Abstract
Since the beginning of December 2022, an unusually high number of cases and deaths of Group A Streptococcus (GAS) infections has been reported in many European countries. GAS infection frequently causes mild diseases such as pharyngitis, tonsillitis, impetigo, cellulitis, and scarlet fever. However, in rare instances, GAS infection can lead to invasive, life-threatening conditions like necrotizing fasciitis and toxic shock syndrome, which are associated with high mortality. The aim of the study was to present the clinical course of invasive Streptococcus pyogenes infections and to highlight the increase in the incidence of severe infections of this etiology, similar to trends observed in other European countries. The study included 11 patients with severe, invasive infections caused by S. pyogenes accompanied by sepsis or septic shock, treated at the 4th Clinical Military Hospital in Wroclaw between December 2022 and May 2023. Among 11 patients, 6 had streptococcal skin and soft tissue infections, 3 had pneumonia caused by S. pyogenes, 1 had streptococcal otitis, and 1 had a knee joint infection. Nine developed septic shock, and three died from fulminant streptococcal toxic shock syndrome (STSS). Physicians should be aware of the increased prevalence of invasive GAS (iGAS) infections; timely diagnosis and effective treatment are crucial to reducing the risk of severe complications, including death.
1. Introduction
Since the beginning of December 2022, an unusually high number of cases and deaths from GAS infections have been reported in many European countries, including the United Kingdom, Ireland, France, the Netherlands, and Sweden. The UK has been the most affected European country by this sudden increase; the rise in invasive GAS infections detected in the UK in children has been several-fold higher than pre-pandemic levels for the equivalent period [1,2]. Since the beginning of 2023, the National Institute of Public Health–National Institute of Hygiene reports from Poland also have shown an increase in Streptococcus pyogenes infections, including invasive, life-threatening conditions like necrotizing fasciitis (NF) and STSS (Figure 1) [3].
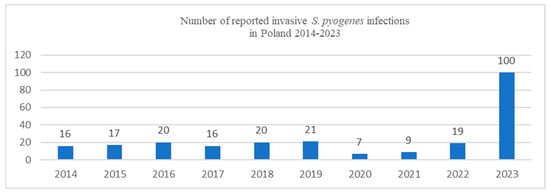
Figure 1.
Number of reported invasive S. pyogenes infections in Poland in 2014–2023 (based on the National Institute of Public Health–National Institute of Hygiene data) [3].
The probable cause of the higher morbidity is increased population susceptibility to infections due to COVID-19 pandemic restrictions. Influenza viruses and RSV (respiratory syncytial virus) cause damage to the respiratory epithelium, which also facilitates bacterial colonization, adherence, and translocation through the epithelial barrier, promoting the way for bacterial infection. Data indicated that the increase in iGAS infections is not associated with new strain of S. pyogenes or the global rise in antibiotic resistance [2,3]. The study, led by London researchers, showed that emm1 strains of S. pyogenes have accounted for >50% of invasive infections in children in England during the 2022–2023 season, and their results indicate that the M1UK lineage remained dominant in England and expanded until the end of 2020 [4,5]. The newly emergent emm1 clade (M1UK) is defined by 27 SNPs and exhibits significantly increased SpeA expression compared to the previously dominant M1global. Epidemiological studies show M1UK is overrepresented in scarlet fever and invasive infections. A newly developed allele-specific PCR assay enables rapid detection of M1UK-specific SNPs to support surveillance efforts [6]. In affected countries, enhanced surveillance for the M1UK sublineage is still warranted [4]. Most cases of invasive GAS infection are observed in adults ≥ 45 years of age, although recent increases in cases also have been linked to younger groups of patients [2,3,4]. Invasive infections often have a rapid and fulminant course; therefore, clinicians, healthcare professionals, and parents should be aware of increased prevalence of iGAS infections; only timely diagnosis and effective treatment may decrease the risk of serious complications, including death [2]. Infection with S. pyogenes can lead to a wide spectrum of clinical manifestations, ranging from mild localized infections to severe, life-threatening invasive diseases. GAS infection frequently causes mild diseases such as pharyngitis, tonsillitis, impetigo, cellulitis, and scarlet fever. However, in rare instances, GAS infection can lead to invasive, life-threatening conditions like necrotizing fasciitis and toxic shock syndrome that are associated with high mortality [7]. In case of deep tissue involvement, the risk of generalized infections, including sepsis and septic shock is higher [8]. Invasive