PLoS ONE 2013, 8, e66818. [Google Scholar] [CrossRef] [PubMed]
- Ratner, A.J.; Aguilar, J.L.; Shchepetov, M.; Lysenko, E.S.; Weiser, J.N. Nod1 Mediates Cytoplasmic Sensing of Combinations of Extracellular Bacteria. Cell Microbiol. 2007, 9, 1343–1351. [Google Scholar] [CrossRef]
- Galdiero, M.; Galdiero, M.; Finamore, E.; Rossano, F.; Gambuzza, M.; Catania, M.R.; Teti, G.; Midiri, A.; Mancuso, G. Haemophilus Influenzae Porin Induces Toll-like Receptor 2-Mediated Cytokine Production in Human Monocytes and Mouse Macrophages. Infect. Immun. 2004, 72, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Paludan, S.R.; Kilian, M.; Ostergaard, L. Live Streptococcus Pneumoniae, Haemophilus Influenzae, and Neisseria Meningitidis Activate the Inflammatory Response Through Toll-like Receptors 2, 4, and 9 in Species-Specific Patterns. J. Leukoc. Biol. 2006, 80, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.E.; Davis, M.J.; Boekschoten, M.V.; Amsen, D.; Dascher, C.C.; Ryffel, B.; Swanson, J.A.; Müller, M.; Blander, J.M. Detection of Prokaryotic mRNA Signifies Microbial Viability and Promotes Immunity. Nature 2011, 474, 385–389. [Google Scholar] [CrossRef]
- Sweet, M.J.; Ramnath, D.; Singhal, A.; Kapetanovic, R. Inducible antibacterial responses in macrophages. Nat. Rev. Immunol. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Polosukhin, V.V.; Cates, J.M.; Lawson, W.E.; Zaynagetdinov, R.; Milstone, A.P.; Massion, P.P.; Ocak, S.; Ware, L.B.; Lee, J.W.; Bowler, R.P.; et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 184, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Staples, K.J.; Taylor, S.; Thomas, S.; Leung, S.; Cox, K.; Pascal, T.G.; Ostridge, K.; Welch, L.; Tuck, A.C.; Clarke, S.C.; et al. Relationships between Mucosal Antibodies, Non-Typeable Haemophilus influenzae (NTHi) Infection and Airway Inflammation in COPD. PLoS ONE 2016, 11, e0167250. [Google Scholar] [CrossRef] [PubMed]
- Baffetta, F.; Buonsanti, C.; Moraschini, L.; Aprea, S.; Canè, M.; Lombardi, S.; Contorni, M.; Rondini, S.; Arora, A.K.; Bardelli, M.; et al. Lung mucosal immunity to NTHi vaccine antigens: Antibodies in sputum of chronic obstructive pulmonary disease patients. Hum. Vaccin. Immunother. 2024, 20, 2343544. [Google Scholar] [CrossRef] [PubMed]
