Quorum Quenching of P. aeruginosa by Portulaca oleracea Methanolic Extract and Its Phytochemical Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Preparation of Methanolic Extract
2.2. Bacterial Samples and Growth Conditions
2.3. Effect of Methanolic Extract on P. aeruginosa
2.3.1. Screening of Antibacterial Activity
2.3.2. Determination of Minimum Inhibitory Concentration (MIC)
2.4. Inhibitory Effects of P. oleracea on the Virulence Factors of P. aeruginosa
2.4.1. Evaluation of P. oleracea Effect on Quorum Sensing
2.4.2. Inhibition of Biofilm Formation
2.4.3. Inhibition of Staphylolytic LasA
2.4.4. Phytochemical Analysis by Gas Chromatography-Mass Spectrometry
2.5. Statistical Analysis
3. Results
3.1. Plant Crude Extract
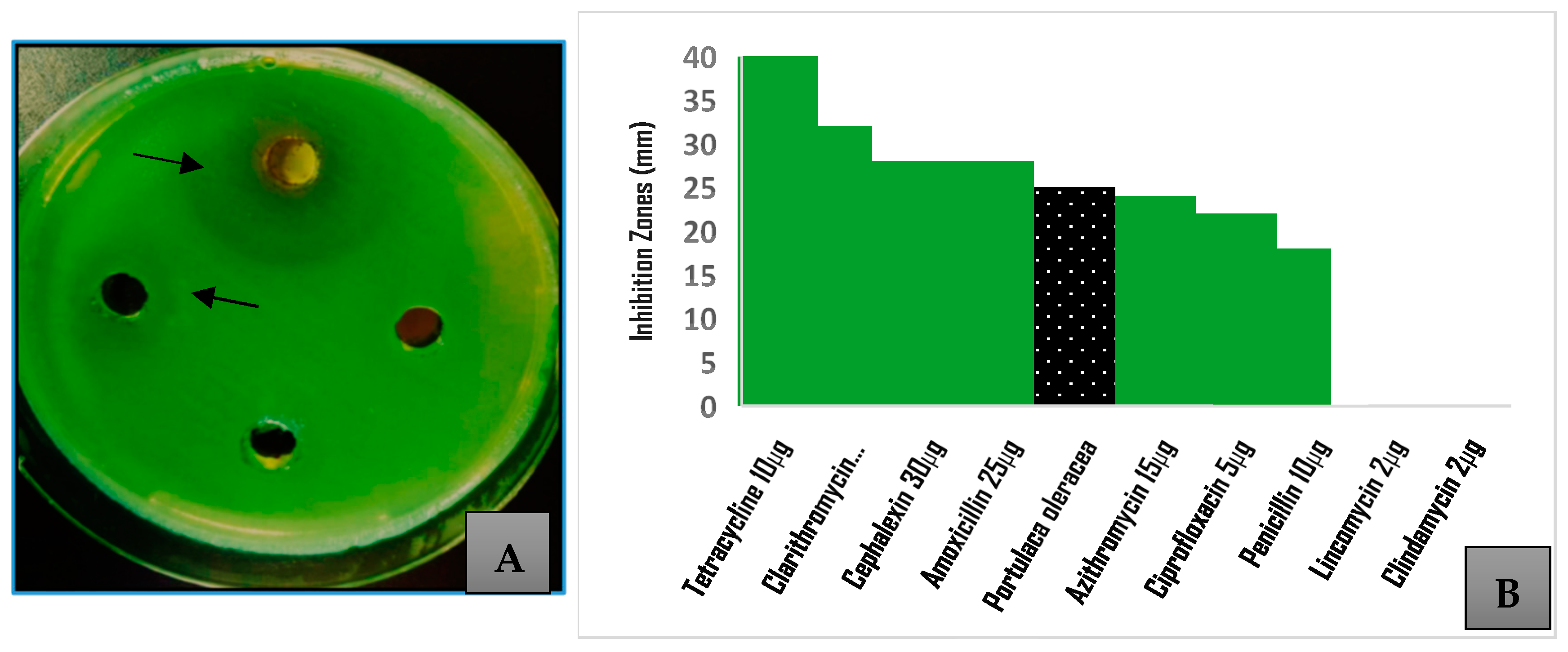
3.2. Effect on Bacterial Growth
3.3. Effects of P. oleracea on the Virulence Factors of P. aeruginosa
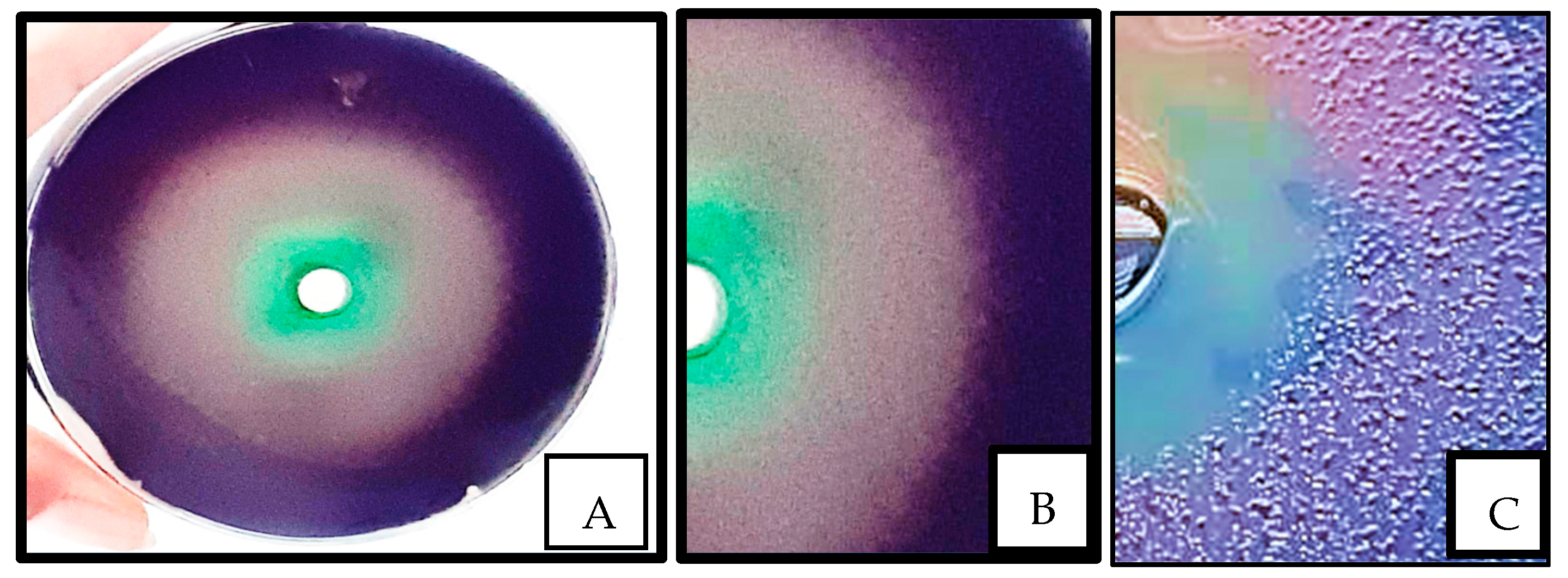
3.3.1. Effect on Quorum Sensing
3.3.2. Biofilm Formation
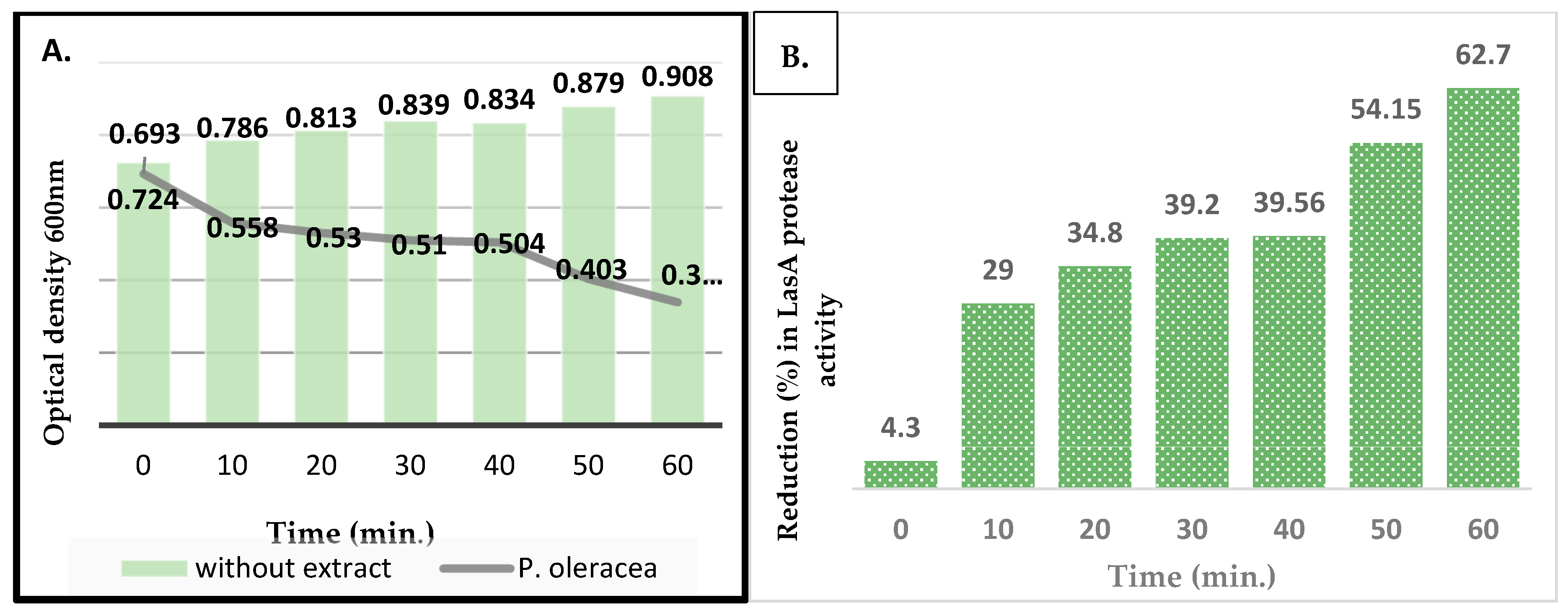
3.3.3. Staphylolytic LasA Inhibition Assay
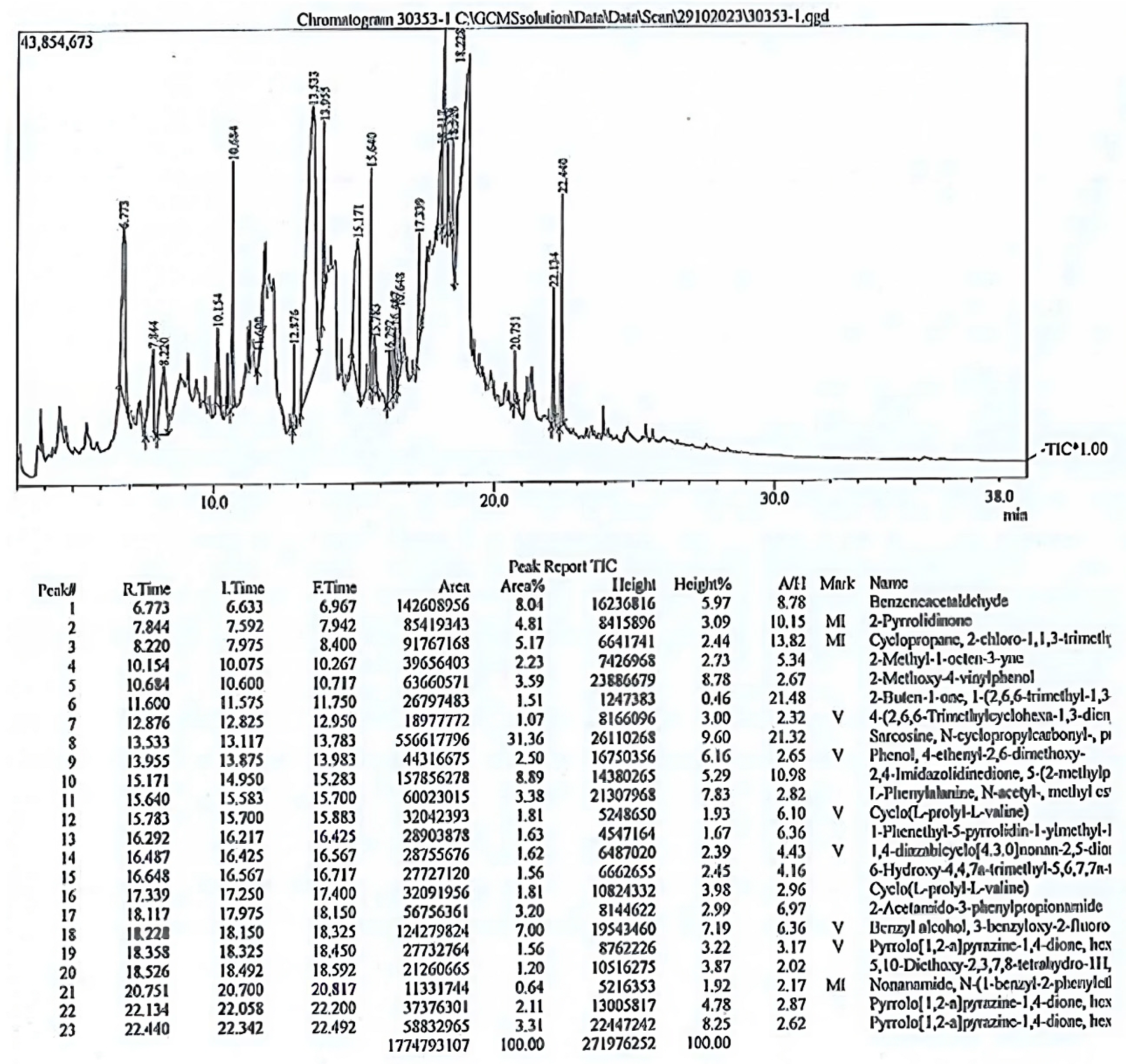
3.4. Phytochemical Analysis by Gas Chromatography-Mass Spectrometry (GC_MS)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Haque, S.; Ahmad, F.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Wahid, M.; Lohani, M.; Khan, S.; Singh, V.; Akhter, N. Developments in Strategies for Quorum Sensing Virulence Factor Inhibition to Combat Bacterial Drug Resistance. Microb. Pathog. 2018, 121, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.A.S.; Bail, L.; Arend, L.N.V.S.; Nogueira, K.D.S.; Tuon, F.F. The Activity of Ceftazidime/Avibactam against Carbapenem-Resistant Pseudomonas aeruginosa. Infect. Dis. 2021, 53, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Litwin, A.; Rojek, S.; Gozdzik, W.; Duszynska, W. Pseudomonas aeruginosa Device Associated—Healthcare Associated Infections and Its Multidrug Resistance at Intensive Care Unit of University Hospital: Polish, 8.5-Year, Prospective, Single-Centre Study. BMC Infect. Dis. 2021, 21. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Bidell, M.R.; Lodise, T.P. Approach to the Treatment of Patients with Serious Multidrug-Resistant Pseudomonas aeruginosa Infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 952–969. [Google Scholar] [CrossRef]