infections can lead to death, even within a few or several hours of the onset of the first symptoms [9,10]. Invasive disease is defined as severe infection accompanied by the isolation of S. pyogenes from physiologically sterile organ tissues, such as blood, cerebrospinal fluid, peritoneal fluid, pleural fluid, synovial fluid, and tissue biopsies [7,8,9,10]. There are three types of invasive GAS disease. STSS is a rapidly progressive disease accompanied by sepsis or septic shock symptoms, including hypotension, fever, and multiple organ failure. The second form of invasive GAS is NF, manifested by rapidly progressive skin and soft tissue infections (SSTIs) that cause necrosis of the muscle fascia and subcutaneous tissues. The third form is organ infections with no symptoms of STSS or NF, although S. pyogenes has been isolated from physiologically sterile tissues. This group includes bacteremia of unknown origin and infections, such as pneumonia, meningitis, peritonitis, osteomyelitis, arthritis, and perinatal infection. About 20% of NF patients and 60-80% of STSS patients are estimated to die [9,10]. S. pyogenes has a wide variety of virulence factors that may cause severe, invasive infections. Bacterial entry and invasion into host cells is possible due to the production of hydrolytic enzymes, such as hyaluronidases, proteases, lipases, collagenases, and nucleases [11]. Cysteine protease SpeB leads to the degradation of intercellular matrix elements. SpeB also participates in the tissue damage characteristic for necrotizing fasciitis and in degradation of kininogen to bradykinin, causing increase permeability of blood vessels. The most important virulence factors include M protein and associated proteins, fibronectin-binding proteins, C5a-peptidase enzyme, and hyaluronic acid. Moreover, extracellular substances, such as O and S streptolysin, proteases, DNAse, streptokinase, cytolytic toxins (hemolysin), and pyrogenic toxins (exotoxins, superantigens), play an important role in progression of invasive GAS disease [11,12]. The main virulence factor and GAS adhesin is the M surface protein, first described by Lancefield (1928) [13]. Its complex structure, properties, and function, as well as its antigenic variability, are unique [14]. More than 220 M protein types are currently known [15]. Anti-M protein antibodies protect from infection with GAS of the same M protein serotype [16]. The primary function of the M protein is prevention of phagocytosis of GAS strains by polymorphonuclear leukocytes and host macrophages. The M protein is responsible for the development of streptococcal toxic shock. After entering the host’s blood, the bacteria release the M protein, which, through the B region, forms complexes with fibrinogen and plasma proteins. Additionally, leukocytes activated by the M protein bind to the vascular endothelium, leading to their destruction, increased permeability, hypotension, intravascular coagulation, and, eventually, multiorgan failure [17,18]. S. pyogenes can also produce several toxins that play a crucial role in the pathogenesis of GAS infections. Toxins include cytolysins and pyrogenic toxins (superantigens). Cytolysins (streptolysin O and S) are membrane-damaging protein toxin [19]. Superantigens are unconventional antigens that cause excessive activation of the immune system by non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release [20]. A timely diagnosis and treatment of severe, invasive infection caused by S. pyogenes may be challenging. Early and aggressive surgical debridement of the affected tissues as well as appropriate intravenous antibiotic therapy is required [10]. In this study, we present our experience in patients with severe invasive S. pyogenes infections.
2. Materials and Methods
2.1. Design and Settings
This single-center, retrospective case series study was conducted at the Department of Anesthesiology and Intensive Therapy, Department of Surgery and Department of Internal Medicine, 4th Military Clinical Hospital in Wroclaw, Poland. The CARE Case Report and Case Series guidelines were followed [21].
2.2. Study Population
The study group consisted of 11 patients treated in the 4th Military Clinical Hospital in Wroclaw between December 2022 and May 2023. This study used retrospective data analysis. Patients with severe infections caused by S. pyogenes accompanied by sepsis or septic shock were enrolled. The inclusion criteria were sepsis or septic shock according to the Sepsis-3 criteria, age > 18, while the exclusion criteria disqualified patients include severe immunosuppression (active cancer, chronic steroid therapy, chemotherapy) or age < 18 years. All septic patients were treated according to the current Surviving Sepsis Campaign guidelines [22].
