Abstract
Adverse events following immunizations (AEFIs) with recombinant zoster vaccine (RZV) are underexplored in fragile populations. This study aims to assess incidence, duration, and characteristics of AEFIs, focusing on the impact of sex, age, and prior Herpes Zoster (HZ) infection in a frail population, including solid organ transplant recipients. We conducted an observational study on patients receiving RZV, and AEFIs were classified as local or systemic and analyzed for incidence, duration, and patterns across groups. We showed that females had a higher incidence of AEFIs (p = 0.02), both local and systemic symptoms, such as swelling +/− redness at the site of injection and fatigue, after the first and second doses. Younger adults experienced more systemic reactions, while older adults reported more local events (e.g., redness and swelling, p = 0.01). Moreover, patients with previous HZ infection exhibited a higher incidence of AEFIs after the second dose (68% vs. 38%, p = 0.001). In conclusion, sex, age, and clinical history significantly influenced AEFI incidence and manifestations. Therefore, it is important to personalize vaccination strategies in frail populations, by tailored administration and monitoring plans, especially in females and individuals with prior HZ infection, to improve vaccine safety and patient outcomes.
1. Introduction
Vaccinations are one of the most effective measures for infectious disease prevention and constitute a key strategy for combating the development and spread of antibiotic resistance [1,2,3,4]. Adjuvanted recombinant vaccines are of great interest in public health because of their ability to enhance the immunogenicity of vaccine antigens, and this characteristic is particularly important in subjects with reduced immune system functions, such as the elderly and immunocompromised individuals, like solid organ transplant recipients [5].
Vaccines could be associated with adverse events, known as adverse events following immunizations (AEFIs), and their incidence and severity can vary depending on individual factors, including sex and age [6,7,8]. Indeed, females exhibit stronger immune responses to vaccines compared to males, often producing two-fold increased antibody titers [6,9]. However, this enhanced immunoreactivity is associated with more frequent and severe AEFIs, as also documented by the Italian Medicine Agency AIFA in its annual report of vaccine post-marketing [10,11]. Various factors can contribute to these differences, such as sex hormone-related changes in immune system composition and functions, favoring T helper 1 (Th1) and B cell-mediated responses in females, while males have enhanced innate responses and pro-inflammatory cytokines levels [12,13,14,15,16]. Moreover, females are more likely to report AEFIs than males [9]. Despite this evidence, sex-based differences in immune responses are still not adequately considered in study design and dose findings of drugs and vaccines, and AEFI incidence, distribution, and risk factors could be under-estimates and data remain limited [5,11]. Age also plays a crucial role in modulating immune responses to vaccines [13], as immunosenescence is frequent in older adults, causing attenuated responses to pathogens and vaccines, as well as AEFI occurrence [17].
Herpes Zoster (HZ) is a disease caused by the reactivation of the varicella-zoster virus (VZV), a Herpes virus that remains latent in the dorsal root ganglia and cranial nerves after primary infection [18]. Viral reactivation is favored by a decline in cell-mediated immunity due to immunosenescence or immunosuppression, such as during acquired or congenital immunodeficiency syndromes or under immunosuppressive therapies. HZ manifests as painful, often unilateral vesicular skin eruptions, and can lead to severe acute complications and/or to long-term syndromes, such as postherpetic neuralgia, negatively affecting quality of life [19]. Therefore, vaccination is a necessary preventive measure to reduce both incidence and severity of HZ and its complications, especially in vulnerable populations. In the elderly, HZ infection is frequently associated with a high rate of hospitalization, up to six-fold compared to younger subjects (up to 25 hospitalizations/100,000 patient/years), and an immunocompromised status can increase this rate, with an in-patient mortality up to two-fold [20]. For example, diabetic patients have a hospitalization rate of up to 15.9 per 100,000, and those subjects aged 65 or older, with a BMI > 30, and poor glycemic control (HbA1c > 8.0%) are even more at risk of rehospitalization (40%) and of post-HZ complications (25% higher risk) [21].
Currently, an inactivated adjuvanted recombinant vaccine against Herpes Zoster (RVZ) is available, providing a high level of protection in the general population and fragile individuals [22]. Although several studies have explored the impact of age and sex on immune responses after vaccinations, detailed characterization of AEFI occurrence and severity in vulnerable populations after adjuvanted recombinant vaccines remains an under-researched area. In this retrospective real-life study, we aimed to analyze incidence and types of AEFIs following RVZ immunization in vulnerable populations, especially in those subjects with prior HZ manifestations. A better understanding of risk factors of AEFI development could optimize and personalize strategies, improving vaccination safety and acceptance.