- Hawdon, N.; Biman, B.; McCready, W.; Brigden, M.; Malik, S.; Vergidis, D.; Kisselgoff, O.; Ulanova, M. Antibody against Haemophilus influenzae protein D in patients with chronic conditions causing secondary immunodeficiency. Vaccine 2012, 30, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Resman, F.; Ristovski, M.; Ahl, J.; Forsgren, A.; Gilsdorf, J.R.; Jasir, A.; Kaijser, B.; Kronvall, G.; Riesbeck, K. Invasive disease caused by Haemophilus influenzae in Sweden 1997–2009; evidence of increasing incidence and clinical burden of non-type b strains. Clin. Microbiol. Infect. 2011, 17, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.Z.; Hu, W.L.; Shang, S.Q.; Li, J.P.; Hong, L.Q.; Yan, J. Serum Concentrations of Antibodies against Outer Membrane Protein P6, Protein D, and T- and B-Cell Combined Antigenic Epitopes of Nontypeable Haemophilus influenzae in Children and Adults of Different Ages. Clin. Vaccine Immunol. 2015, 23, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Martinovich, K.M.; Rahman, T.; de Gier, C.; Seppanen, E.J.; Orami, T.; Granland, C.M.; Francis, J.; Yoannes, M.; Corscadden, K.J.; Ford, R.; et al. Differences in Pneumococcal and Haemophilus influenzae Natural Antibody Development in Papua New Guinean Children in the First Year of Life. Front. Immunol. 2021, 12, 725244. [Google Scholar] [CrossRef] [PubMed]

| Label | Site of Isolation | Invasiveness | Sequence Type (ST) 1 | Notes |
|---|---|---|---|---|
| 08-252 | Blood | yes | 389 | Source: Dr. Tsang’s collection |
| 08-254 | Blood | yes | 599 | Source: Dr. Tsang’s collection |
| 375 | Middle ear effusion | no | 3 | Described in [15] |
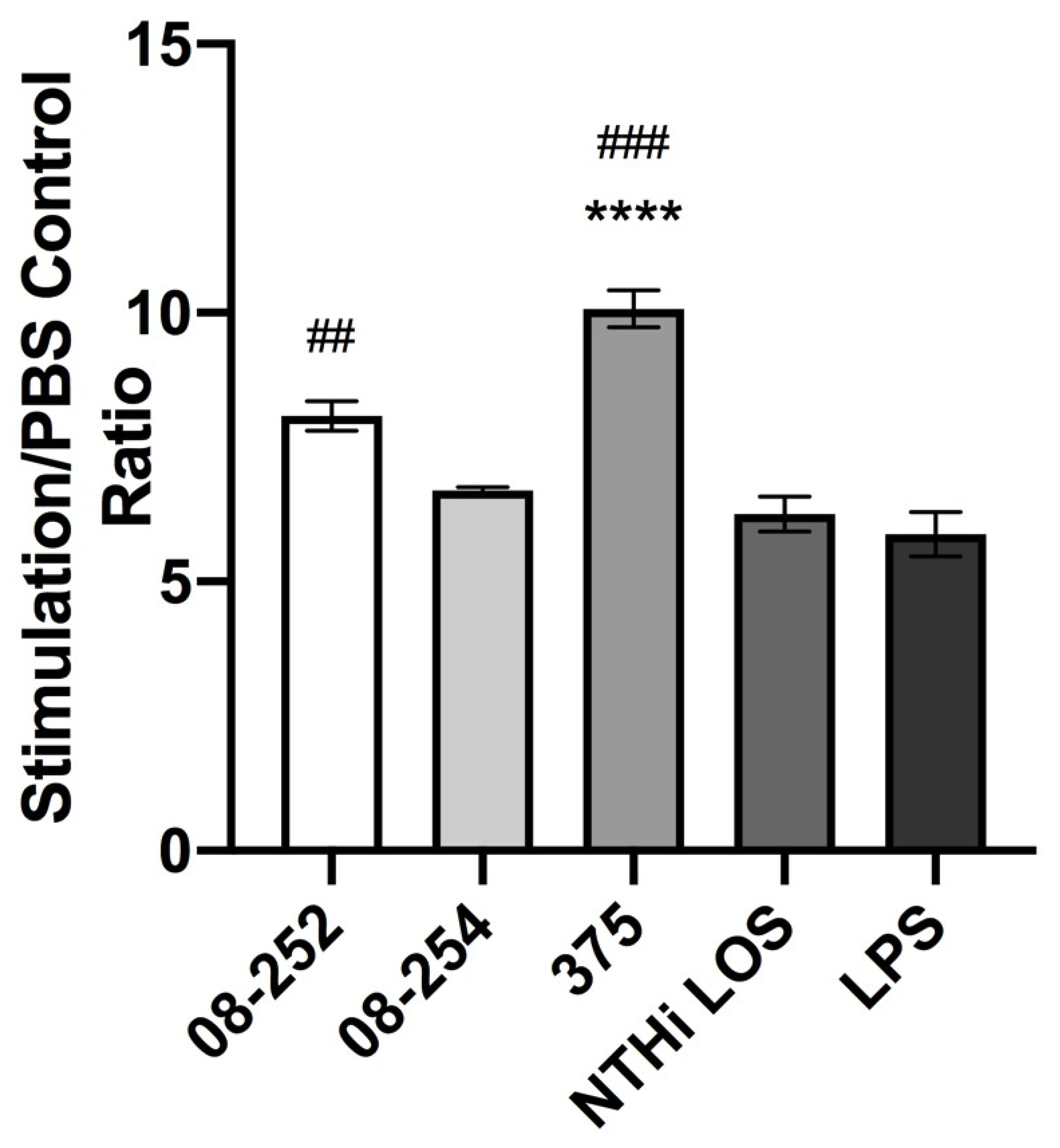
| Stimulation | MFI | Stimulation/PBS Control Ratio |
|---|---|---|
| H. influenzae NTHi 08-252 | 96,730.5 ± 6538 | 8.08 ± 0.55 |
| H. influenzae NTHi 08-254 | 59,459.4 ± 10,027 | 6.69 ± 0.10 |
| H. influenzae NTHi 375 | 59,768.5 ± 9427 | 10.07 ± 0.60 ** |
| NTHi LOS | 77,743.5 ± 6827 | 6.26 ± 0.56 |
| E. coli LPS | 73,447.7 ± 6538 | 5.88 ± 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huska, B.; Ulanova, M. Inflammatory Responses to Non-Typeable Haemophilus influenzae Clinical Isolates from Invasive and Non-Invasive Infections. Pathogens 2025, 14, 210. https://doi.org/10.3390/pathogens14030210
Huska B, Ulanova M. Inflammatory Responses to Non-Typeable Haemophilus influenzae Clinical Isolates from Invasive and Non-Invasive Infections. Pathogens. 2025; 14(3):210. https://doi.org/10.3390/pathogens14030210
Chicago/Turabian StyleHuska, Brenda, and Marina Ulanova. 2025. "Inflammatory Responses to Non-Typeable Haemophilus influenzae Clinical Isolates from Invasive and Non-Invasive Infections" Pathogens 14, no. 3: 210. https://doi.org/10.3390/pathogens14030210
APA StyleHuska, B., & Ulanova, M. (2025). Inflammatory Responses to Non-Typeable Haemophilus influenzae Clinical Isolates from Invasive and Non-Invasive Infections. Pathogens, 14(3), 210. https://doi.org/10.3390/pathogens14030210