2.3. Data Collection
Sociodemographic, clinical, and biochemical data at the time of admission were recorded. The Acute Physiology and Chronic Health Evaluation II (APACHE II) [23] and Sequential Organ Failure Assessment (SOFA) scores [24] were calculated at the initial time of admission. Data on the following parameters were collected: age, sex, serum creatinine, urea concentration, procalcitonin, C-reactive protein, lactate, electrolytes, morphology, arterial, and venous blood gas analysis, and parameters assessing the coagulation system as well as the liver injury and function. Additionally, the results of blood, urine, bronchoalveolar, and nose and pharynx cultures were collected. The antibiotics used, the number of days of treatment, the length of hospital stay, the type of surgical treatment, and mortality were analyzed.
2.4. Microbiology Procedure
2.4.1. Blood Culture
Blood culture bottles BacT/ALERT FN PLUS and BacT/ALERT FA PLUS (bioMérieux, Marcy-l’Étoile, France) were incubated in a BacT/ALERT 3D instrument (bioMérieux, Marcy-l’Étoile, France) at 37 °C in a 5-day protocol. After bacterial growth was detected, the positive blood cultures were Gram-stained, streaked onto Columbia agar, chocolate agar, MacConkey, and Schaedler agar (bioMérieux, Marcy-l’Étoile, France) for overnight incubation at 37 °C [25].
2.4.2. Throat Swab Culture
Throat swabs were placed in Amies transport media and used to inoculate Columbia agar, chocolate agar, MacConkey agar, Mannitol Salt agar, and Sabouraud agar (bioMérieux, Marcy-l’Étoile, France) for overnight incubation at 37 °C with 5% CO2 [25].
2.4.3. Bronchoalveolar Lavage Culture
Bronchoalveolar lavage (BAL) aspirate was taken with a sterile catheter from the trachea immediately after intubation by a newly inserted endotracheal tube. For a quantitative culture of bacteria in BAL fluid, 10 µL loops of the aspirate were plated on Columbia agar, chocolate agar, MacConkey agar, Mannitol salt agar, and Sabouraud agar (bioMérieux, Marcy-l’Étoile, France) and incubated in 5% CO2 overnight at 37 °C [25].
2.4.4. Skin Swabs and Tissue Biopsies
Skin swabs were taken and stored in Amies transport media (bioMérieux, Marcy-l’Étoile, France), and tissue biopsies were stored in a thioglycollate broth (bioMérieux, Marcy-l’Étoile, France). A semi-quantitative technique was used to assess bacterial growth in the swabs. Samples were inoculated on Columbia agar, chocolate agar, MacConkey agar, Mannitol salt agar, and Sabouraud agar (bioMérieux, Marcy-l’Étoile, France) for overnight incubation at 37 °C with 5% CO2 [25].
2.4.5. Gram-Stain
Two sets of smears were prepared from each of the positive detected blood culture bottles and then run in the PREVI Color Gram system (bioMérieux, Marcy-l’Étoile, France).
2.4.6. Identification and Antimicrobial Susceptibility Testing
Identification of bacterial isolates was performed via matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF) (VITEK MS, bioMérieux, Marcy-l’Étoile, France). The VITEK-2 automated system and AST-ST03 panel (bioMérieux, Marcy-l’Étoile, France) were used for antimicrobial susceptibility testing.
2.4.7. Molecular Procedure
The BioFire Blood Culture Identification Panel 2 BCID2 (bioMérieux, Marcy-l’Étoile, France) testing was performed according to the manufacturer’s guidelines. Specifically, 200 μL of the positive blood culture sample was mixed with the supplied sample dilution buffer and used to inoculate a pre-hydrated panel pouch. The BioFire Pneumonia Plus Panel (bioMérieux, Marcy-l’Étoile, France) was used for the diagnosis of lower respiratory tract infections. In this case, the specimen analyzed was sputum, and the sample was prepared according to the testing protocol. The provided sample swab from the test kit was used to collect the sputum sample, which was then prepared following the specific instructions outlined in the testing protocol. The BioFire BCID2 and Pneumonia Plus Panel pouch was then loaded onto the BioFire FilmArray TORCH System (bioMérieux, Marcy-l’Étoile, France) for nucleic acid extraction, amplification, and analysis.
2.5. Ethical Considerations
The study protocol was approved by the Bioethics Committee of the Lower Silesia Medical Chamber in Wrocław, Poland (approval no.: 1/BNR/2023 and approval date: 12 July 2023). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and Good Clinical Practice. Informed consent was obtained from all patients. The CARE Case Report and Case Series guidelines were followed [21].