2. Materials and Methods
2.1. Study Design
In this observational real-life study, a total of 174 frail Caucasian adults (mean age, 57 ± 11.8 years; range, 28–84 years) who received RZV at the “Ruggi” hospital site, University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Salerno, Italy, between 2022 and 2024 were included. The RZV schedule consisted of two intramuscular doses of 0.5 mL each, with an interval of 2 to 6 months between each administration, as per Italian guidelines and technical datasheet. Vaccination was free of charge as per clinical Italian practice and was administered at the site hospital Ruggi following the Italian Health Institute’s recommendations. No other vaccinations were administered together with RZV, and at least one month elapsed between different vaccine doses. After vaccination, patients were monitored by periodical out-patient visits. The inclusion criteria were as follows: age ≥ 18 years old; presence of severe immunodeficiency conditions, such as hematological malignances, hematopoietic stem cell or solid organ transplantation, or human immunodeficiency virus (HIV) infection; the presence of geriatric frailty, defined as individuals aged 65 years or older with reduced physiological reserve and resilience; the presence of comorbidities that posed an indication for RZV, including type 2 diabetes, obesity, or autoimmune diseases; the completion of the vaccination cycle; and signed informed consent. The exclusion criteria were the following: no indications for RZV; incomplete vaccination; and refusal to sign informed consent. Clinical characteristics are summarized in Table 1.

Table 1.
Patients’ characteristics at enrollment.
This study followed the Declaration of Helsinki and was approved by local Ethics Committee “Campania Sud”, Naples, Italy (n. 185_r.p.s.o./2022).
2.2. Demographic and AEFI Data Collection
A specific Case Report Form (CRF) was used to collect patients’ data, including underlying conditions and previous HZ disease, and type, duration, and treatments used for AEFIs and severe AEFIs (SAEFIs) after first and second dose administration. AEFIs were classified according to the Italian and European guidelines [23,24].
2.3. Statistical Analysis
Data were collected in a spreadsheet and analyzed using SPSS 23.0 statistics package. Continuous variables were presented as mean ± standard deviation (SD). Two-group comparisons were performed using an unpaired or paired t-test for normally distributed data, while categorical variables were analyzed using Chi square test or Fisher’s exact test for multiple groups. A logistic regression analysis was performed to investigate the effects of several factors on AEFI occurrence. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Females More Frequently Report Mild/Moderate AEFIs After First RZV Dose
To explore sex-related differences in AEFI incidence, types, and duration, patients were stratified by sex (females, N = 51, 29%; and males, N = 123, 71%). In both groups, kidney transplant recipients were the most represented (76% females, and 70.7% males), while HIV infection was exclusively present in males (10.6%), as well as geriatric frailty (4.1%) (Table 2). As expected, females more frequently complained of AEFIs after the first dose of RZV (N = 28; 55%) compared to males (N = 44; 35.8%) (p = 0.02), without differences in duration (75 ± 74.8 h vs. 66 ± 114.8 h, females vs. males; p = 0.7), either as a local or systemic event (all p > 0.05) (Table 2). Paracetamol was used to treat AEFIs (100% in both females and males). Conversely, local and systemic AEFIs showed a variable distribution between sexes (Figure 1). In detail, females more frequently reported local AEFIs after the first RZV dose compared to males, especially swelling and/or redness at the injection site (14% vs. 2%, females vs. males; p = 0.0036), while males were more likely to experience pain at the injection site (77% vs. 64%, males vs. females; p = 0.088). For systemic AEFIs, females more commonly reported fatigue compared to males (29% vs. 16%, females vs. males; p = 0.0488) (Figure 1A).

Table 2.
Incidence and duration of adverse events following immunization (AEFI) stratified by sex.
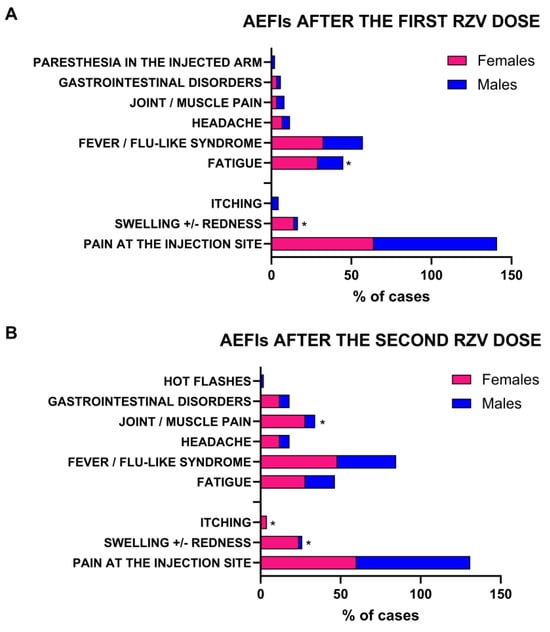
Figure 1.