- Rezzoagli, C.; Archetti, M.; Mignot, I.; Baumgartner, M.; Kümmerli, R. Combining Antibiotics with Antivirulence Compounds Can Have Synergistic Effects and Reverse Selection for Antibiotic Resistance in Pseudomonas aeruginosa. PLoS Biol. 2020, 18, e3000805. [Google Scholar] [CrossRef]
- Duplantier, M.; Lohou, E.; Sonnet, P. Quorum Sensing Inhibitors to Quench, P. aeruginosa Pathogenicity. Pharmaceuticals 2021, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; Maeda, T.; Mandujano-Tinoco, E.A.; Tomás, M.; Pérez-Eretza, B.; García-Contreras, S.J.; Wood, T.K.; García-Contreras, R. Role of Quorum Sensing in Bacterial Infections. World J. Clin. Cases 2015, 3, 575. [Google Scholar] [CrossRef]
- Schuster, M.; Joseph Sexton, D.; Diggle, S.P.; Peter Greenberg, E. Acyl-Homoserine Lactone Quorum Sensing: From Evolution to Application. Annu. Rev. Microbiol. 2013, 67, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Abu-Niaaj, L.F.; Al-Daghistani, H.I.; Katampe, I.; Abu-Irmaileh, B.; Bustanji, Y.K. Pomegranate Peel: Bioactivities as Antimicrobial and Cytotoxic Agents. Food Sci. Nutr. 2024, 12, 2818–2832. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Elansary, H.O. Editorial: Bioactive Compounds, Functional Ingredients, Antioxidants, and Health Benefits of Edible Plants. Front. Plant Sci. 2024, 15, 1420069. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Saadat, S.; Khazdair, M.R.; Gholamnezhad, Z.; El-Seedi, H.; Boskabady, M.H. Phytochemical Characteristics and Anti-Inflammatory, Immunoregulatory, and Antioxidant Effects of Portulaca oleracea, L.: A Comprehensive Review. Evid.-Based Complement. Altern. Med. 2023, 2023, 2075444. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghistani, H.I.; Abu-Niaaj, L.F.; Bustanji, Y.; Al-Hamaideh, K.D.; Al-Salamat, H.; Nassar, M.N.; Jaber, H.M.; Amer, N.H.; Abu-Irmaileh, B.; Al-Nuaimi, A.H.D. Antibacterial and Cytotoxicity Evaluation of Arum Hygrophilum Bioss. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7306–7316. [Google Scholar] [CrossRef] [PubMed]