3. Results
3.1. General Characteristics
A total of 11 patients (5 women and 6 men) with ages ranging from 21 to 74 years old, with confirmed severe, invasive infections caused by S. pyogenes, were enrolled in our analysis. The characteristics of the patients are presented in Table 1.

Table 1.
Characteristics of the patients.
3.2. Patient Diagnostic and Treatment Data
Overall, 6 out of 11 patients initially had streptococcal skin and soft tissue infections affecting area of chest (2) and lower limbs (4) (Figure 2 and Figure 3).
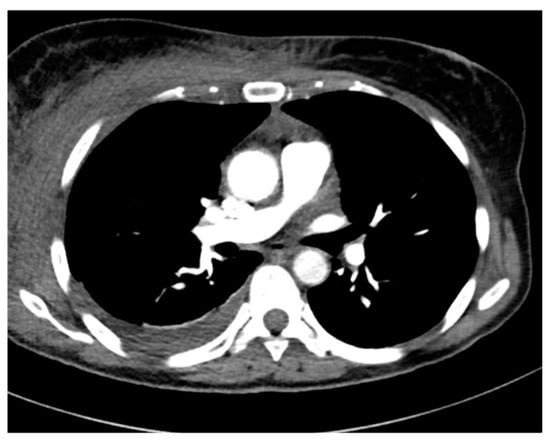
Figure 2.
Chest CT. Cellulitis and mediastinitis caused by S. pyogenes.
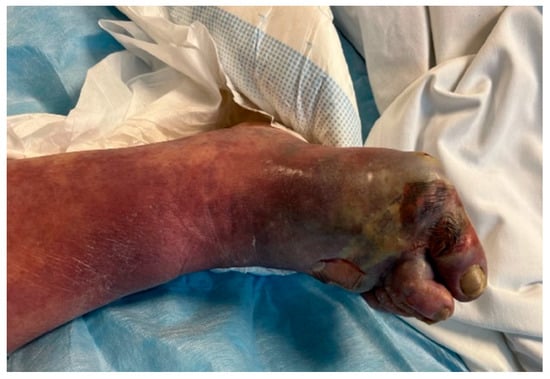
Figure 3.
Lower leg phlegmon caused by S. pyogenes.
We also reported three cases of pneumonia caused by S. pyogenes (Figure 4, Figure 5 and Figure 6), including one case of pleural empyema.
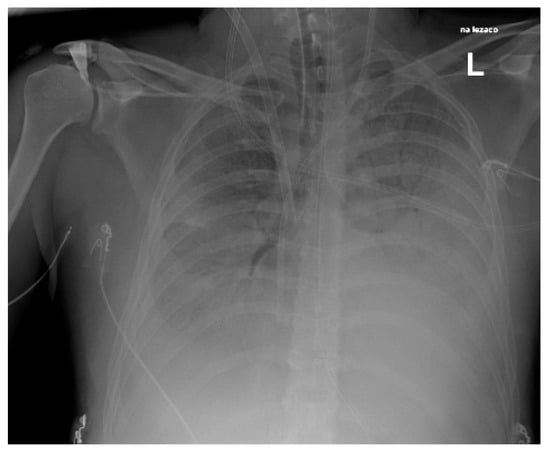
Figure 4.
Chest X-Ray. Patient with S. pyogenes and AH1N1 pneumoniae.
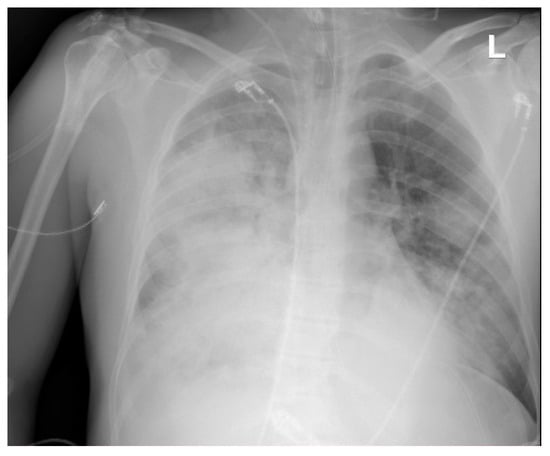
Figure 5.
