Abstract
Quorum sensing (QS) is a molecular communication mechanism among bacterial cells. It is critical in regulating virulence factors, motility, antibiotic resistance, and biofilm formation. Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen linked to healthcare-associated infections, food poisoning, and biofilm formation. Treating infections caused by pathogenic bacteria has become a challenge due to the development of multi-antibiotic resistance upon continuous exposure of bacteria to antibiotics. An alternative strategy to conventional antimicrobials to decrease the bacterial pathogenicity is QS inhibition, also known as quorum quenching. Using plant-derived compounds is an environmentally friendly strategy to block the bacterial QS and inhibit bacterial growth. Portulaca oleracea is a popular plant in different countries and is also used in traditional medicine. It is widely consumed raw in salads and as garnishes, though it can be cooked as a vegetarian dish. This study evaluates the antimicrobial activity of the methanolic extract of P. oleracea and its effectiveness in blocking or attenuating the QS of P. aeruginosa. The agar well diffusion method used for screening the antibacterial activity showed a significant growth inhibition of P. aeruginosa by the extract at 500 mg/mL with a minimum inhibitory concentration of 31.25 mg/mL. A bioindicator bacterium, Chromobacterium violaceum CV026, was used to determine the effect of the methanolic extract on the QS of P. aeruginosa. The results indicated a significant reduction in biofilm formation, pyocyanin production, and LasA staphylolytic activity. The phytochemical analysis by Gas Chromatography–Mass Spectrometry showed that the methanolic extract contained several phenols, alkaloids, esters, and other compounds previously reported to have antibacterial and antioxidant effects. These findings highlight the effectiveness of P. oleracea methanolic extract in attenuating the QS and virulence factors of P. aeruginosa. This study suggests that P. oleracea is an important source of natural antimicrobials and its use would be beneficial in food and pharmaceutical applications.
1. Introduction
Quorum sensing (QS) is a molecular communication mechanism among bacterial cells. It is a cell density-based intercellular communication system that regulates the expression of bacterial virulence factors, motility, and biofilm formation [1]. An innovative strategy to decrease the level of bacterial pathogenicity is to inhibit QS, which is known as quorum quenching [2]. Pseudomonas aeruginosa is an opportunistic pathogen linked to conjunctivitis, otitis media, and other healthcare-acquired infections such as ventilator-associated pneumonia, post-surgical infection, and burn-wound contamination [3,4]. Treatment can be challenging due to Pseudomonas’ adaptation to environmental changes, which leads to the development of multi-antibiotic resistance, biofilm formation, and modification of the gene expression of several virulence factors regulated by QS [5]. The virulence factors are mainly exotoxin A, glycolipid biosurfactant (rhamnolipids), siderophores, pigments, and some critical enzymes such as elastase, alkaline protease, and lipases [6,7]. Studies have indicated that the QS regulatory network reacts to environmental stress in bacterial populations. It relies on the secretion and perception of small autoinducer signaling molecules known as acyl homoserine lactones (AHLs), which stimulate bacterial cells to sense and regulate the expression of virulence factors [8,9]. The QS network of P. aeruginosa is a multi-layered hierarchy consisting of three main interconnected signaling mechanisms: lasA, rhl, and pqs [1]. The coordinated expression of these genes is triggered when the level of AHLs is above a threshold concentration, activating specific transcription factors [10]. Quorum quenching and interference with the QS system are novel eco-friendly strategies to prevent antibiotic resistance by preventing microbial growth without causing bacterial stress from antibiotic exposure.
Plants and herbs are rich in secondary metabolites, which possess a wide range of physiological activities, including antimicrobial, anticancer, antioxidant, and antispasmodic effects [11,12,13,14,15,16]. These bioactivities are due to different categories of natural compounds, such as polyphenols, flavones, quinones, terpenoids, alkaloids, and volatile oils [13,14,15,16,17]. Many plant extracts have been reported to inhibit microbial growth, interfere with quorum sensing, and prevent biofilm formation in Gram-negative bacteria, including P. aeruginosa [11,18,19,20]. For example, acyl homoserine lactones (AHLs) interfere with the autoinducer receptors in bacteria and disrupt cell signaling [21,22]. Studies have suggested that the bioactivity of some natural compounds is due to their structural similarity to cellular enzymes or their substrates, making them function as analogs to such molecules [15].
Portulaca oleracea L. (Purslane) is eaten fresh or cooked for its nutritional value, and it is commonly used to treat multiple microbial infections. Chemical studies have indicated that Purslane contains polyphenols, alkaloids, α-tocopherol, ascorbic acid, omega-3 fatty acids, β-carotene, and glutathione, in addition to a high content of minerals such as calcium, iron, zinc, magnesium, and potassium [13,23,24,25,26,27]. Several natural products have shown anti-inflammatory, antioxidant, anticancer, hypocholesterolemia, and bronchodilator properties [24,28,29,30,31,32,33]. However, to the authors’ knowledge, no study has yet identified the mechanism for such activities. This study evaluates the effect of the methanolic extract of P. oleracea on QS by P. aeruginosa cells and its influence on the biofilm formation and expression of other virulence factors of this bacterium.
2. Materials and Methods
All chemicals were purchased from Thermo Fisher Scientific (Waltham, MA, USA), unless mentioned otherwise.
2.1. Plant Collection and Preparation of Methanolic Extract
Portulaca oleracea was purchased from local stores in Amman, Jordan. The plant was authenticated by the National Agricultural Research Center in Jordan. The leaves were collected and thoroughly washed with distilled water to remove impurities. They were then air-dried at room temperature, ground in an electric grinder, and stored in an airtight container until needed. A methanolic extract was prepared by soaking 500 g of ground material in 1 L of methanol at room temperature with continuous agitation for three days (Figure 1). The mixture was then filtered using Whatman No. 1 filter paper to remove larger particles, and the filtrate was concentrated through vacuum evaporation at 45 °C to yield a dry material referred to as the “crude”. For antimicrobial activity screening, a stock solution of the crude was prepared at a concentration of 500 mg/mL by dissolving the desired amount of the dry material in a physiological buffer solution (PBS). The serial solutions used in this study were prepared using this stock solution.

Figure 1.
Portulaca oleracea (Purslane) (A); air-dried plant (B); leaves soaked in methanol (C).