- Abu-Niaaj, L.; Abu-Zarga, M.; Abdalla, S. Isolation and Inhibitory Effects of Eupatilin, a Flavone Isolated from Artemisia monosperma Del., on Rat Isolated Smooth Muscle. Int. J. Pharmacogn. 1996, 34, 134–140. [Google Scholar] [CrossRef]
- Abu-Niaaj, L.; Abu-Zarga, M.; Sabri, S.; Abdalla, S. Isolation and Biological Effects of 7-O-Methyleriodictyol, a Flavanone Isolated from Artemisia monosperma, on Rat Isolated Smooth Muscles. Planta Medica 1993, 59, 42–45. [Google Scholar] [CrossRef]
- Abu-Niaaj, L.; Katampe, I. Isolation and Characterization of Flavones from Artemisia monosperma. Pharmacogn. J. 2018, 10, 1018–1023. [Google Scholar] [CrossRef]
- Qin, T.; Chen, K.; Xi, B.; Pan, L.; Xie, J.; Lu, L.; Li, K. In Vitro Antibiofilm Activity of Resveratrol against Aeromonas hydrophila. Antibiotics 2023, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents from Apocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist. Updates 2020, 51, 100695. [Google Scholar] [CrossRef]
- El-Mahdy, A.M. Effect of Resveratrol on Quorum Sensing and Some Virulence Characteristics of Pseudomonas aeruginosa Isolated from Manoura University Hospitals. J. Microbiol. 2017, 46, 98–111. [Google Scholar]
- Hossain, M.A.; Lee, S.-J.; Park, N.-H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.-W.; Park, S.-C. Impact of Phenolic Compounds in the Acyl Homoserine Lactone-Mediated Quorum Sensing Regulatory Pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal–Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Tao, X.; Ying, X.; Stien, D. Two Amide Glycosides from Portulaca oleracea L. and Its Bioactivities. Nat. Prod. Res. 2019, 35, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Ajam, F.; Rakhshandeh, H.; Askari, V.R. A Pharmacological Review on Portulaca oleracea L.: Focusing on Anti-Inflammatory, Anti-Oxidant, Immuno-Modulatory and Antitumor Activities. J. Pharmacopunct. 2019, 22, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M. Portulaca oleracea L. A Review. J. Pharm. Res. 2011, 4, 3044–3048. [Google Scholar]