Chest X-ray. Pneumonia caused by S. pyogenes. Patient on veno-venous extracorporeal membrane oxygenation (VV-ECMO).
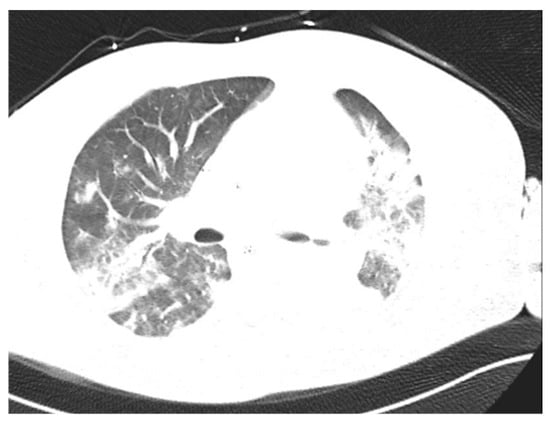
Figure 6.
Chest CT. Patient with S. pyogenes pneumonia.
Moreover, there was one case of streptococcal otitis with para-cerebral abscess (Figure 7) and one infection of the knee joint.
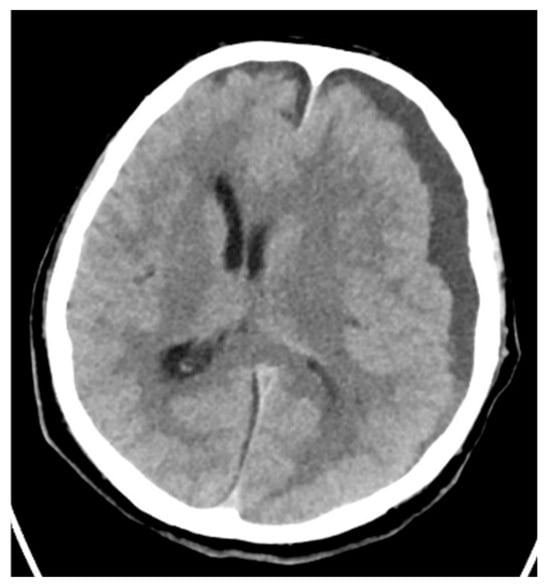
Figure 7.
Brain CT. Cerebral abscess caused by S. pyogenes.
Nine patients developed septic shock. Due to the S-AKI, five of the patients in our study underwent extracorporeal elimination therapy, including Oxiris filter (twice), SepteX (once), and CytoSorb (once). No effect of this therapy on the course of treatment was observed. One patient required extracorporeal membrane oxygenation due to acute respiratory distress syndrome during S. pyogenes pneumonia. The fulminant course of STSS led to the death of three patients. Patients detailed diagnostic and treatment data are presented in Table 2.

Table 2.
Patient diagnostic and treatment data.
The detailed results of the admission laboratory tests are presented in Table 3.

Table 3.
Laboratory results on the day of admission.
4. Discussion
Sepsis and septic shock are conditions with high mortality and morbidity caused by a systemic infection that leads to organ dysfunction [26]. Approximately 663,000 new cases of invasive GAS disease have been reported each year, with 163,000 deaths per year [8]. Our study presents a case series of sepsis and septic shock caused by S. pyogenes. In many countries after the COVID-19 pandemic, there was an alarming increase in mild GAS infections, such as strep throat and scarlet fever, across all age groups [2]. However, the number of severe invasive S. pyogenes infections in young, previously unaffected people has increased significantly. Since the beginning of 2023, the National Institute of Public Health–National Institute of Hygiene reports from Poland also have shown an increase in invasive S. pyogenes infections [3].