2.2. Bacterial Samples and Growth Conditions
The antibacterial activity of P. oleracea and its antibiofilm effectiveness were evaluated against Pseudomonas aeruginosa (ATCC27853) (American Type Culture Collection, Manassas, VA, USA). The effect of the extract on QS was assessed on a clinical isolate of P. aeruginosa collected from sputum for another study, and the concentration of its produced pyocyanin was 36.448 (μg/mL) (IRB # BAU/24/11/2022-2023). Staphylococcus aureus (ATCC 25923) was used as a reference organism in the LasA assay. Upon use, a bacterial inoculum in LB broth was incubated overnight at 30 °C [34]. The biosensor strain used was Chromobacterium violaceum CV026 (Carolina Biological Supplies, Burlington, NC, USA), a Tn5 mutant strain derived from wild-type C. violaceum (CV31532). This mutant bacterium cannot produce N-acyl homoserine lactones (AHLs), a class of small signaling molecules involved in bacterial quorum sensing, however, it remains responsive to exogenous AHLs, such as N-hexanoyl-L-homoserine lactone (C6-AHL) and N-butanoyl-L-homoserine lactone (C4-HSL) [22]. It produces a purple-violet pigment called violacein, which indicates cellular communications [35]. AHLs are signaling molecules involved in bacterial quorum sensing by regulating gene expression in Gram-negative bacteria. For use, C. violaceum was inoculated in LB containing 1% tryptone, 0.5% yeast extract, and 1% NaCl and incubated at 30 °C for 48 h [36], and the optical density at OD600 was adjusted to 0.1–0.2 (equivalent to 0.5 McFarland, which represents 1.5 × 108 CFU/mL).
2.3. Effect of Methanolic Extract on P. aeruginosa
2.3.1. Screening of Antibacterial Activity
The inhibitory effect of the P. oleracea methanolic extract against P. aeruginosa was assessed according to the guidelines of the Clinical and Laboratory Standards Institute [37].
A solution of the crude extract at a concentration of 500 mg/mL was used for the evaluation, and the effect was compared to standard antibiotics. Mueller–Hinton (MH) agar plates were used, and 8 mm diameter wells were made in the agar plates using a sterile borer. In the MH broth, a bacterial inoculum was grown overnight at 37 °C, and the OD600 was standardized to 0.1. A sterile cotton applicator was immersed in the standardized culture and swabbed uniformly on the MH agar plates. Then, 125 μL of the stock solution of the plant extract was placed in a well, while the PBS was placed in a control well because it was the solution used to dissolve the crude. Plates were incubated at 37 °C for 24 h, and the diameters of inhibition zones were measured in millimeters (mm). The inhibition was reported as an average reading of three replicates. The inhibition of bacterial growth was compared to that caused by standard antibiotics. For the comparison study, disks of the selected antibiotics were distributed on the surfaces of agar plates swabbed with P. aeruginosa and incubated overnight at 37 °C before measuring the diameters of inhibition zones in mm. The antibiotic disks contained penicillin G (10 μg), clarithromycin (15 μg), ciprofloxacin (5 μg), lincomycin (2 μg), cephalexin (30 μg), amoxicillin (25 μg), tetracycline (10 μg), azithromycin (15 μg), and clindamycin (2 μg).
2.3.2. Determination of Minimum Inhibitory Concentration (MIC)
The MIC of the P. oleracea crude (dry) of the methanolic extract was determined using a series of six (6) two-fold dilutions (250, 125, 62.5, 31.25, 15.62, and 7.81 mg/mL) prepared from the 500 mg/mL stock; then, they were filtered through 0.45 µm filters. In the MH agar, wells of 8 mm were made; a well for each dilution plus a well for the control. A standardized bacterial inoculum was spread on the agar surface, and 125 μL of each dilution or the control solution (PBS) was placed in the wells. The agar plates were incubated at 37 °C overnight before measuring the inhibition zones in mm.
2.4. Inhibitory Effects of P. oleracea on the Virulence Factors of P. aeruginosa
For the assays below, the sub-MIC was used, and this concentration is defined as the extract’s concentration before the MIC that allowed for bacterial growth. The growth of P. aeruginosa was confirmed by incubating a bacterial inoculum in MH broth to grow overnight at 37 °C, while the absorbance at OD600 was measured every 2 h.
2.4.1. Evaluation of P. oleracea Effect on Quorum Sensing
To determine the potential of the methanolic extract as an inhibitor for quorum sensing in P. aeruginosa, the mutant bacterium C. violaceum CV026 was used as a biosensor for cell density, indicating QS [34]. This bacterium secretes a purple pigment called violacein, but only when exposed to exogenous 3-oxo-C6-HSL and short-chain AHLs [38]. Thus, a reduction in violacein production indicates an interruption of quorum sensing or complete quorum quenching. The assay was performed by topping the MH agar plates with a thin-layer of agar containing C. violaceum prepared by adding 10 mL of a culture of this bacterium to 200 mL of MH semisolid agar supplemented with 2 mL of 10 μM synthetic AHL called Acetyl-L-homoserine lactone (CAS 51524-71-1) from Santa Cruz Biotechnology, Inc. (Dallas, Texas, U.S.A.). The agar mixture was poured onto pre-warmed MH agar plates and left to solidify at room temperature before making 8 mm wells in the agar. The wells contained either 125 μL of sub-MIC of plant extract or a control. In addition to PBS as a control, Furanone or methanol were used as positive and negative controls, respectively. Inhibitory zones smaller than 10 mm indicated moderate activity, while zones bigger than 10 mm indicated potent activity [39]. The assay was also performed by placing 125 μL tetracycline (10 µg) in a well as a control to evaluate the antibiotic effect on QS activity.
2.4.2. Inhibition of Biofilm Formation
The effectiveness of the plant extract as an inhibitor for biofilm formation was evaluated according to Adeyemo et al., with modifications [40]. In a microtiter plate, a culture of P. aeruginosa was grown in Tryptic Soy broth at 37 °C overnight. The plate was centrifuged at 4500 rpm for 15 min and washed twice with PBS; then, the bacterial cells were resuspended in LB broth to obtain an OD600 of 0.1–0.2. To each well in a 96-microtiter plate, 180 μL of sterile LB broth was added; then, 150 μL of standardized bacterial culture (approximately 1.5 × 108 CFU/mL) was added and mixed well. A 50 µL sub-MIC solution was added making the total volume 380 μL per well, while the control well had 50 µL of PBS (the solution used to prepare the sub-MIC). Biofilm formation was initiated by incubating the 96-well plate at 37 °C overnight, and then the cell density was determined quantitatively using a crystal violet stain. The plate was washed gently three times with sterile distilled water and dried. Following this, 200 μL of 0.2% crystal violet was added to each well, and the microtiter plate was incubated at room temperature for 15 min. The plate was washed to remove the excess stain, and 100 μL of 95% ethanol was added and mixed before reading the absorbance at 570 nm. The experiment was performed in triplicate, and data were presented as averages. The percentage of biofilm inhibition was calculated using the following equation:
Inhibition of Biofilm Formation (%) = [(OD570 of Control − OD570 of Treated wells)/OD570 of Control] × 100
2.4.3. Inhibition of Staphylolytic LasA
The assessment of the effect of the plant extract on the activity of LasA of P. aeruginosa was carried out according to the method of Alasil et al. with modifications [34]. LasA is an extracellular protease secreted by P. aeruginosa that has multiple roles in bacterial virulence, including the lysis of Staphylococcus aureus. It is measured by the ability of a P. aeruginosa supernatant to lyse boiled cells of S. aureus.