- Liu, L.; Howe, P.; Zhou, Y.F.; Xu, Z.Q.; Hocart, C.; Zhan, R. Fatty Acids and Beta-Carotene in Australian Purslane (Portulaca oleracea) Varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tleubayeva, M.I.; Datkhayev, U.M.; Alimzhanova, M.; Ishmuratova, M.Y.; Korotetskaya, N.V.; Abdullabekova, R.M.; Flisyuk, E.V.; Gemejiyeva, N.G. Component Composition and Antimicrobial Activity of CO2 Extract of Portulaca oleracea, Growing in the Territory of Kazakhstan. Sci. World J. 2021, 2021, 5434525. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Kianmehr, M. Anti-Asthmatic Effects of Portulaca oleracea and Its Constituents, a Review. J. Pharmacopunct. 2019, 22, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial Attributes of Apigenin, Isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 175851. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Moniem AE, A.; Al-Quraishy, S.; Saleh, R.A. Antioxidant Effect of Purslane (Portulaca oleracea) and Its Mechanism of Action. J. Med. Plants Res. 2011, 5, 1589–1593. [Google Scholar]
- Bae, J.-H. Antimicrobial Effect of Portulaca oleracea Extracts on Food-Borne Pathogens. Prev. Nutr. Food Sci. 2004, 9, 306–311. [Google Scholar] [CrossRef]
- Alasil, S.M.; Omar, R.; Ismail, S.; Yusof, M.Y. Antibiofilm Activity, Compound Characterization, and Acute Toxicity of Extract from a Novel Bacterial Species of Paenibacillus. Int. J. Microbiol. 2014, 2014, 649420. [Google Scholar] [CrossRef] [PubMed]
- Sylwia, J.; Martin, J. Fast Screening Method for Detection of Acyl-HSL-Degrading Soil Isolates. J. Microbiol. Methods 2004, 57, 415–420. [Google Scholar] [CrossRef]
- Bali, E.B.; Türkmen, K.E.; Erdönmez, D.; Sağlam, N. Comparative Study of Inhibitory Potential of Dietary Phytochemicals against Quorum Sensing Activity of and Biofilm Formation by Chromobacterium Violaceum 12472, and Swimming and Swarming Behaviour of Pseudomonas aeruginosa PAO1. Food Technol. Biotechnol. 2019, 57, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Lewis, J.S., II; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J., Jr.; Limbago, B.; et al. M100 Performance Standards for Antimicrobial Susceptibility Testing a CLSI Supplement for Global Application, 30th ed.; CLSI: Malvern, PA, USA, 2020; Available online: http://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 19 January 2025).