Incidence of adverse events following immunization (AEFI) stratified by sex after first and second recombinant zoster vaccine (RZV). *, p value < 0.05.
After the second vaccine dose, males presented more AEFIs than those observed after the first dose (N = 50; 41%); however, females tended to display adverse events more frequently (N = 28; 55%) (p = 0.09). Total symptom duration was similar in both sexes (51 ± 40.4 h vs. 48 ± 42.7 h, females vs. males; p = 0.8), regardless of being local or systemic (all p > 0.05) (Table 2). Paracetamol was used to treat AEFIs (100% in both females and males). As observed for AEFI distribution after the first dose, local and systemic symptoms showed a variable distribution between the sexes. In particular, females more frequently reported local AEFIs after the second RZV dose compared to males, especially swelling and/or redness at the injection site (24% vs. 2%, females vs. males; p < 0.0001) and itching (4% vs. 0%, females vs. males; p = 0.0272). For systemic AEFIs, females more commonly experienced muscle/joint pain after the second dose compared to males (28% vs. 6%, females vs. males; p = 0.0002) (Figure 1B). No significant variations between sexes for the remaining symptoms were observed (all p > 0.05).
3.2. Young Adults More Frequently Report Local and Systemic AEFIs
Next, to investigate the effects of age on AEFI development after RZV, patients were divided in three groups based on age at vaccination: young adults aged 28–39 years; adults aged 40–65 years; and older adults aged > 65 years. After the administration of the first dose, total AEFI incidence tended to progressively decrease with age, as older adults complained of an AEFI only in 30% of cases compared to 54% in young adults and 44% in adults (all p > 0.05). This trend could be explained with the phenomenon of immunosenescence in older adults, that could also be related to the lower incidence of both systemic and local adverse events (17% and 26%, respectively; all p > 0.05) (Table 3). Total duration did not significantly differ between age groups, either as local or systemic symptoms (all p > 0.05). Based on manifestations, young adults tended to more frequently complain of local (54% vs. 35% vs. 26%; p = 0.11) and systemic AEFIs (38% vs. 27% vs. 17%; p = 0.21) compared to adults and elderly. In particular, redness and/or swelling at the injection site was significantly more frequent in young adults compared to adults and elderly (23% vs. 1% vs. 2%, respectively; p = 0.004). No other significant differences were observed between age groups in types of AEFI manifestations.

Table 3.
Incidence, duration, and manifestations of AEFIs stratified by age.
Similarly, after the administration of the second dose, total AEFI incidence was significantly higher in younger subjects, as older adults reported an AEFI only in 37% of cases compared to 69% in young adults and 45% in adults (p = 0.04), with lower incidence of both systemic and local adverse events (37% and 26%, respectively; p = 0.11 and p = 0.12) (Table 3). Total duration did not significantly differ between age groups, either as local or systemic symptoms (all p > 0.05), as observed for AEFIs after the first dose. Based on manifestations, young adults tended to more frequently complain of local (54% vs. 37% vs. 26%; p = 0.11) and systemic AEFIs (69% vs. 42% vs. 37%; p = 0.12) compared to adults and elderly. No significant differences were observed for local AEFIs, while young adults more commonly reported fever or flu-like syndrome compared to adults and elderly (55% vs. 42% vs. 30%, respectively; p = 0.04). No other significant differences were observed between age groups in types of AEFI manifestations, as well as duration of local and systemic AEFIs (Table 3).
3.3. Previous AEFI Occurrence Does Not Increase Severity and Incidence of Adverse Events After Second RZV Dose
Subsequently, we thought to study the safety of RZV in subjects who have experienced an AEFI after the first dose, and we investigated incidence, type, and duration of a second AEFI in this subgroup of patients. Among all 174 vaccinated subjects, 49 of them (28%) after both the first and second doses, while 31 patients (18%) reported an AEFI only after the second administration, and 71 (41%) reported no cases. To explore incidence and severity of AEFIs during the second dose, we compared subjects who experienced an AEFI after both the first and second administration (Group I/II AEFI) with those who reported an AEFI only after the second dose (Group II AEFI) (Table 4).

Table 4.