Briefly, an overnight culture of P. aeruginosa was grown in LB medium at 37 °C in a shaker. The culture was divided into 10 mL aliquots, to which 1 mL of fresh LB medium containing a sub-MIC dilution of Portulaca was added to a final concentration of 1 mg/mL. After approximately 12 h, when the bacterial culture was expected to be in a late stationary phase, the culture was centrifuged at 10,000× g for 10 min, and the supernatant was used in the assay. The control was the cell supernatant without the addition of the plant extract. An overnight culture of S. aureus was boiled for 10 min and centrifuged for 10 min at 13,000 rpm, and the pellet was resuspended in 10 mM of sodium–phosphate buffer (Na2PO4 with pH 4.5). The culture was read at OD600 and standardized to 0.1 (0.5 McFarland). A 100 µL aliquot of the P. aeruginosa supernatant with or without the plant crude extract was added to 900 µL of the boiled S. aureus suspension. The positive control was 2(5H)-Furanone, and LB broth was used as a negative control. The OD600 was recorded every 10 min for 1 h, and the staphylolytic LasA activity was expressed as the OD600 change per hour per μg of protein.
2.4.4. Phytochemical Analysis by Gas Chromatography-Mass Spectrometry
The analysis of the chemical composition of the P. oleracea methanolic extract was carried out by Gas Chromatography–Mass Spectrometry (GC-MS). The system consisted of an HP 5890 series II plus GC and 5972 quadrupole mass selective detector (MSD) coupled to a Vectra XM2 4/100i computer workstation. A sample of the crude extract was reconstituted in 1 mL of dichloromethane ≥ 99.8% (v/v) (Aldrich Chemical, St Louis, MO, USA). The sample passed through glass wool to remove the remaining solid materials, and 2.0 μL was transferred into an autosampler glass vials with Teflon caps for analysis. The column used was a DB-5 ms column, with dimensions of 30 cm × 0.25 μm × 0.25 mm. The instrument settings were as follows: the analysis time was 50 min, the injector temperature was set to 250 °C with the injection mode set to Spitless, and the temperatures of the ion source and interface were set to 200 °C and 280 °C, respectively. The identification of components via the mentioned software was carried out to compare the spectra of unknown compounds to standard database spectra from a library. The relative amount of each compound was expressed as a percentage of the total peak area in the chromatogram, based on the retention time.
2.5. Statistical Analysis
All the bioassays were performed in triplicate, and the data were presented as mean values with or without the standard deviation (SD). Data analysis was performed with SPSS software version 19.0 (Chicago, IL, USA). The statistical difference between different test conditions was determined using Student’s t-test. The difference was considered significant when p < 0.05.
3. Results
3.1. Plant Crude Extract
The evaporation of the 500 g plant filtrate in 1 L of methanol resulted in approximately 64 g of crude extract. The yield calculated using the formula below was 12.77%
3.2. Effect on Bacterial Growth
The methanolic extract at a concentration of 500 mg/mL was evaluated for its antibacterial activity against P. aeruginosa. The agar well diffusion method showed a growth inhibition of 25 ± 2.1 mm. Figure 2 shows that the inhibition zone was higher than that caused by several antibiotics used to treat P. aeruginosa infections (p = 0.001), including penicillin, ciprofloxacin, azithromycin, lincomycin, and clindamycin. The inhibition was 62% of that caused by tetracycline, which exhibited the highest inhibition (40 mm) of bacterial growth among the tested antibiotics. The MIC was determined to be 31.25 mg/mL, making the sub-MIC 62.5 mg/mL. This sub-MIC solution was used in the assays performed to evaluate the effect of the Portulaca methanolic extract on the expression of the virulence factors.
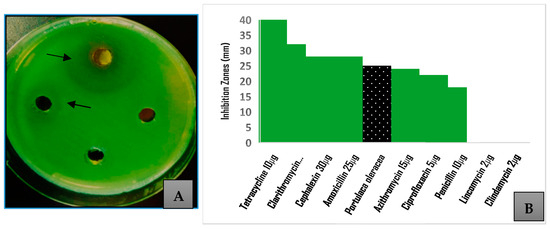
Figure 2.
Antibacterial activity screening of Portulaca oleracea methanolic extract; (A) inhibition zones exhibited by different concentrations of the crude extract against Pseudomonas aeruginosa (arrows); (B) comparison of inhibition caused by 500 mg/mL of crude extract to that exhibited by selected antibiotics.
3.3. Effects of P. oleracea on the Virulence Factors of P. aeruginosa
3.3.1. Effect on Quorum Sensing
A clinical isolate of P. aeruginosa was used to determine the effect of the extract on QS. Figure 3 shows that the sub-MIC exhibited significant inhibition of violacein, with an inhibition zone of 68 mm, as indicated by the appearance of a colorless murky halo zone around the well. When the screening was performed using tetracycline, a clear inhibition zone appeared around the well, indicating bacteriostatic activity, while the region surrounding the well containing the sub-MIC extract showed normal bacterial growth.
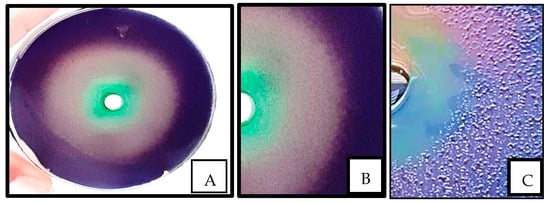
Figure 3.
Evaluation of quorum sensing; (A) whole plate revealing anti-QS activity of Portulaca oleracea extract using Chromobacterium violaceum; (B) a confluent layer of bacteria, which lost their ability to produce violacein upon exposure to P. oleracea; (C) the growth inhibition zone surrounding the well having tetracycline (10 μL).
3.3.2. Biofilm Formation
The assessment of the attachment phase of the biofilm in response to the plant extract was carried out using the sub-MIC. The biofilm formation was significantly reduced (67.08%) by the tested concentration of the methanolic extract (p < 0.05). This reduction was indicated by the decreased absorbance at OD570 nm for the plant-treated well (0.186 ± 0.040) compared to that measured for the control (0.565 ± 0.012).