- Joo, H.-S.; Deyrup, S.T.; Shim, S.H. Endophyte-Produced Antimicrobials: A Review of Potential Lead Compounds with a Focus on Quorum-Sensing Disruptors. Phytochem. Rev. 2020, 20, 543–568. [Google Scholar] [CrossRef]
- Zaki, A.A. Assessment of Anti-Quorum Sensing Activity for Some Ornamental and Medicinal Plants Native to Egypt. Sci. Pharm. 2013, 81, 251–258. [Google Scholar] [CrossRef]
- Adeyemo, R.O.; Famuyide, I.M.; Dzoyem, J.P.; Lyndy Joy, M. Anti-Biofilm, Antibacterial, and Anti-Quorum Sensing Activities of Selected South African Plants Traditionally Used to Treat Diarrhoea. Evid. Based Complement. Altern. Med. 2022, 2022, e1307801. [Google Scholar] [CrossRef]
- Fagbemi, K.O.; Aina, D.A.; Adeoye-Isijola, M.O.; Naidoo, K.K.; Coopoosamy, R.M.; Olajuyigbe, O.O. Bioactive Compounds, Antibacterial and Antioxidant Activities of Methanol Extract of Tamarindus Indica Linn. Sci. Rep. 2022, 12, 9432. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Gutwald, R.; Wiedmann-Al-Ahmad, M.; Haberstroh, J.; Obermeyer, J.; Kuenz, A.; Betz, H.; Wolter, F.; Duttenhoefer, F.; Schmelzeisen, R.; et al. Effect of Gabapentin-Lactam and Gamma-Aminobutyric Acid/Lactam Analogs on Proliferation and Phenotype of Ovine Mesenchymal Stem Cells. Int. J. Oral Maxillofac. Implant. 2013, 28, e230–e238. [Google Scholar] [CrossRef] [PubMed]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.-R.; Yoo, D.; Wang, M.-H.; Oh, D.-H. Bioactive Potential of 2-Methoxy-4-Vinylphenol and Benzofuran from Brassica oleracea L. Var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods 2020, 9, 568. [Google Scholar] [CrossRef]
- Singh, N.; Mansoori, A.; Jiwani, G.; Solanke, A.U.; Thakur, T.K.; Kumar, R.; Chaurasiya, M.; Kumar, A. Antioxidant and Antimicrobial Study of Schefflera vinosa Leaves Crude Extracts against Rice Pathogens. Arab. J. Chem. 2021, 14, 103243. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Klimo, K.; Hümmer, W.; Hölzer, J.; Petermann, A.; Garreta-Rufas, A.; Böhmer, F.D.; Schreier, P. Identification of 3-Hydroxy-β-Damascone and Related Carotenoid-Derived Aroma Compounds as Novel Potent Inducers of Nrf2-Mediated Phase 2 Response with Concomitant Anti-Inflammatory Activity. Mol. Nutr. Food Res. 2009, 53, 1237–1244. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Antispasmodic Activity of β-Damascenone and E-Phytol Isolated from Ipomoea Pes-Caprae. Planta Medica 1992, 58, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Cernel, N.; Zitka, O.; Skalickova, S.; Gumulec, J.; Sztalmachova, M.; Rodrigo, M.A.; Socher, M.; Masarik, J.; Adam, M.; Hubalek, V.; et al. Effect of Sarcosine on Antioxidant Parameters and Metallothionein Content in the PC-3 Prostate Cancer Cell Line. Oncol. Rep. 2013, 29, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Bouali, N.; Ahmad, I.; Patel, H.; Alhejaili, E.B.; Hamadou, W.S.; Badraoui, R.; Lajimi, R.H.; Alreshidi, M.; Siddiqui, A.J.; Adnan, M.; et al. GC–MS Screening of the Phytochemical Composition of Ziziphus Honey: ADME Properties and in Vitro/in Silico Study of Its Antimicrobial Activity. J. Biomol. Struct. Dyn. 2023, 42, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Y.; Selzer, M.E.; Pincus, J.H. Phenytoin: Mechanisms of Its Anticonvulsant Action. Ann. Neurol. 1986, 20, 171–184. [Google Scholar] [CrossRef]
- Dahiya, R.; Kumar, A.; Yadav, R. Synthesis and Biological Activity of Peptide Derivatives of Iodoquinazolinones/Nitroimidazoles. Molecules 2008, 13, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tafolla, L.; Padrón, J.M.; Mendoza, G.; Luna-Rodríguez, M.; Fernández, J.J.; Norte, M.; Trigos, Á. Antiproliferative Activity of Biomass Extract from Pseudomonas cedrina. Electron. J. Biotechnol. 2019, 40, 40–44. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kumar, A.; Singh, U.K. Tetrazoles: Synthesis and Biological Activity. Immunol. Endocr. Metab. Agents Med. Chem. 2018, 18, 3–21. [Google Scholar] [CrossRef]