Incidence, duration, and manifestations in patients with AEFIs after first and second RZV dose (Group I/II AEFI) or only after second administration (Group II AEFI).
Group I/II AEFI tended to exhibit a higher incidence of systemic AEFIs (96%) and lower local symptoms (69%) compared to Group II AEFI (84% and 87%, respectively; p = 0.06 and p = 0.07). These findings suggested that patients who experience an AEFI after the first dose could develop a more robust (and potentially more reactogenic) immune response after the second dose. For specific symptoms, injection site pain was the most common local AEFI, occurring more frequently in Group II (77%) than in Group I/II (61%), although the difference did not reach statistical significance (p = 0.1). Among systemic AEFI, the most frequent symptoms included fever, fatigue, and headache, with similar distributions between the two groups (Table 4). However, no differences were observed between groups in terms of total duration, local and systemic AEFI persistence, and types of manifestations (all p > 0.05). After the second dose, medications to alleviate AEFI symptoms were more commonly employed in Group I/II AEFI compared to Group II AEFI (35% vs. 16%; p = 0.07), and paracetamol was the only drug used (Table 4).
3.4. Impact of Previous Herpes Zoster Infection on Prevalence and Duration of AEFI
Finally, we investigated the efficacy and safety of RZV in patients who had a clinical history of HZ and the impact of this vaccination on reducing viral reactivation. For this purpose, patients were stratified based on previous HZ infection, and 78% of subjects (N = 136) reported no prior HZ, while 22% of the total cohort (N = 38) had a clinical history of HZ. However, none of these subjects reported HZ infection in the 12 months before the vaccination. Following the first dose, overall AEFI incidence was similar between groups (40% vs. 45%, without and with previous HZ; p = 0.6), indicating that a previous HZ infection could be not related to AEFI development after RZV. However, once experienced, patients with a clinical history of HZ were more likely to have systemic symptoms (82% vs. 56%, with and without previous HZ; p = 0.05) (Table 5). No differences were observed between groups in terms of total duration, local and systemic AEFI persistence, and types of manifestations (all p > 0.05). After the second dose, the incidence of AEFIs was significantly higher in subjects with previous HZ compared to those without a clinical history (68% vs. 38%, respectively; p = 0.001) (Table 5). This finding suggested that patients with a previous HZ infection could have a greater antigen susceptibility and an enhanced immune response, likely because the second vaccine dose could act as a “third” booster to their immune system. No differences were observed between groups in terms of total duration, local and systemic AEFI persistence, and types of manifestations (all p > 0.05).

Table 5.
Incidence, duration, and manifestations stratified by clinical history of Herpes Zoster (HZ) infection.
Finally, we confirmed the effects of these variables on the occurrence of AEFIs after the first and second RZV doses by multivariate logistic regression analysis (Table 6). In particular, female sex (p = 0.0415) and the presence of other comorbidities (p = 0.0194), including rheumatological disorders, hematological malignancies, diabetes, or immunological diseases, were significantly associated with the occurrence of AEFIs after the first vaccine dose. Conversely, age (p = 0.0300), clinical history of HZ infection (p = 0.0378), regardless of the number of previous reactivations (p = 0.5100), and the occurrence of AEFIs after the first dose (p = 0.0007) were significantly associated with the development of AEFIs after the second RZV administration.

Table 6.
Multivariate logistic regression analysis.
4. Discussion
Vaccination is the most important preventive measure in public health to reduce infectious disease-related mortality and morbidity worldwide [25]. HZ results from the reactivation of VZV, which is latent in the sensory ganglia after primary infection [26]. Viral reactivations are usually caused by reduced immunosurveillance due to impaired cellular-mediated immunity related to aging, immunosenescence, and immunosuppressive status [27]. Therefore, HZ can frequently occur in frail populations, and its related complications, such as post-herpetic neuralgia, can significantly impact the quality of life of these subjects [28]. The individual lifetime risk of developing HZ increases with age, especially after 50 years of age with two-thirds of all cases, and post-herpetic neuralgia can persist for more than 1 year in 10% of affected subjects, mainly the elderly (60–70% of all cases of post-HZ neuralgia) [29]. Moreover, HZ-related mortality is often under-considered. It poses a public health problem, as in Europe, the median mortality incidence in those aged ≥ 50 years is 0.039 per 100 000, and is particularly higher in females, although in some European Countries, such as Spain, Italy, France, and Denmark, males are more affected [30]. HZ-related mortality also increases with aging, with higher incidence in subjects aged 70–74 years old [27]. Therefore, HZ vaccination can dramatically abolish mortality and morbidity in general and frail populations.