3.3.3. Staphylolytic LasA Inhibition Assay
Figure 4 shows the reduction in activity for LasA protease with time for the bacterial cultures inoculated with the sub-MIC of the plant extract. The OD600 readings indicated a significant time-dependent decrease in the activity of LasA protease upon treating cells with the plant extract. The OD600 for the negative control (LB) was 0.635, compared to 0.207 for the positive control, which was 2(5H)-Furanone.
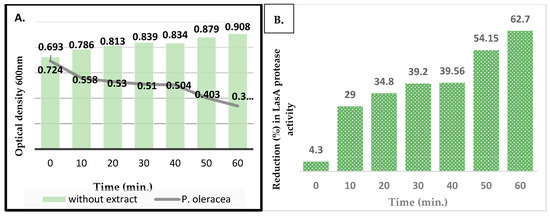
Figure 4.
The activity of LasA protease of P. aeruginosa with and without P. oleracea methanolic extract; (A) The OD600 for the activity of LasA protease at different time intervals; (B) the percentage of reduction in activity of LasA protease with time in the presence of the extract.
3.4. Phytochemical Analysis by Gas Chromatography-Mass Spectrometry (GC_MS)
The GC-MS analysis of the phytochemical composition of the methanol extract of P. oleracea indicated the presence of twenty-two compounds (Table 1). The mass spectrum of the compounds is presented in Figure 5.

Table 1.
GC-MS spectral analysis of Portulaca oleracea methanolic extract.
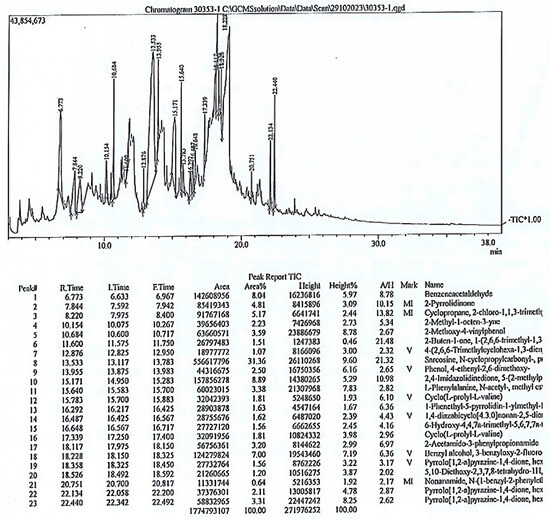
Figure 5.
GC-MS chromatogram of Portulaca aleracea methanolic extract.
4. Discussion
P. aeruginosa is a common leading cause of infections in healthcare settings and the second leading cause of pneumonia in patients supported by ventilators in the United States [60]. The pathogenicity of this bacterium is largely attributed to several virulence factors that enhance the colonization and invasion of the host tissues and thus its survival. P. aeruginosa is a biofilm-forming bacterium that utilizes QS signals to establish colonies and evade the host immune system [61]. Pathogens, such as P. aeruginosa, capable of biofilm formation pose a significant challenge in the medical field due to the exhibition of high resistance to both antibiotics and host immune responses [62,63,64]. It was reported that disrupting biofilm formation and reducing virulence factors regulated by QS is a great approach to controlling microbial infection. Therefore, attenuating or blocking the expression of virulence factors is an innovative strategy to replace traditional antibiotics to decrease microbial pathogenicity. The quorum sensing system controls these factors as it represents cellular communication at the molecular level. This study suggests that the methanolic extract of Portulaca oleracea caused quorum quenching, as indicated by the decreased expression of the virulence factors, the production of pyocyanin, the LasA activity, and biofilm formation. Studies have reported that extracts of several plants contain inhibitors for QS [65,66], while others have been recognized for their inhibitory effect on bacterial growth and biofilm formation [11,18,67,68]. Such bioactivities have been attributed to natural products such as polyphenols, tannins, and alkaloids [14,15,16,17,18]. It has been reported that some phenols compete with AHLs for QS receptor binding, causing the inhibition of the QS system [69,70,71], while alkaloids have been reported as homologs to AHLs [72].
P. oleracea is recognized by the World Health Organization as a popular medicinal plant used for treating microbial infections [73,74]. This plant is rich in nutritional value with no reported side effects on health [75]. The methanolic extract of Portulaca showed an abundance of polyphenols, which are antioxidants and exhibit antimicrobial properties [76]. The antimicrobial activity of different extracts of Portulaca was reported in comparison to Candida albicans and several Gram-positive and Gram-negative bacteria, including P. aeruginosa [28,30,33,77].
QS-inhibitory molecules are interesting when addressing P. aeruginosa infections due to their ability to mitigate inflammation-induced damage and counteract the growing issue of antimicrobial resistance [78]. Biological compounds typically disrupt the bacterial AHL-QS system in three ways: inhibiting the synthesis of signaling molecules through the LuxI-encoded AHL synthase, degrading or modifying the signaling molecules, or targeting the LuxR signal receptor [79]. Our study reports a significant reduction in biofilm formation due to P. aeruginosa, with a decrease of 67.08% when cultured in the presence of the P. oleracea extract. The inhibitory effect of this methanolic extract may be due to small molecules being extracted by methanol that possibly interfere with the AHL signaling molecules, either through their degradation or modification by specific proteins or by antagonizing their activity.
The phytochemical analysis by GC-MS revealed a diverse array of secondary metabolites in the P. oleracea methanolic extract, encompassing phenols, ketones, fatty acids, alkaloids, and inorganic and organic compounds (Table 1). The analysis showed the richness of the plant in several compounds previously reported for their antibacterial and antioxidant activities. Among these bioactive compounds was pyrrolo [1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) (C14H16N2O2), which has previously been reported for its significant anti-quorum sensing activity against P. aeruginosa. Its effective inhibition of biofilm formation contributed to modifications in the architecture of the biofilm, thus hindering bacterial adherence and subsequent biofilm development while preserving cell viability within the biofilm matrix. Furthermore, this compound has been reported to decrease the motility of P. aeruginosa cells and reduce the expression of virulence factors such as pyocyanin, rhamnolipid, and other enzymes including elastase and proteases [80]. Another compound that might contribute to the inhibitory effect of Portulaca on QS is diketopiperazine. This compound has been reported as an inhibitor of the QS system in both C. violaceum CV026 and P. aeruginosa PAO1 [81]. It showed the inhibition of biofilm formation, a reduction in pyocyanin and elastase production in P. aeruginosa PAO1, and a reduced production of violacein in C. violaceum CV026 [82]. A third compound that might contribute to the inhibitory effect of Portulaca on the QS system is 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT). This compound belongs to a class of heterocyclic compounds known as furans, which possess a wide range of biological properties [54]. It has been reported that minor alterations in the substitution patterns of furan derivatives can lead to significant changes in their biological activity. This is evident in many agents with a core furan ring, representing antimicrobial compounds that also exhibit anti-inflammatory, analgesic, antidepressant, anxiolytic, anti-glaucoma, antihypertensive, diuretic, anti-ulcer, anti-aging, and anticancer properties [83]. The phytochemical analysis in this study also detected several phenols in the methanolic extract of Portulaca. The hydroxyl groups in the phenolic compounds were reported to disrupt transport across the bacterial cell membrane by altering the electron flow and inhibiting ATP synthesis, leading to cell death [84]. Some studies showed that specific polyphenols demonstrate anti-QS activity in Pseudomonas putida [85,86], while others indicated that phenolic compounds often demonstrate inhibitory effects on biofilm formation, swarming motility, and the production of some virulence factors, such as adhesion, proteolytic activity, and elastase [87,88,89].