- Dhanabalan, S.; Muthusamy, K.; Iruthayasamy, J.; Kumaresan, P.V.; Ravikumar, C.; Kandasamy, R.; Natesan, S.; Periyannan, S. Unleashing Bacillus Species as Versatile Antagonists: Harnessing the Biocontrol Potentials of the Plant Growth-Promoting Rhizobacteria to Combat Macrophomina phaseolina Infection in Gloriosa superba. Microbiol. Res. 2024, 283, 127678. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.K.A.; Kim, S.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and Isolated 6-Hydroxy-4,4,7a-Trimethyl-5,6,7,7a-Tetrahydrobenzofuran-2(4H)-One (HTT); LPS-Induced Inflammation Attenuation via Suppressing NF-ΚB, MAPK and Oxidative Stress through Nrf2/HO-1 Pathways in RAW 264.7 Macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Sulaiman, M.; Hassan, Y.; Taskin Tok, T.; Noundow, X.S. Synthesis, Antibacterial Activity and Docking Studies of Benzyl Alcohol Derivatives. J. Turk. Chem. Soc. Sect. A Chem. 2020, 7, 481–488. [Google Scholar] [CrossRef]
- Dash, S.; Jin, C.; Lee, O.O.; Xu, Y.; Qian, P.-Y. Antibacterial and Antilarval-Settlement Potential and Metabolite Profiles of Novel Sponge-Associated Marine Bacteria. J. Ind. Microbiol. Biotechnol. 2009, 36, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.I.; Babiker, E.H.; Saeed, H.A. Streptomyces: Isolation, Optimization of Culture Conditions and Extraction of Secondary Metabolites. Int. Curr. Pharm. J. 2016, 5, 27–32. [Google Scholar] [CrossRef]
- Manimaran, M.; Kannabiran, K. Marine Streptomyces Sp. VITMK1 Derived Pyrrolo [1, 2-A] Pyrazine-1, 4-Dione, Hexahydro-3-(2-Methylpropyl) and Its Free Radical Scavenging Activity. Open Bioact. Compd. J. 2017, 5, 23–30. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An Antibiotic Agent Pyrrolo [1,2-a]Pyrazine-1,4-Dione,Hexahydro Isolated from a Marine Bacteria Bacillus tequilensis MSI45 Effectively Controls Multi-Drug Resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef]
- Gellatly, S.L.; Hancock, R.E.W. Pseudomonas aeruginosa: New Insights into Pathogenesis and Host Defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef]
- Hemmati, J.; Nazari, M.; Abolhasani, F.S.; Ahmadi, A.; Asghari, B. In Vitro Investigation of Relationship between Quorum-Sensing System Genes, Biofilm Forming Ability, and Drug Resistance in Clinical Isolates of Pseudomonas aeruginosa. BMC Microbiol. 2024, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Heyndrickx, M.; Flint, S.; Liu, W.; Chen, W.; Zhao, J.; Zhang, H. Community-Wide Changes Reflecting Bacterial Interspecific Interactions in Multispecies Biofilms. Crit. Rev. Microbiol. 2021, 47, 338–358. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Rollet, C.; Gal, L.; Guzzo, J. Biofilm-Detached Cells, a Transition from a Sessile to a Planktonic Phenotype: A Comparative Study of Adhesion and Physiological Characteristics in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2008, 290, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Qaralleh, H.; Saghir SA, M.; Al-Limoun, M.O.; Dmor, S.M.; Khleifat, K.; Al-Ahmad, B.E.M.; Al-Omari, L.; Tabana, Y.; Mothana, R.A.; Al-Yousef, H.; et al. Effect of Matricaria aurea Essential Oils on Biofilm Development, Virulence Factors and Quorum Sensing-Dependent Genes of Pseudomonas aeruginosa. Pharmaceuticals 2024, 17, 386. [Google Scholar] [CrossRef] [PubMed]
- Naga, N.G.; El-Badan, D.E.; Ghanem, K.M.; Shaaban, M.I. It Is the Time for Quorum Sensing Inhibition as Alternative Strategy of Antimicrobial Therapy. Cell Commun. Signal. 2023, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Al Qaisi, Y.T.; Khleifat, K.M.; Oran, S.A.; Al Tarawneh, A.A.; Qaralleh, H.; Al-Qaisi, T.S.; Farah, H.S. Ruta graveolens, Peganum harmala, and Citrullus colocynthis Methanolic Extracts Have in Vitro Protoscolocidal Effects and Act against Bacteria Isolated from Echinococcal Hydatid Cyst Fluid. Arch. Microbiol. 2022, 204, 228. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.u.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Santos, C.A.; Almeida, F.A.; Quecán, B.X.V.; Pereira, P.A.P.; Gandra, K.M.B.; Cunha, L.R.; Pinto, U.M. Bioactive Properties of Syzygium cumini (L.) Skeels Pulp and Seed Phenolic Extracts. Front. Microbiol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of Biofilm Formation and Quorum Sensing Mediated Phenotypes by Berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef] [PubMed]