Among our cases, 9 were treated in the Intensive Care Unit, 1 in the Department of Internal Medicine, and 1 in the Department of Surgery. Three patients developed pneumonia, six patients developed skin and soft tissue infections, including two lower leg phlegmon, one patient developed septic arthritis, and one patient developed otitis with para-cerebral abscess. In all cases of infection, broad-spectrum empirical antibiotic therapy was used due to the initial severe condition of the patients. The β-lactam penicillin is still the gold standard for antibiotic treatment of GAS infections [26,27]. β-lactams target penicillin-binding proteins to block peptidoglycan cross-linking in metabolically active bacteria, leading to bacterial death. Despite extensive use for decades, there has been minimal change in the susceptibility of GAS to penicillin [28]. All strains of S. pyogenes remain uniformly sensitive to this antibiotic. The second antibiotic used to treat S. pyogenes infection is clindamycin, which is bacteriostatic and can reduce the production of toxic proteins and virulence factors [29]. Clindamycin promotes phagocyte killing and the inhibition of toxins and superantigens by inhibiting M protein synthesis [30,31,32]. The Infectious Diseases Society of America recommends treating necrotizing GAS infections with penicillin and clindamycin. Surgical debridement is a crucial element of treatment in the case of an invasive form of the condition [10,27,31]. In the cases described in this study, seven patients underwent surgical procedures, all of whom survived. Non-surgical preparation in one case required amputation of the lower leg. In the remaining cases, tissue incision and drainage were performed, except in the case of a patient with a middle ear infection and para-cerebral abscess who underwent surgery to remove the abscess and drain the ear. In four patients, it was impossible to perform surgical treatment due to the localization of the infection within the lungs or the involvement of the chest wall muscles. In one of the cases, the mediastinum was involved. Three of these patients died. We observed that despite the implementation of broad-spectrum antibiotic therapy and performing of the primary source surgical debridement, the condition of the patients initially deteriorated. In patient No. 7, whose prior site of infection was the pectoralis major muscle on the left side, piperacillin with tazobactam, linezolid, and clindamycin was started within 1 h of stay in the emergency department. However, on the fourth day, despite intensive treatment, muscle fasciotomy and drainage were performed due to the patient’s rapidly deteriorating condition, and tissue biopsies were taken simultaneously for microbiological examination. Despite antibiotic treatment, S. pyogenes was detected in the cultures of the patient’s tissues. Twelve hours after surgery, the patient’s condition improved significantly. Analysis of laboratory test results in patients with invasive S. pyogenes infection revealed the presence of leukopenia, neutropenia, and lymphopenia in those who had a preceding viral infection. These patients did not have a history of previous immunodeficiencies, and their complete blood count revealed significantly reduced levels of white blood cells, including neutrophils and lymphocytes. Leukopenia following viral infection can occur due to various pathophysiological mechanisms. Viruses often induce apoptosis in lymphocytes, particularly T lymphocytes. Apoptosis of T lymphocytes can occur due to direct viral DNA damage or altered gene expression related to cell survival. This leads to a decrease in the number of circulating T lymphocytes and subsequent leukopenia. Viral infections also trigger the release of various pro-inflammatory cytokines, such as interferons, interleukins, and tumor necrosis factors [33]. These cytokines can influence leukocytes’ production and function, resulting in a decrease in their circulating counts. Finally, some viruses can directly affect the hematopoietic system and disrupt the processes of cell formation and maturation. For example, the influenza virus can specifically target and affect immune cell production in the bone marrow [33,34]. This can result in a decreased production of lymphocytes, including B cells, T cells, and natural killer cells, which are crucial for mounting an effective immune response against viral infections. The leukopenia was likely temporary; however, these patients had a severely compromised immune system that was unable to fight off the invasive S. pyogenes infection [33,34,35]. Surgical intervention should be the treatment of choice for invasive GAS skin and soft tissue infections. Inflammation, tissue edema, tissue toxin-mediated necrosis, and thrombosis can reduce antibiotic penetration. Tissue incision and drainage lead to decreased tissue pressure, improved blood supply, and thus better distribution of antibiotics to infected tissues. Due to the high mortality rate of 30% or more of invasive GAS infections, treatment should be initiated immediately to reduce the risk of death [27,36]. Penicillin is still the drug of choice, and treatment delay and inadequacy are associated with lower survival rates [28]. In addition, clindamycin should be implemented for treatment [29,30]. Due to the possibility of resistance to macrolide, lincosamide, and streptogramin B, linezolid can be used, which, similarly to clindamycin, inhibits the production of proteins and toxins while effectively penetrating the tissues [37]. Due to the S-AKI, five of the patients in our study underwent extracorporeal elimination therapy, including Oxiris filter (twice), SepteX (once), and CytoSorb (once). No effect of this therapy on the course of treatment was observed. One patient required extracorporeal membrane oxygenation due to acute respiratory distress syndrome during S. pyogenes pneumonia.
5. Conclusions
Physicians should be aware of increased prevalence of iGAS infections, only timely diagnosis and effective treatment including surgical debridement and proper antibiotic therapy (penicillin and clindamycin) may decrease the risk of serious complications, including death. The preliminary data of winter 2023/2024 indicate that there is a close association between iGAS cases and winter respiratory viruses, close monitoring, and investigation of iGAS activity should be continuing during coming years.