This study showed a significant reduction in biofilm formation caused by P. oleracea methanolic extract. This inhibitory effect might be due to numerous extracted small phenols attenuating QS regulation in P. aeruginosa, as indicated by the large inhibitory zones formed and the lack of violacein produced by C. violaceum. Additionally, the phytochemical analysis showed the presence of several imidazolidine derivatives. Such compounds were reported as inhibitors of P. aeruginosa virulence factors, including protease, hemolysin, and pyocyanin [90]. This study reported that clinical isolates from patients with chronic infections may lead to the accumulation of mutations in QS genes, suggesting that a strain-specific response to QS inhibitors should be considered upon evaluating the anti-virulence properties of such compounds.
In conclusion, the findings of this study suggest the potential use of P. oleracea in managing infections caused by P. aeruginosa by inhibiting quorum sensing, and biofilm formation, and reducing the production of pyocyanin and LasA proteases. Natural compounds exhibiting quorum quenching may be combined with conventional antibiotics to enhance their efficacy as antimicrobials. To our knowledge, this study is the first to identify potential inhibitors of quorum sensing in P. oleracea which can suppress the growth of P. aeruginosa without causing stressful conditions. This study highlights P. oleracea as an important source of natural antimicrobials for possible use as a food additive, and in other pharmaceutical applications. Further investigation on the composition of additional active compounds in P. oleracea and their antimicrobial activity is recommended.
Author Contributions
H.I.A.-D.: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing—original draft; reviewing and editing the final manuscript; S.M.M.: data curation; formal analysis; validation; software; writing—original draft. K.A.S.: data curation; formal analysis; validation; software; writing—original draft. L.F.A.-N.: conceptualization; data curation; formal analysis; investigation; methodology; resources; validation; visualization; writing—original draft; reviewing and editing the final manuscript. S.Z.: data curation; validation; visualization. R.M.A.-A.: investigation. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Abdul Hameed Shoman Foundation for Research, Grant No. 4/2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the technical support provided by Al-Ahliyya Amman University, Central State University, and Al-Balqa Applied University.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant Derived Natural Products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm Activity and Molecular Mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Haque, S.; Ahmad, F.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Wahid, M.; Lohani, M.; Khan, S.; Singh, V.; Akhter, N. Developments in Strategies for Quorum Sensing Virulence Factor Inhibition to Combat Bacterial Drug Resistance. Microb. Pathog. 2018, 121, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.A.S.; Bail, L.; Arend, L.N.V.S.; Nogueira, K.D.S.; Tuon, F.F. The Activity of Ceftazidime/Avibactam against Carbapenem-Resistant Pseudomonas aeruginosa. Infect. Dis. 2021, 53, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Litwin, A.; Rojek, S.; Gozdzik, W.; Duszynska, W. Pseudomonas aeruginosa Device Associated—Healthcare Associated Infections and Its Multidrug Resistance at Intensive Care Unit of University Hospital: Polish, 8.5-Year, Prospective, Single-Centre Study. BMC Infect. Dis. 2021, 21. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Bidell, M.R.; Lodise, T.P. Approach to the Treatment of Patients with Serious Multidrug-Resistant Pseudomonas aeruginosa Infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 952–969. [Google Scholar] [CrossRef]
- Rezzoagli, C.; Archetti, M.; Mignot, I.; Baumgartner, M.; Kümmerli, R. Combining Antibiotics with Antivirulence Compounds Can Have Synergistic Effects and Reverse Selection for Antibiotic Resistance in Pseudomonas aeruginosa. PLoS Biol. 2020, 18, e3000805. [Google Scholar] [CrossRef]
- Duplantier, M.; Lohou, E.; Sonnet, P. Quorum Sensing Inhibitors to Quench, P. aeruginosa Pathogenicity. Pharmaceuticals 2021, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; Maeda, T.; Mandujano-Tinoco, E.A.; Tomás, M.; Pérez-Eretza, B.; García-Contreras, S.J.; Wood, T.K.; García-Contreras, R. Role of Quorum Sensing in Bacterial Infections. World J. Clin. Cases 2015, 3, 575. [Google Scholar] [CrossRef]