In our previous investigation, we confirmed the efficacy and safety of this novel RZV in a real-life setting, comprising immunocompromised subjects, especially solid organ and hematopoietic stem cell transplant recipients [22]. Indeed, the AEFI occurrence rate is similar to that reported in clinical trials and in immunocompetent subjects, and events are usually of mild or moderate severity, requiring local treatments or nonsteroidal anti-inflammatory drugs [31,32,33]. In this retrospective real-life study, we confirmed and expanded the safety of RZV in a frail population, and we showed that females were more susceptible to developing AEFIs compared to males, especially local symptoms after the first dose and joint and/or muscle pain after the second administration. This higher AEFI incidence in females after RZV is in accordance with published data on other vaccinations, as females have a more active cellular-mediated immune response compared to males, and this is linked to common and/or severe AEFI occurrence, as also documented annually by local and international medicine agencies [10]. For example, in Italy, AEFIs after COVID-19 immunization were predominantly reported by females (76%), as well as in the US (females, 78.7% of total reported side effects, by US-CDC reports) [34]. This discrepancy could be related to an increased attitude of females to report mild to moderate side effects; however, severe adverse reactions, that are principally reported by healthcare professionals, such as anaphylaxis, are more common in females (80% of cases from 1990 to 2016 by CDC reports) [35]. Therefore, our results confirmed the general observations of increased incidence of AEFIs in females after any vaccination [6,9,22,36,37,38,39,40]. For example, females are at an increased risk of injection site and systemic reactions following influenza vaccines compared to males, regardless of age [37], as well as anaphylaxis, occurring in females in up to 83% of cases after various vaccinations [39], or immediate hypersensitivity after administration of the monovalent 2009 pandemic influenza A (H1N1) vaccine, that is four times more common in females, especially in those aged 30–39 years old [40]. Moreover, after at least one dose of the AZD1222 vaccine against SARS-CoV-2, the most common AEFIs are moderate fever (69.4%), muscle aches (68.6%), fatigue/sleepiness (62.5%), body aches (59.4%), headache (58.5%), pain at the injection site (58.3%), and chills (45.7%), and females are more likely to report gastrointestinal symptoms and other AEFIs compared to males [41]. Similarly, in our cohort receiving RZV, the most commonly reported AEFIs were fever, pain at the injection site, and fatigue, especially in females.
Sex-related differences in immune responses are principally caused by sex hormones and genetic/epigenetic mechanisms that modulate immune system composition and functions [12,42,43,44]. For example, seasonal TIV with a half dose of vaccine can induce protective antibody titers in females, with similar levels observed in males after a full dose vaccination [45]. Moreover, mRNA-based vaccines seem to be more effective in males compared to females, although AEFIs can occur more frequently in women [46]. Despite these known differences, design and dose finding studies do not consider sex-related immune system variations. For RZV, in a phase IV study, 4381 adverse events were reported in the first eight months of marketing, with fever (23.6% of cases) and pain at the injection site (22.5%) occurring more frequently [47]. A similar safety profile was also observed in patients with rheumatoid arthritis under biologic agents or JAK inhibitors [48]. However, no results stratified by sex have been reported yet [48]. Here, we showed that females were more likely to report local and systemic AEFIs following the first and second RZV dose compared to males, especially swelling and/or redness at the injection site, fatigue, and joint and/or muscle pain, while males were more likely to report pain at the injection site, especially after the first dose.
Age disparities in AEFI occurrence and severity were reported for influenza and COVID-19 vaccines, with young adults and adults more frequently reporting AEFIs, while older adults aged 65 years or older have a higher incidence of severe side effects and deaths following either quadrivalent virus-like particle vaccination or quadrivalent inactivated vaccination [49,50]. Our results are consistent with age and gender disparities in AEFIs, as our young adults more commonly reported side effects compared to the elderly, especially systemic symptoms. These data emphasized the importance of adopting targeted monitoring strategies by age groups, preparing young individuals for a higher incidence of systemic symptoms, and implementing preventive measures to reduce discomfort in older adults.
Finally, we showed that patients with a clinical history of HZ infection had an increased incidence of AEFIs, particularly after the second dose of the vaccine, likely because of enhanced immune reactivity mediated by antigenic memory stimulated by the vaccine. However, duration and clinical manifestations were similar to those observed in patients without a clinical history of HZ. This observation could be of importance in vulnerable subgroups, such as patients with comorbidities or frailty and a prior HZ infection, to better plan a vaccine schedule, dosage, and monitoring.