Author Contributions
Conceptualization: P.L., J.J. and N.S.; methodology: P.L., N.S. and J.J.; formal analysis: P.L., N.S., M.B. (Martyna Biała), M.B. (Marzenna Bartoszewicz), L.Ł. and J.J.; investigation: P.L., N.S. and J.J.; data curation: P.L., N.S. and J.J.; visualization: P.L., N.S., M.B. (Martyna Biała), M.B. (Marzenna Bartoszewicz) and J.J.; software: P.L., N.S., L.Ł. and J.J.; writing—original draft: P.L., N.S., J.J. and M.B. (Martyna Biała); writing—review and editing: M.B. (Marzenna Bartoszewicz) and L.Ł.; supervision: J.J.; project administration: P.L. and N.S.; funding acquisition: P.L. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by the Bioethics Committee of the Lower Silesia Medical Chamber in Wrocław, Poland (approval no.: 1/BNR/2023 and approval date: 12 July 2023). The study was conducted in accordance with the guidelines of the Declaration of Helsinki and Good Clinical Practice. The CARE Case Report and Case Series guidelines were followed.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Centre for Disease Prevention and Control (ECDC). Increase in Invasive Group A Streptococcal Infections Among Children in Europe, Including Fatalities. 2022. Available online: https://www.ecdc.europa.eu/en/news-events/increase-invasive-group-streptococcal-infections-among-children-europe-including (accessed on 29 July 2023).
- World Health Organization. Disease Outbreak News; Increased Incidence of Scarlet Fever and Invasive Group A Streptococcus Infection—Multi-Country. 15 December 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON429 (accessed on 29 July 2023).
- National Institute of Public Health—National Institute of Hygiene. Department of Epidemiology and Surveillance of Infectious Diseases. Laboratory of Monitoring and Epidemiological Analysis [In Polish]. Available online: http://wwwold.pzh.gov.pl/oldpage/epimeld/index_p.html?fbclid=IwAR2cc9IHwAVjTkWLEsKOXYZVEs37nqO4NO1ZGqH-VF5GPmZ3YR9gM3_IvRg (accessed on 5 June 2023).
- Ramos Amador, J.T.; Berzosa Sánchez, A.; Illán Ramos, M. Group A Streptococcus invasive infection in children: Epidemiologic changes and implications. Rev. Esp. Quimioter. 2023, 36 (Suppl. 1), 33–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhi, X.; Li, H.; Li, H.; Loboda, Z.; Charles, S.; Vieira, A.; Huse, K.; Jauneikaite, E.; Reeves, L.; Mok, K.Y.; et al. Emerging Invasive Group A Streptococcus M1UK Lineage Detected by Allele-Specific PCR, England, 2020. Emerg Infect Dis. 2023, 29, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Das, S.; Hayes, A.J.; Bertolla, O.M.; Davies, M.R.; Walker, M.J.; Whiley, D.M.; Irwin, A.D.; Tickner, J.A. A rapid molecular detection tool for toxigenic M1UK Streptococcus pyogenes. J. Infect. Dis. 2024, jiae437. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.; Green, M. Group A streptococcus. Semin. Pediatr. Infect. Dis. 2006, 17, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Hoge, C.W.; Schwartz, B.; Talkington, D.F.; Breiman, R.F.; MacNeill, E.M.; Englender, S.J. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. A retrospective population-based study. JAMA 1993, 269, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Babiker, A.; Kadri, S.S. ICU Management of Invasive β-Hemolytic Streptococcal Infections. Infect. Dis. Clin. N. Am. 2022, 36, 861–887. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.W. Pathogenesis of Group A Streptococcal Infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Carothers, K.E.; Liang, Z.; Mayfield, J.; Donahue, D.L.; Lee, M.; Boggess, B.; Ploplis, V.A.; Castellino, F.J.; Lee, S.W. The Streptococcal Protease SpeB Antagonizes the Biofilms of the Human Pathogen Staphylococcus aureus USA300 through Cleavage of the Staphylococcal SdrC Protein. J. Bacteriol. 2020, 202, e00008-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lancefield, R.C. The Antigenic Complex of Streptococcus haemolyticus. J. Exp. Med. 1928, 47, 91–103. [Google Scholar] [CrossRef]