- Schuster, M.; Joseph Sexton, D.; Diggle, S.P.; Peter Greenberg, E. Acyl-Homoserine Lactone Quorum Sensing: From Evolution to Application. Annu. Rev. Microbiol. 2013, 67, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Abu-Niaaj, L.F.; Al-Daghistani, H.I.; Katampe, I.; Abu-Irmaileh, B.; Bustanji, Y.K. Pomegranate Peel: Bioactivities as Antimicrobial and Cytotoxic Agents. Food Sci. Nutr. 2024, 12, 2818–2832. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; Elansary, H.O. Editorial: Bioactive Compounds, Functional Ingredients, Antioxidants, and Health Benefits of Edible Plants. Front. Plant Sci. 2024, 15, 1420069. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Saadat, S.; Khazdair, M.R.; Gholamnezhad, Z.; El-Seedi, H.; Boskabady, M.H. Phytochemical Characteristics and Anti-Inflammatory, Immunoregulatory, and Antioxidant Effects of Portulaca oleracea, L.: A Comprehensive Review. Evid.-Based Complement. Altern. Med. 2023, 2023, 2075444. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghistani, H.I.; Abu-Niaaj, L.F.; Bustanji, Y.; Al-Hamaideh, K.D.; Al-Salamat, H.; Nassar, M.N.; Jaber, H.M.; Amer, N.H.; Abu-Irmaileh, B.; Al-Nuaimi, A.H.D. Antibacterial and Cytotoxicity Evaluation of Arum Hygrophilum Bioss. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7306–7316. [Google Scholar] [CrossRef] [PubMed]
- Abu-Niaaj, L.; Abu-Zarga, M.; Abdalla, S. Isolation and Inhibitory Effects of Eupatilin, a Flavone Isolated from Artemisia monosperma Del., on Rat Isolated Smooth Muscle. Int. J. Pharmacogn. 1996, 34, 134–140. [Google Scholar] [CrossRef]
- Abu-Niaaj, L.; Abu-Zarga, M.; Sabri, S.; Abdalla, S. Isolation and Biological Effects of 7-O-Methyleriodictyol, a Flavanone Isolated from Artemisia monosperma, on Rat Isolated Smooth Muscles. Planta Medica 1993, 59, 42–45. [Google Scholar] [CrossRef]
- Abu-Niaaj, L.; Katampe, I. Isolation and Characterization of Flavones from Artemisia monosperma. Pharmacogn. J. 2018, 10, 1018–1023. [Google Scholar] [CrossRef]
- Qin, T.; Chen, K.; Xi, B.; Pan, L.; Xie, J.; Lu, L.; Li, K. In Vitro Antibiofilm Activity of Resveratrol against Aeromonas hydrophila. Antibiotics 2023, 12, 686. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents from Apocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist. Updates 2020, 51, 100695. [Google Scholar] [CrossRef]
- El-Mahdy, A.M. Effect of Resveratrol on Quorum Sensing and Some Virulence Characteristics of Pseudomonas aeruginosa Isolated from Manoura University Hospitals. J. Microbiol. 2017, 46, 98–111. [Google Scholar]
- Hossain, M.A.; Lee, S.-J.; Park, N.-H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.-W.; Park, S.-C. Impact of Phenolic Compounds in the Acyl Homoserine Lactone-Mediated Quorum Sensing Regulatory Pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal–Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Tao, X.; Ying, X.; Stien, D. Two Amide Glycosides from Portulaca oleracea L. and Its Bioactivities. Nat. Prod. Res. 2019, 35, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.B.; Ajam, F.; Rakhshandeh, H.; Askari, V.R. A Pharmacological Review on Portulaca oleracea L.: Focusing on Anti-Inflammatory, Anti-Oxidant, Immuno-Modulatory and Antitumor Activities. J. Pharmacopunct. 2019, 22, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M. Portulaca oleracea L. A Review. J. Pharm. Res. 2011, 4, 3044–3048. [Google Scholar]
- Liu, L.; Howe, P.; Zhou, Y.F.; Xu, Z.Q.; Hocart, C.; Zhan, R. Fatty Acids and Beta-Carotene in Australian Purslane (Portulaca oleracea) Varieties. J. Chromatogr. A 2000, 893, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tleubayeva, M.I.; Datkhayev, U.M.; Alimzhanova, M.; Ishmuratova, M.Y.; Korotetskaya, N.V.; Abdullabekova, R.M.; Flisyuk, E.V.; Gemejiyeva, N.G. Component Composition and Antimicrobial Activity of CO2 Extract of Portulaca oleracea, Growing in the Territory of Kazakhstan. Sci. World J. 2021, 2021, 5434525. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Kianmehr, M. Anti-Asthmatic Effects of Portulaca oleracea and Its Constituents, a Review. J. Pharmacopunct. 2019, 22, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Nayaka, H.B.; Londonkar, R.L.; Umesh, M.K.; Tukappa, A. Antibacterial Attributes of Apigenin, Isolated from Portulaca oleracea L. Int. J. Bacteriol. 2014, 2014, 175851. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Moniem AE, A.; Al-Quraishy, S.; Saleh, R.A. Antioxidant Effect of Purslane (Portulaca oleracea) and Its Mechanism of Action. J. Med. Plants Res. 2011, 5, 1589–1593. [Google Scholar]
- Bae, J.-H. Antimicrobial Effect of Portulaca oleracea Extracts on Food-Borne Pathogens. Prev. Nutr. Food Sci. 2004, 9, 306–311. [Google Scholar] [CrossRef][Green Version]
- Alasil, S.M.; Omar, R.; Ismail, S.; Yusof, M.Y. Antibiofilm Activity, Compound Characterization, and Acute Toxicity of Extract from a Novel Bacterial Species of Paenibacillus. Int. J. Microbiol. 2014, 2014, 649420. [Google Scholar] [CrossRef] [PubMed]
- Sylwia, J.; Martin, J. Fast Screening Method for Detection of Acyl-HSL-Degrading Soil Isolates. J. Microbiol. Methods 2004, 57, 415–420. [Google Scholar] [CrossRef]
- Bali, E.B.; Türkmen, K.E.; Erdönmez, D.; Sağlam, N. Comparative Study of Inhibitory Potential of Dietary Phytochemicals against Quorum Sensing Activity of and Biofilm Formation by Chromobacterium Violaceum 12472, and Swimming and Swarming Behaviour of Pseudomonas aeruginosa PAO1. Food Technol. Biotechnol. 2019, 57, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Lewis, J.S., II; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J., Jr.; Limbago, B.; et al. M100 Performance Standards for Antimicrobial Susceptibility Testing a CLSI Supplement for Global Application, 30th ed.; CLSI: Malvern, PA, USA, 2020; Available online: http://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 19 January 2025).