- Quecán, B.X.V.; Rivera, M.L.C.; Pinto, U.M. Bioactive Phytochemicals Targeting Microbial Activities Mediated by Quorum Sensing. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 397–416. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J.C. Dietary Phytochemicals as Quorum Sensing Inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Liu, G.; Liu, A.; Yang, C.; Zhou, C.; Zhou, Q.; Li, H.; Yang, H.; Mo, J.; Zhang, Z.; Li, G.; et al. Portulaca oleracea L. Organic Acid. Extract Inhibits Persistent Methicillin-Resistant Staphylococcus aureus in Vitro and in Vivo. Front. Microbiol. 2023, 13, 1076154. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A Review on Bioactive Phytochemicals and Ethnopharmacological Potential of Purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- OECD. Test No.423: Acute Oral Toxicity-Acute Toxic Class Method; Guidelines for Testing of Chemicals; OECD: Paris, France, 2011. [Google Scholar]
- Desta, Z.Y.; Cherie, D.A. Determination of Antioxidant and Antimicrobial Activities of the Extracts of Aerial Parts of Portulaca quadrifida. Chem. Cent. J. 2018, 12, 146. [Google Scholar] [CrossRef]
- Keser, F.; Karatepe, M.; Keser, S.; Tekin, S.; Türkoğlu, İ.; Kaygili, O.; Demir, E.; Ökkeş, Y.; Sandal, S.; Kırbag, S. In Vitro Biological Activities and Phytochemical Contents of Portulaca oleracea L. (Purslane). J. Phys. Chem. Funct. Mater. 2021, 4, 1–7. [Google Scholar]
- Londonkar, R.; Kamble, A. Hepatotoxic and Invivo Antioxidant Potential of Pandanus odoratissimus against Carbon Tetrachloride Induced Liver Injury in Rats. Orient. Pharm. Exp. Med. 2011, 11, 229–234. [Google Scholar] [CrossRef]
- Luo, J.; Kong, J.; Dong, B.; Huang, H.; Wang, K.; Hou, C.; Liang, Y.; Li, B.; Chen, Y.; Wu, L. Baicalein Attenuates the Quorum Sensing-Controlled Virulence Factors of Pseudomonas aeruginosa and Relieves the Inflammatory Response in P. aeruginosa-Infected Macrophages by Downregulating the MAPK and NFκB Signal-Transduction Pathways. Drug Des. Dev. Ther. 2016, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Mishra, A.; Jha, B. 3-Benzyl-Hexahydro-Pyrrolo [1,2-a]Pyrazine-1,4-Dione Extracted from Exiguobacterium indicum Showed Anti-Biofilm Activity against Pseudomonas aeruginosa by Attenuating Quorum Sensing. Front. Microbiol. 2019, 10, 1269. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Makarov, V.; Brackman, G.; Israyilova, A.; Azzalin, A.; Forneris, F.; Riabova, O.; Savina, S.; Coenye, T.; et al. Discovery of New Diketopiperazines Inhibiting Burkholderia cenocepacia Quorum Sensing in Vitro and in Vivo. Sci. Rep. 2016, 6, 32487. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A Diketopiperazine Factor from Rheinheimera aquimaris QSI02 Exhibits Anti-Quorum Sensing Activity. Sci. Rep. 2016, 6, 39637. [Google Scholar] [CrossRef] [PubMed]
- Saeid, H.; Al-sayed, H.; Bader, M. A Review on Biological and Medicinal Significance of Furan. AlQalam J. Med. Appl. Sci. 2023, 6, 44–58. [Google Scholar]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Karatuna, O.; Yagci, A. Analysis of Quorum Sensing-Dependent Virulence Factor Production and Its Relationship with Antimicrobial Susceptibility in Pseudomonas aeruginosa Respiratory Isolates. Clin. Microbiol. Infect. 2010, 16, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-Sensing. Z. Naturforsch. C 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from Infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Yin, H.; Deng, Y.; Wang, H.; Liu, W.; Zhuang, X.; Chu, W. Tea Polyphenols as an Antivirulence Compound Disrupt Quorum-Sensing Regulated Pathogenicity of Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 16158. [Google Scholar] [CrossRef]
- Mohamed, B.; Abdel-Samii, Z.K.; Abdel-Aal, E.H.; Abbas, H.A.; Shaldam, M.A.; Ghanim, A.M. Synthesis of Imidazolidine-2,4-Dione and 2-Thioxoimidazolidin-4-One Derivatives as Inhibitors of Virulence Factors Production in Pseudomonas aeruginosa. Arch. Pharm. 2020, 353, e1900352. [Google Scholar] [CrossRef]