Our study has some limitations: (i) the observational nature of the designed investigation and inclusion of a relatively small sample size with some biases related to disease-specific prevalence (e.g., HIV infection was predominant in males, while kidney transplant recipients were females); (ii) a short follow-up for the identification of long-term vaccine-related complications; and (iii) the lack of a control group without comorbidities and other immunocompromised conditions.
5. Conclusions
In conclusion, incidence and characteristics of AEFIs after RZV can be influenced by several factors, such as sex, age, and previous HZ infection. Indeed, sex-related differences in immune responses are caused by hormone effects on immune cell maturation and distribution, favoring innate responses and inflammation in males and adaptive immunity in females. This physiological diversity is associated with a higher risk of severe infectious diseases in males compared to females, while they show a better response to vaccination with a lower incidence of AEFIs. Moreover, age also modifies immune system composition and activation, as immunosenescence frequently occurs with aging, making immune responses less efficient. In our study, we confirmed that sex and age are principal risk factors of AEFIs in females after RZV, and younger subjects have less pronounced immune system activation compared to the elderly. In addition, individuals with a history of HZ infection could exhibit altered immune responses due to prior sensitization, which could influence the severity and frequency of AEFIs. Our study emphasizes the importance of considering sex, age, and clinical history when planning a vaccine schedule, dosage, and monitoring, particularly in vulnerable populations. Differences in incidence and types of AEFIs between sexes and age groups highlight the need to tailor vaccine administration, to optimize its efficacy and minimize discomfort and side effects. A targeted focus on frail populations, for whom currently available data are limited, could significantly contribute to improving vaccination planning and AEFI management. Future studies on larger cohorts stratified by clinical and demographic characteristics will be crucial to confirm our observations and to identify potential predictive factors of AEFI occurrence.
Author Contributions
Conceptualization, M.C.; methodology, M.C., G.M., M.R., S.C. (Salvatore Calabrese), F.C., M.B., W.L., S.C. (Simona Caruccio), C.I., C.G., M.B.M., G.G., B.S., E.A.V. and V.G.; formal analysis, M.C., V.G., G.M., M.R., S.C. (Salvatore Calabrese), F.C., M.B., S.C. (Simona Caruccio), C.I., C.G., G.G., B.S. and M.B.M.; investigation, M.C., M.R., S.C. (Salvatore Calabrese), S.C. (Simona Caruccio), F.C., C.G., M.B., C.I., M.B.M., B.S., G.G. and G.M.; resources, W.L. and E.A.V.; data curation, M.C. and V.G.; writing—original draft preparation, M.C. and V.G.; writing—review and editing, A.F. and F.D.C.; funding acquisition, A.F. and F.D.C.; project administration, A.F. and F.D.C.; supervision, W.L., E.A.V., A.F. and F.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Patients received informed consent obtained in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of “Campania Sud”, Naples, Italy (No. 185 r.p.s.o./2022), 15 December 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within this article.
Acknowledgments
Sincere thanks to M. Tomeo, Department of Medicine and Surgery, University of Salerno, Baronissi Campus, Italy; and P. S. Caiazzo, Risk Management Unit, University Hospital “San Giovanni di Dio e Ruggi d’Aragona”, Salerno, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Costanzo, V.; Roviello, G.N. The Potential Role of Vaccines in Preventing Antimicrobial Resistance (AMR): An Update and Future Perspectives. Vaccines 2023, 11, 333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rappuoli, R.; Pizza, M.; Del Giudice, G.; De Gregorio, E. Vaccines, new opportunities for a new society. Proc. Natl. Acad. Sci. USA 2014, 111, 12288–12293. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Maaten, S.; Upshur, R.E.G.; Patrick, D.M.; Marra, F. The Effect of Universal Influenza Immunization on Antibiotic Prescriptions: An Ecological Study. Clin. Infect. Dis. 2009, 49, 750–756. [Google Scholar] [CrossRef]
- Ozgur, S.K.; Beyazova, U.; Kemaloglu, Y.K.; Maral, I.; Sahin, F.; Camurdan, A.D.; Kizil, Y.; Dinc, E.; Tuzun, H. Effectiveness of Inactivated Influenza Vaccine for Prevention of Otitis Media in Children. Pediatr. Infect. Dis. J. 2006, 25, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iss.it/vaccini/-/asset_publisher/EqhqPGnWTDJo/content/differenze-di-genere-in-risposta-alle-vaccinazioni (accessed on 19 November 2024).