- Ghosh, P. The nonideal coiled coil of M protein and its multifarious functions in pathogenesis. Adv. Exp. Med. Biol. 2011, 715, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Kuliyev, E.; Nizet, V.; Ghosh, P. A conserved 3D pattern in a Streptococcus pyogenes M protein immunogen elicits M-type crossreactivity. J. Biol. Chem. 2023, 299, 104980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venkatesan, P. Rise in group A streptococcal infections in England. Lancet Respir. Med. 2023, 11, e16. [Google Scholar] [CrossRef] [PubMed]
- Szczypa, K.; Wilemska, J.; Hryniewicz, W.; Sitkiewicz, I. The mechanisms of virulence of Streptococcus pyogenes. Adv. Microbiol. 2012, 51, 3–15. (In Polish) [Google Scholar]
- Valderrama, J.A.; Nizet, V. Group A Streptococcus encounters with host macrophages. Future Microbiol. 2018, 13, 119–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nizet, V. Streptococcal beta-hemolysins: Genetics and role in disease pathogenesis. Trends Microbiol. 2002, 10, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Proft, T.; Fraser, J.D. Streptococcal Superantigens: Biological properties and potential role in disease. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK333435/ (accessed on 29 July 2023).
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013, 2, 38–43. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; Rhodes, A.; Piquilloud, L.; Hernandez, G.; Takala, J.; Gershengorn, H.B.; Tavares, M.; Coopersmith, C.M.; Myatra, S.N.; Singer, M.; et al. The Sequential Organ Failure Assessment (SOFA) Score: Has the time come for an update? Crit. Care 2023, 27, 15. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L. (Ed.) Clinical Microbiology Procedures Handbook, 4th ed.; American Society for Microbiology: Washington, DC, USA, 2016. [Google Scholar]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein EJ, C.; Gorbach, S.L.; Hirschmann, J.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Macris, M.H.; Hartman, N.; Murray, B.; Klein, R.F.; Roberts, R.B.; Kaplan, E.L.; Horn, D.; Zabriskie, J.B. Studies of the continuing susceptibility of group A streptococcal strains to penicillin during eight decades. Pediatr. Infect. Dis. J. 1998, 17, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.M.; Kelly, J.A. Clindamycin-induced suppression of toxic-shock syndrome-associated exotoxin production. J. Infect. Dis. 1984, 149, 471. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, C.G.; Peterson, P.K.; Schmeling, D.; Kim, Y.; Mathews, J.; Wannamaker, L.; Quie, P.G. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J. Clin. Investig. 1981, 67, 1249–1256. [Google Scholar] [CrossRef]
- Mascini, E.M.; Jansze, M.; Schouls, L.M.; Verhoef, J.; Van Dijk, H. Penicillin and clindamycin differentially inhibit the production of pyrogenic exotoxins A and B by group A streptococci. Int. J. Antimicrob. Agents 2001, 18, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Sriskandan, S.; Faulkner, L.; Hopkins, P. Streptococcus pyogenes: Insight into the function of the streptococcal superantigens. Int. J. Biochem. Cell Biol. 2007, 39, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Derichard, A.; Hindy-Francois, C.; Jugie, M.; Hamza, J. Pancytopenia associated with influenza A infection. Presse Med. 2013, 42, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, H.; Song, P.; Zhang, Z.; Chen, J.; Zhou, Y.-H. Dynamic changes of lymphocyte counts in adult patients with severe pandemic H1N1 influenza A. J. Infect. Public. Health 2019, 12, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, J.; Yang, L.; Zhou, F.; Li, H.; Cao, B. Influenza Virus in Community-Acquired Pneumonia: Current Understanding and Knowledge Gaps. Semin. Respir. Crit. Care Med. 2020, 41, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.W. Comprehensive review of morbidity and mortality trends for rheumatic fever, streptococcal disease, and scarlet fever: The decline of rheumatic fever. Rev Infect Dis 1989, 11, 928–953. [Google Scholar] [CrossRef]
- Oppegaard, O.; Rath, E. Treatment of Necrotizing Soft Tissue Infections: Antibiotics. In Necrotizing Soft Tissue Infections: Clinical and Pathogenic Aspects Advances in Experimental Medicine and Biology; Norrby-Teglund, A., Svensson, M., Skrede, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 87–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).