- Joo, H.-S.; Deyrup, S.T.; Shim, S.H. Endophyte-Produced Antimicrobials: A Review of Potential Lead Compounds with a Focus on Quorum-Sensing Disruptors. Phytochem. Rev. 2020, 20, 543–568. [Google Scholar] [CrossRef]
- Zaki, A.A. Assessment of Anti-Quorum Sensing Activity for Some Ornamental and Medicinal Plants Native to Egypt. Sci. Pharm. 2013, 81, 251–258. [Google Scholar] [CrossRef]
- Adeyemo, R.O.; Famuyide, I.M.; Dzoyem, J.P.; Lyndy Joy, M. Anti-Biofilm, Antibacterial, and Anti-Quorum Sensing Activities of Selected South African Plants Traditionally Used to Treat Diarrhoea. Evid. Based Complement. Altern. Med. 2022, 2022, e1307801. [Google Scholar] [CrossRef]
- Fagbemi, K.O.; Aina, D.A.; Adeoye-Isijola, M.O.; Naidoo, K.K.; Coopoosamy, R.M.; Olajuyigbe, O.O. Bioactive Compounds, Antibacterial and Antioxidant Activities of Methanol Extract of Tamarindus Indica Linn. Sci. Rep. 2022, 12, 9432. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Gutwald, R.; Wiedmann-Al-Ahmad, M.; Haberstroh, J.; Obermeyer, J.; Kuenz, A.; Betz, H.; Wolter, F.; Duttenhoefer, F.; Schmelzeisen, R.; et al. Effect of Gabapentin-Lactam and Gamma-Aminobutyric Acid/Lactam Analogs on Proliferation and Phenotype of Ovine Mesenchymal Stem Cells. Int. J. Oral Maxillofac. Implant. 2013, 28, e230–e238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Barathikannan, K.; Wei, S.; Kim, J.-R.; Yoo, D.; Wang, M.-H.; Oh, D.-H. Bioactive Potential of 2-Methoxy-4-Vinylphenol and Benzofuran from Brassica oleracea L. Var. capitate f, rubra (Red Cabbage) on Oxidative and Microbiological Stability of Beef Meat. Foods 2020, 9, 568. [Google Scholar] [CrossRef]
- Singh, N.; Mansoori, A.; Jiwani, G.; Solanke, A.U.; Thakur, T.K.; Kumar, R.; Chaurasiya, M.; Kumar, A. Antioxidant and Antimicrobial Study of Schefflera vinosa Leaves Crude Extracts against Rice Pathogens. Arab. J. Chem. 2021, 14, 103243. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Klimo, K.; Hümmer, W.; Hölzer, J.; Petermann, A.; Garreta-Rufas, A.; Böhmer, F.D.; Schreier, P. Identification of 3-Hydroxy-β-Damascone and Related Carotenoid-Derived Aroma Compounds as Novel Potent Inducers of Nrf2-Mediated Phase 2 Response with Concomitant Anti-Inflammatory Activity. Mol. Nutr. Food Res. 2009, 53, 1237–1244. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Antispasmodic Activity of β-Damascenone and E-Phytol Isolated from Ipomoea Pes-Caprae. Planta Medica 1992, 58, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Cernel, N.; Zitka, O.; Skalickova, S.; Gumulec, J.; Sztalmachova, M.; Rodrigo, M.A.; Socher, M.; Masarik, J.; Adam, M.; Hubalek, V.; et al. Effect of Sarcosine on Antioxidant Parameters and Metallothionein Content in the PC-3 Prostate Cancer Cell Line. Oncol. Rep. 2013, 29, 2459–2466. [Google Scholar] [CrossRef] [PubMed]
- Bouali, N.; Ahmad, I.; Patel, H.; Alhejaili, E.B.; Hamadou, W.S.; Badraoui, R.; Lajimi, R.H.; Alreshidi, M.; Siddiqui, A.J.; Adnan, M.; et al. GC–MS Screening of the Phytochemical Composition of Ziziphus Honey: ADME Properties and in Vitro/in Silico Study of Its Antimicrobial Activity. J. Biomol. Struct. Dyn. 2023, 42, 1368–1380. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Y.; Selzer, M.E.; Pincus, J.H. Phenytoin: Mechanisms of Its Anticonvulsant Action. Ann. Neurol. 1986, 20, 171–184. [Google Scholar] [CrossRef]
- Dahiya, R.; Kumar, A.; Yadav, R. Synthesis and Biological Activity of Peptide Derivatives of Iodoquinazolinones/Nitroimidazoles. Molecules 2008, 13, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tafolla, L.; Padrón, J.M.; Mendoza, G.; Luna-Rodríguez, M.; Fernández, J.J.; Norte, M.; Trigos, Á. Antiproliferative Activity of Biomass Extract from Pseudomonas cedrina. Electron. J. Biotechnol. 2019, 40, 40–44. [Google Scholar] [CrossRef]
- Kaushik, N.; Kumar, N.; Kumar, A.; Singh, U.K. Tetrazoles: Synthesis and Biological Activity. Immunol. Endocr. Metab. Agents Med. Chem. 2018, 18, 3–21. [Google Scholar] [CrossRef]
- Dhanabalan, S.; Muthusamy, K.; Iruthayasamy, J.; Kumaresan, P.V.; Ravikumar, C.; Kandasamy, R.; Natesan, S.; Periyannan, S. Unleashing Bacillus Species as Versatile Antagonists: Harnessing the Biocontrol Potentials of the Plant Growth-Promoting Rhizobacteria to Combat Macrophomina phaseolina Infection in Gloriosa superba. Microbiol. Res. 2024, 283, 127678. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.K.A.; Kim, S.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and Isolated 6-Hydroxy-4,4,7a-Trimethyl-5,6,7,7a-Tetrahydrobenzofuran-2(4H)-One (HTT); LPS-Induced Inflammation Attenuation via Suppressing NF-ΚB, MAPK and Oxidative Stress through Nrf2/HO-1 Pathways in RAW 264.7 Macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Sulaiman, M.; Hassan, Y.; Taskin Tok, T.; Noundow, X.S. Synthesis, Antibacterial Activity and Docking Studies of Benzyl Alcohol Derivatives. J. Turk. Chem. Soc. Sect. A Chem. 2020, 7, 481–488. [Google Scholar] [CrossRef]
- Dash, S.; Jin, C.; Lee, O.O.; Xu, Y.; Qian, P.-Y. Antibacterial and Antilarval-Settlement Potential and Metabolite Profiles of Novel Sponge-Associated Marine Bacteria. J. Ind. Microbiol. Biotechnol. 2009, 36, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.I.; Babiker, E.H.; Saeed, H.A. Streptomyces: Isolation, Optimization of Culture Conditions and Extraction of Secondary Metabolites. Int. Curr. Pharm. J. 2016, 5, 27–32. [Google Scholar] [CrossRef]
- Manimaran, M.; Kannabiran, K. Marine Streptomyces Sp. VITMK1 Derived Pyrrolo [1, 2-A] Pyrazine-1, 4-Dione, Hexahydro-3-(2-Methylpropyl) and Its Free Radical Scavenging Activity. Open Bioact. Compd. J. 2017, 5, 23–30. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An Antibiotic Agent Pyrrolo [1,2-a]Pyrazine-1,4-Dione,Hexahydro Isolated from a Marine Bacteria Bacillus tequilensis MSI45 Effectively Controls Multi-Drug Resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef]
- Gellatly, S.L.; Hancock, R.E.W. Pseudomonas aeruginosa: New Insights into Pathogenesis and Host Defenses. Pathog. Dis. 2013, 67, 159–173. [Google Scholar] [CrossRef]