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M. Vaccine immunogenetics: Bedside to bench to population. Vaccine 2008, 26, 6183–6188. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M. Application of pharmacogenomics to vaccines. Pharmacogenomics 2009, 10, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Anticoli, S.; D’Ambrosio, A.; Giordani, L.; Viora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. Dell’istituto Super. Sanita 2016, 52, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.aifa.gov.it/en/-/aifa-pubblica-il-rapporto-vaccini (accessed on 2 January 2025).
- Global Manual on Surveillance of Adverse Events Following Immunization. Available online: https://www.who.int/publications/i/item/9789241507769 (accessed on 24 November 2024).
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M.A. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mittal, S.; Rawat, C.; Gupta, A.; Solanki, H.K.; Singh, R.K. Adverse Events Following Immunization Among Children Under Two Years of Age: A Prospective Observational Study from North India. Cureus 2023, 15, e38356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klein, S.L. Immune cells have sex and so should journal articles. Endocrinology 2012, 153, 2544–2550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Kroehl, M.E.; Johnson, M.J.; Hammes, A.; Reinhold, D.; Lang, N.; Levin, M.J. Comparative Immune Responses to Licensed Herpes Zoster Vaccines. J. Infect. Dis. 2018, 218 (Suppl. S2), S81–S87. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. Npj Vaccines 2024, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.A.; Patel, B.C. Herpes Zoster. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Johnson, R.W. Herpes zoster and postherpetic neuralgia. Expert Rev. Vaccines 2010, 9 (Suppl. S3), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Roustand, L.; Schmidt, A.; Jacquemet, P.; de Wazières, B.; Vabre, C.; Nishimwe, M.; Faure, E. Clinical profile of herpes zoster-related hospitalizations and complications: A French population-based database study. J. Infect. 2024, 89, 106330. [Google Scholar] [CrossRef] [PubMed]
- Giorda, C.B.; Picariello, R.; Tartaglino, B.; Nada, E.; Romeo, F.; Costa, G.; Gnavi, R. Hospitalisation for herpes zoster in people with and without diabetes: A 10-year-observational study. Diabetes Res. Clin. Pract. 2024, 210, 111603. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.; Giudice, V.; Moccia, G.; Longanella, W.; Caruccio, S.; Tremiterra, G.; Sinopoli, P.; Benvenuto, D.; Serio, B.; Malatesta, F.; et al. Safety of Adjuvanted Recombinant Herpes Zoster Virus Vaccination in Fragile Populations: An Observational Real-Life Study. Vaccines 2024, 12, 990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adverse Event Following Immunization. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/aefi/serious-aefi (accessed on 30 November 2024).
- Hartwig, S.; Siegel, J.; Schneider, P.J. Preventability and severity assessment in reporting adverse drug reactions. Am. J. Hosp. Pharm. 1992, 49, 2229–2232. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/health-topics/vaccines-and-immunization?utm_#tab=tab_1 (accessed on 3 January 2025).
- Depledge, D.P.; Sadaoka, T.; Ouwendijk, W.J.D. Molecular Aspects of Varicella-Zoster Virus Latency. Viruses 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bricout, H.; Haugh, M.; Olatunde, O.; Prieto, R.G. Herpes zoster-associated mortality in Europe: A systematic review. BMC Public Health 2015, 15, 466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schutzer-Weissmann, J.; Farquhar-Smith, P. Post-herpetic neuralgia—A review of current management and future directions. Expert Opin. Pharmacother. 2017, 18, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Schmader, K. Herpes zoster in older adults. Clin. Infect. Dis. 2001, 32, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Pinchinat, S.; Cebrián-Cuenca, A.M.; Bricout, H.; Johnson, R.W. Similar herpes zoster incidence across Europe: Results from a systematic literature review. BMC Infect. Dis. 2013, 13, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullane, K.M.; Morrison, V.A.; Camacho, L.H.; Arvin, A.; McNeil, S.A.; Durrand, J.; Campbell, B.; Su, S.C.; Chan, I.S.F.; Parrino, J.; et al. Safety and efficacy of inactivated varicella zoster virus vaccine in immunocompromised patients with malignancies: A two-arm, randomised, double-blind, phase 3 trial. Lancet Infect. Dis. 2019, 19, 1001–1012. [Google Scholar] [CrossRef]
- Stefanizzi, P.; Moscara, L.; Palmieri, C.; Martinelli, A.; Di Lorenzo, A.; Venerito, V.; Germinario, C.A.; Tafuri, S. Safety profile of recombinant adjuvanted anti-herpes zoster vaccine (RZV) in high-risk groups: Data from active surveillance program. Puglia (Italy), 2021–2023. Vaccine 2024, 42, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Zeevaert, R.; Thiry, N.; Maertens de Noordhout, C.; Roberfroid, D. Efficacy and safety of the recombinant zoster vaccine: A systematic review and meta-analysis. Vaccine X 2023, 15, 100397. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/vaccine-safety-systems/monitoring/covid-19.html (accessed on 3 January 2025).