| Peak No. | R-Time (min) | Area (%) | Identification of Compound | Molecular Weight (g/mol) | Chemical Class | Formula | Activities | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.773 | 8.04 | Benzene acetaldehyde | 120.15 | Aldehyde | C8H8O | Antioxidant, mutagenic, antimicrobial | [41] |
| 2 | 7.844 | 4.81 | 2-pyrrolidinone | 85.10 | Lactam | C4H7NO | Induces cell line proliferation | [42] |
| 3 | 8.220 | 5.17 | Cyclopropane,2-chloro-1,1,3-trimethyl | 118.60 | Alkyl halide | C6H11Cl | ||
| 4 | 10.154 | 2.23 | 2-Methyl-1-octen-3-yne | 122.2 | Alkyne | C9H14 | ||
| 5 | 10.684 | 3.59 | 2-Methoxy-4-vinylphenol | 115 | Phenol | C9H10O2 | Antimicrobial, antioxidant, anti-inflammatory, analgesic, antigermination, antiproliferative | [43,44] |
| 6 | 11.600 | 1.51 | Trans-beta-Damascenone | 190.28 | Ketone | C13H18O | Anti-inflammatory, anticancer antispasmodic activity | [45,46] |
| 7 | 12.87 | 1.07 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl) but-3-en-2-one) | 190.28 | Ketone | C13H18O | Antioxidant | [44] |
| 8 | 13.533 | 31.36 | Sacrosine, N-cyclopropylcarbonyl-, propyl ester | 199 | Ester | C10H17NO3 | Reduces cell viability | [47] |
| 9 | 13.955 | 2.5 | 2,6-Dimethoxy-4-ethyl-phenol | 180 | Phenol | C10H12O3 | Antioxidant capacity | |
| 10 | 15.171 | 8.89 | 2,4-Imidazolidinedione,5-(2-methlypropyl) | 156 | Imidazolidine | C7H12N2O2 | Antimicrobial, anticonvulsant | [48,49] |
| 11 | 15.640 | 3.38 | N-acetyl-3-phenylalanine methyl ester | 221 | Ester | C12H15NO3 | Antimicrobial | [50] |
| 12 | 15.783 | 1.81 | Cyclo(L-prolyl-L-valine) | 196 | Diketopiperazine | C10H16N2O2 | Antiproliferative activity | [51] |
| 13 | 16.292 | 1.63 | 1-Phenyethyl-5-pyrrolidin-l-ylmethyl-1H-tetrazole | 257 | Tetrazole | C14H19N5 | Anti-inflammatory, antidiabetic, anticancer, antibacterial activity | [52] |
| 14 | 16.487 | 1.62 | 1,4-diazabicyclo [4.3.0]nonan-2,5-dione,3-methyl | 168 | Pyrimidine | C8H12N2O2 | Antifungal, antimicrobial activity | [53] |
| 15 | 16.648 | 1.56 | 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT) | 196 | Furan | C11H16O3 | Anti-inflammatory | [54] |
| 16 | 17.339 | 1.81 | Cyclo(L-prolyl-L-valine) | 196 | Diketopiperazine | C10H16N2O2 | Antiproliferative activity | [51] |
| 17 | 18.117 | 3.20 | 2-Acetamido-3-phenylpropionamide | 206 | Phenylpropanamide | C16H14N2O2 | ||
| 18 | 18.228 | 7.00 | Benzyl alcohol, 3-benzyloxy-2-flouro | 232 | Alcohol | C14H13FO2 | Antibacterial | [51] |
| 19 | 18.358 | 1.56 | Pyrrolo [1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) | 210 | Diketopiperazine | C11H18N2O2 | Antioxidant, antifungal | [55] |
| 20 | 18.526 | 1.2 | 5,10-Diethoxy-2,3,7,8 tetrahydro-1H,6H-dipyrrolo [1,2-a:1′,2′-d] pyrazine | 250 | Pyrazine | C14H22N2O2 | Antimicrobial | [56] |
| 21 | 20.751 | 0.64 | Nonanamide, N-(1-benzyl, 2-phenylethyl) | 351 | Nonanamide | C24H33NO | Antimicrobial | [57] |
| 22 | 22.134 | 2.11 | Pyrrolo [1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) | 244 | Pyrazine | C14H16N2O2 | Antioxidant, antibacterial | [58,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Daghistani, H.I.; Matalqah, S.M.; Shadid, K.A.; Abu-Niaaj, L.F.; Zein, S.; Abo-Ali, R.M. Quorum Quenching of P. aeruginosa by Portulaca oleracea Methanolic Extract and Its Phytochemical Profile. Pathogens 2025, 14, 163. https://doi.org/10.3390/pathogens14020163
Al-Daghistani HI, Matalqah SM, Shadid KA, Abu-Niaaj LF, Zein S, Abo-Ali RM. Quorum Quenching of P. aeruginosa by Portulaca oleracea Methanolic Extract and Its Phytochemical Profile. Pathogens. 2025; 14(2):163. https://doi.org/10.3390/pathogens14020163
Chicago/Turabian StyleAl-Daghistani, Hala I., Sina M. Matalqah, Khalid A. Shadid, Lubna F. Abu-Niaaj, Sima Zein, and Raeda M. Abo-Ali. 2025. "Quorum Quenching of P. aeruginosa by Portulaca oleracea Methanolic Extract and Its Phytochemical Profile" Pathogens 14, no. 2: 163. https://doi.org/10.3390/pathogens14020163
APA StyleAl-Daghistani, H. I., Matalqah, S. M., Shadid, K. A., Abu-Niaaj, L. F., Zein, S., & Abo-Ali, R. M. (2025). Quorum Quenching of P. aeruginosa by Portulaca oleracea Methanolic Extract and Its Phytochemical Profile. Pathogens, 14(2), 163. https://doi.org/10.3390/pathogens14020163