- Hemmati, J.; Nazari, M.; Abolhasani, F.S.; Ahmadi, A.; Asghari, B. In Vitro Investigation of Relationship between Quorum-Sensing System Genes, Biofilm Forming Ability, and Drug Resistance in Clinical Isolates of Pseudomonas aeruginosa. BMC Microbiol. 2024, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Heyndrickx, M.; Flint, S.; Liu, W.; Chen, W.; Zhao, J.; Zhang, H. Community-Wide Changes Reflecting Bacterial Interspecific Interactions in Multispecies Biofilms. Crit. Rev. Microbiol. 2021, 47, 338–358. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Rollet, C.; Gal, L.; Guzzo, J. Biofilm-Detached Cells, a Transition from a Sessile to a Planktonic Phenotype: A Comparative Study of Adhesion and Physiological Characteristics in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2008, 290, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Qaralleh, H.; Saghir SA, M.; Al-Limoun, M.O.; Dmor, S.M.; Khleifat, K.; Al-Ahmad, B.E.M.; Al-Omari, L.; Tabana, Y.; Mothana, R.A.; Al-Yousef, H.; et al. Effect of Matricaria aurea Essential Oils on Biofilm Development, Virulence Factors and Quorum Sensing-Dependent Genes of Pseudomonas aeruginosa. Pharmaceuticals 2024, 17, 386. [Google Scholar] [CrossRef] [PubMed]
- Naga, N.G.; El-Badan, D.E.; Ghanem, K.M.; Shaaban, M.I. It Is the Time for Quorum Sensing Inhibition as Alternative Strategy of Antimicrobial Therapy. Cell Commun. Signal. 2023, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Al Qaisi, Y.T.; Khleifat, K.M.; Oran, S.A.; Al Tarawneh, A.A.; Qaralleh, H.; Al-Qaisi, T.S.; Farah, H.S. Ruta graveolens, Peganum harmala, and Citrullus colocynthis Methanolic Extracts Have in Vitro Protoscolocidal Effects and Act against Bacteria Isolated from Echinococcal Hydatid Cyst Fluid. Arch. Microbiol. 2022, 204, 228. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, C.L.; Kiselev, H.Y.u.; Budanova, E.V.; Zamyatnin, A.A.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef]
- Santos, C.A.; Almeida, F.A.; Quecán, B.X.V.; Pereira, P.A.P.; Gandra, K.M.B.; Cunha, L.R.; Pinto, U.M. Bioactive Properties of Syzygium cumini (L.) Skeels Pulp and Seed Phenolic Extracts. Front. Microbiol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of Biofilm Formation and Quorum Sensing Mediated Phenotypes by Berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef] [PubMed]
- Quecán, B.X.V.; Rivera, M.L.C.; Pinto, U.M. Bioactive Phytochemicals Targeting Microbial Activities Mediated by Quorum Sensing. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 397–416. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J.C. Dietary Phytochemicals as Quorum Sensing Inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef]
- Liu, G.; Liu, A.; Yang, C.; Zhou, C.; Zhou, Q.; Li, H.; Yang, H.; Mo, J.; Zhang, Z.; Li, G.; et al. Portulaca oleracea L. Organic Acid. Extract Inhibits Persistent Methicillin-Resistant Staphylococcus aureus in Vitro and in Vivo. Front. Microbiol. 2023, 13, 1076154. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A Review on Bioactive Phytochemicals and Ethnopharmacological Potential of Purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- OECD. Test No.423: Acute Oral Toxicity-Acute Toxic Class Method; Guidelines for Testing of Chemicals; OECD: Paris, France, 2011. [Google Scholar]
- Desta, Z.Y.; Cherie, D.A. Determination of Antioxidant and Antimicrobial Activities of the Extracts of Aerial Parts of Portulaca quadrifida. Chem. Cent. J. 2018, 12, 146. [Google Scholar] [CrossRef]
- Keser, F.; Karatepe, M.; Keser, S.; Tekin, S.; Türkoğlu, İ.; Kaygili, O.; Demir, E.; Ökkeş, Y.; Sandal, S.; Kırbag, S. In Vitro Biological Activities and Phytochemical Contents of Portulaca oleracea L. (Purslane). J. Phys. Chem. Funct. Mater. 2021, 4, 1–7. [Google Scholar]
- Londonkar, R.; Kamble, A. Hepatotoxic and Invivo Antioxidant Potential of Pandanus odoratissimus against Carbon Tetrachloride Induced Liver Injury in Rats. Orient. Pharm. Exp. Med. 2011, 11, 229–234. [Google Scholar] [CrossRef]
- Luo, J.; Kong, J.; Dong, B.; Huang, H.; Wang, K.; Hou, C.; Liang, Y.; Li, B.; Chen, Y.; Wu, L. Baicalein Attenuates the Quorum Sensing-Controlled Virulence Factors of Pseudomonas aeruginosa and Relieves the Inflammatory Response in P. aeruginosa-Infected Macrophages by Downregulating the MAPK and NFκB Signal-Transduction Pathways. Drug Des. Dev. Ther. 2016, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Mishra, A.; Jha, B. 3-Benzyl-Hexahydro-Pyrrolo [1,2-a]Pyrazine-1,4-Dione Extracted from Exiguobacterium indicum Showed Anti-Biofilm Activity against Pseudomonas aeruginosa by Attenuating Quorum Sensing. Front. Microbiol. 2019, 10, 1269. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Makarov, V.; Brackman, G.; Israyilova, A.; Azzalin, A.; Forneris, F.; Riabova, O.; Savina, S.; Coenye, T.; et al. Discovery of New Diketopiperazines Inhibiting Burkholderia cenocepacia Quorum Sensing in Vitro and in Vivo. Sci. Rep. 2016, 6, 32487. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A Diketopiperazine Factor from Rheinheimera aquimaris QSI02 Exhibits Anti-Quorum Sensing Activity. Sci. Rep. 2016, 6, 39637. [Google Scholar] [CrossRef] [PubMed]
- Saeid, H.; Al-sayed, H.; Bader, M. A Review on Biological and Medicinal Significance of Furan. AlQalam J. Med. Appl. Sci. 2023, 6, 44–58. [Google Scholar]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Karatuna, O.; Yagci, A. Analysis of Quorum Sensing-Dependent Virulence Factor Production and Its Relationship with Antimicrobial Susceptibility in Pseudomonas aeruginosa Respiratory Isolates. Clin. Microbiol. Infect. 2010, 16, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-Sensing. Z. Naturforsch. C 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from Infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Yin, H.; Deng, Y.; Wang, H.; Liu, W.; Zhuang, X.; Chu, W. Tea Polyphenols as an Antivirulence Compound Disrupt Quorum-Sensing Regulated Pathogenicity of Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 16158. [Google Scholar] [CrossRef]
- Mohamed, B.; Abdel-Samii, Z.K.; Abdel-Aal, E.H.; Abbas, H.A.; Shaldam, M.A.; Ghanim, A.M. Synthesis of Imidazolidine-2,4-Dione and 2-Thioxoimidazolidin-4-One Derivatives as Inhibitors of Virulence Factors Production in Pseudomonas aeruginosa. Arch. Pharm. 2020, 353, e1900352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).