- Su, J.R.; Moro, P.L.; Ng, C.S.; Lewis, P.W.; Said, M.A.; Cano, M.V. Anaphylaxis after vaccination reported to the Vaccine Adverse Event Reporting System, 1990–2016. J. Allergy Clin. Immunol. 2019, 143, 1465–1473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Tadount, F.; Lo, E.; Sadarangani, M.; Wei, S.Q.; Rafferty, E.; Quach, C.; MacDonald, S.E. Sex differences in adverse events following seasonal influenza vaccines: A meta-analysis of randomized controlled trials. J. Epidemiol Community Health 2023, 77, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Stromme, M.; Moyassari, S.; Chadha, A.S.; Tartaglia, M.C.; Szoeke, C.; Ferretti, M.T. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp. Clin. Trials 2022, 115, 106700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampath, V.; Rabinowitz, G.; Shah, M.; Jain, S.; Diamant, Z.; Jesenak, M.; Rabin, R.; Vieths, S.; Agache, I.; Akdis, M.; et al. Vaccines and allergic reactions: The past, the current COVID-19 pandemic, and future perspectives. Allergy 2021, 76, 1640–1660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halsey, N.A.; Griffioen, M.; Dreskin, S.C.; Dekker, C.L.; Wood, R.; Sharma, D.; Jones, J.F.; LaRussa, P.S.; Garner, J.; Berger, M.; et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine 2013, 31, 6107–6112. [Google Scholar] [CrossRef]
- Tran, V.N.; Nguyen, H.A.; Le, T.T.A.; Truong, T.T.; Nguyen, P.T.; Nguyen, T.T.H. Factors influencing adverse events following immunization with AZD1222 in Vietnamese adults during first half of 2021. Vaccine 2021, 39, 6485–6491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosato, E.; Sciarra, F.; Anastasiadou, E.; Lenzi, A.; Venneri, M.A. Revisiting the physiological role of androgens in women. Expert Rev. Endocrinol. Metab. 2022, 17, 547–561. [Google Scholar] [CrossRef]
- Sciarra, F.; Franceschini, E.; Campolo, F.; Gianfrilli, D.; Pallotti, F.; Paoli, D.; Isidori, A.M.; Venneri, M.A. Disruption of Circadian Rhythms: A Crucial Factor in the Etiology of Infertility. Int. J. Mol. Sci. 2020, 21, 3943. [Google Scholar] [CrossRef]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Candore, G. Sex and gender affect immune aging. Front. Aging 2023, 4, 1272118. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.J.; Nelson, M.R.; Klote, M.M.; VanRaden, M.J.; Huang, C.Y.; Cox, N.J.; Klimov, A.; Keitel, W.A.; Nichol, K.L.; Carr, W.W.; et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): Age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008, 168, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Spini, A.; Giudice, V.; Brancaleone, V.; Morgese, M.G.; De Francia, S.; Filippelli, A.; Ruggieri, A.; Ziche, M.; Ortona, E.; Cignarella, A.; et al. Sex-tailored pharmacology and COVID-19: Next steps towards appropriateness and health equity. Pharmacol. Res. 2021, 173, 105848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hesse, E.M. Postlicensure Safety Surveillance of Recombinant Zoster Vaccine (Shingrix)—United States, October 2017–June 2018. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Venerito, V.; Stefanizzi, P.; Cantarini, L.; Lavista, M.; Galeone, M.G.; Di Lorenzo, A.; Iannone, F.; Tafuri, S.; Lopalco, G. Immunogenicity and Safety of Adjuvanted Recombinant Zoster Vaccine in Rheumatoid Arthritis Patients on Anti-Cellular Biologic Agents or JAK Inhibitors: A Prospective Observational Study. Int. J. Mol. Sci. 2023, 24, 6967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, L.A.; Gurtman, A.; van Cleeff, M.; Jansen, K.U.; Jayawardene, D.; Devlin, C.; Scott, D.A.; Emini, E.A.; Gruber, W.C.; Schmoele-Thoma, B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine 2013, 31, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and Gender Disparities in Adverse Events Following COVID-19 Vaccination: Real-World Evidence Based on Big Data for Risk Management. Front. Med. 2021, 8, 700014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).