Biocontrol Potential of Trichoderma spp. Against Phytophthora ramorum
Abstract
1. Introduction
- Analysis of Temperature-Dependent Growth Rate: We began by performing a study of temperature-dependent growth rate on 51 Trichoderma isolates, hypothesizing that the growth rate of Trichoderma correlates with its biocontrol efficacy against P. ramorum. Trichoderma isolates that grow and colonize substrates rapidly could compete more effectively against P. ramorum.
- Dual-Culture Assay for Direct Interaction: Next, we assessed the direct interaction, or mycoparasitism, of Trichoderma with P. ramorum. This assay used a dual-culture approach to measure the rates at which Trichoderma overgrew and killed an existing P. ramorum culture through direct interaction.
- Antibiosis Microplate Assay for Antagonistic Effects: Additionally, we investigated the antibiosis of different Trichoderma isolates with respect to P. ramorum by testing sterile culture filtrates of Trichoderma isolates in a novel in vitro microplate assay to assess their antagonistic effects on P. ramorum germination and growth.
2. Materials and Methods
2.1. Analysis of Temperature-Dependent Growth Rate
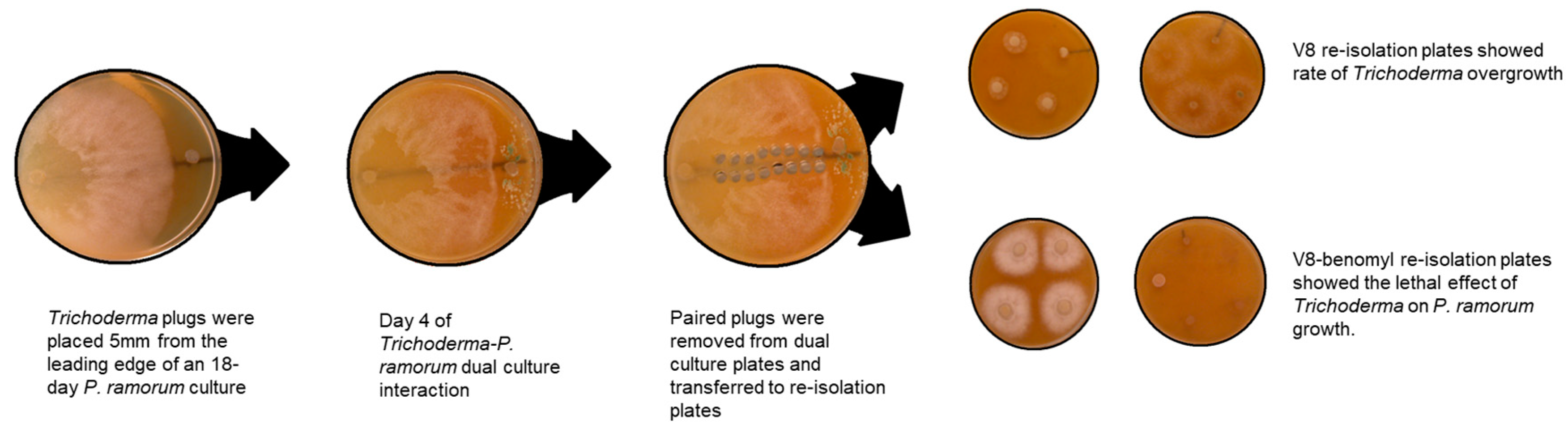
2.2. Dual Culture Assay for Direct Interaction
2.3. Antibiosis Microplate Assay for Antagonistic Effect
2.3.1. Phytophthora Ramorum Sporangia Production
2.3.2. Production of Trichoderma Filtrates
2.3.3. Antibiosis Microplate Assay
2.4. Statistical Analyses
3. Results
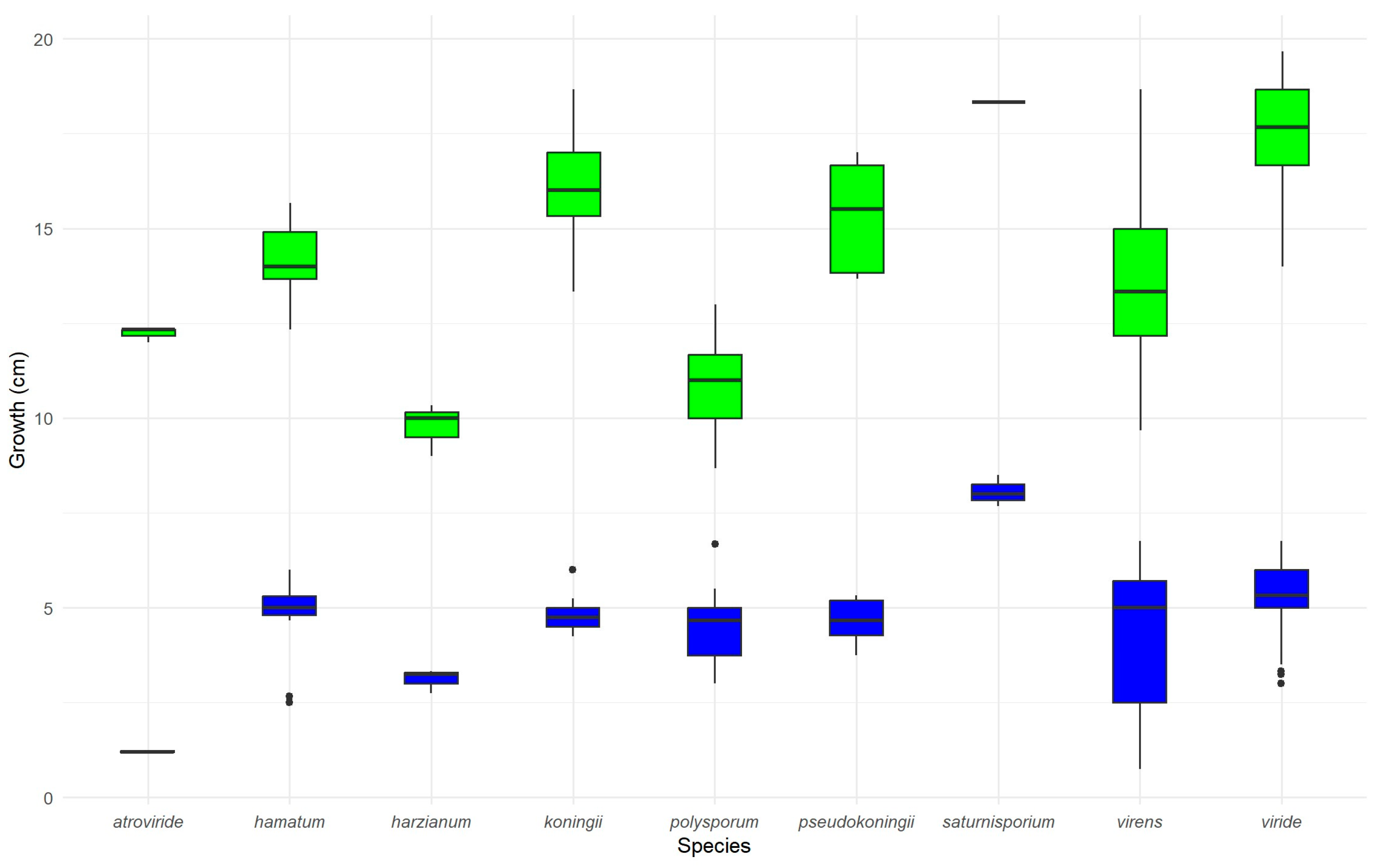
3.1. Analysis of Temperature-Dependent Growth Rate
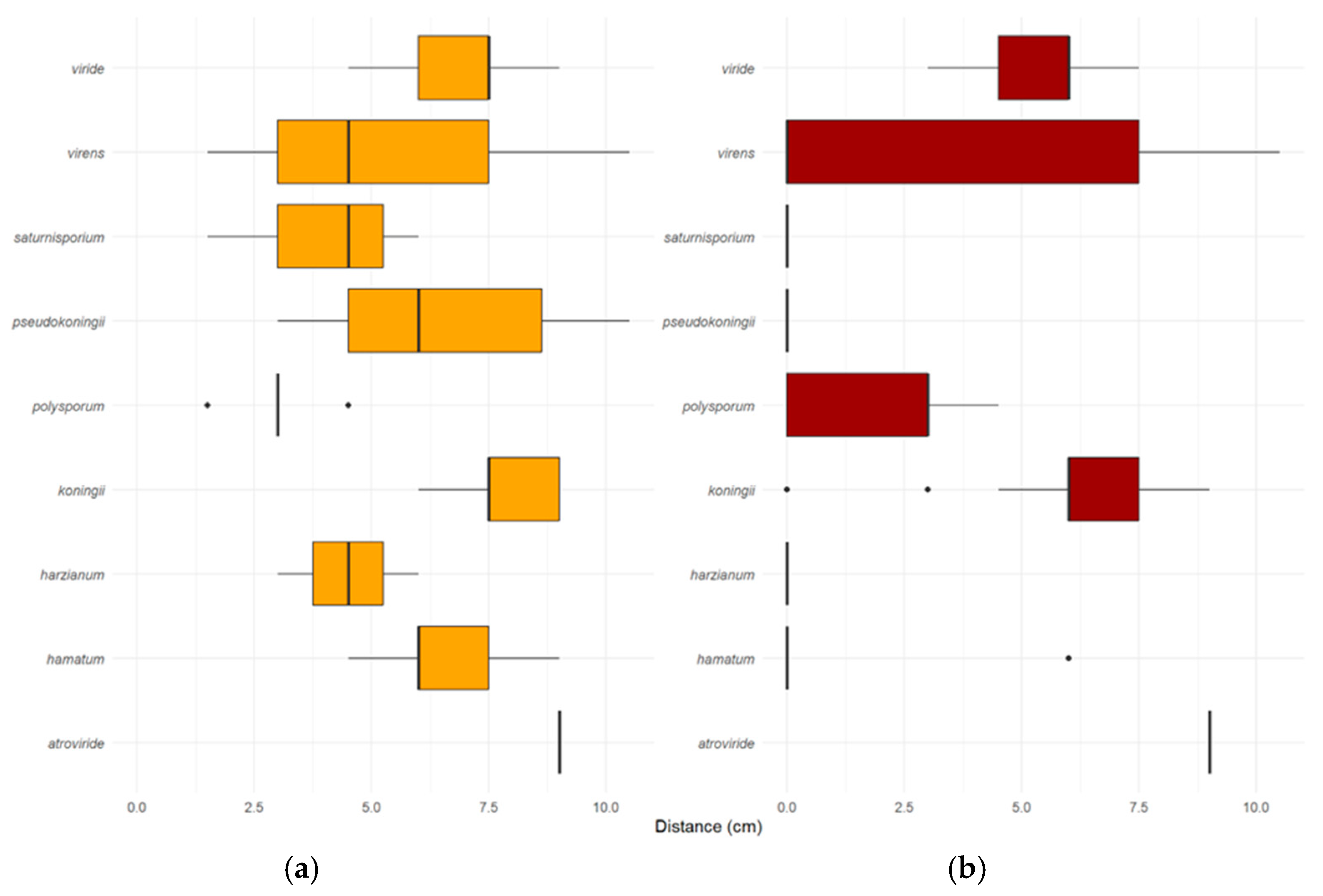
3.2. Dual Culture Assay for Direct Interaction-Rate of Overgrowth
3.3. Dual Culture Assay for Direct Interaction-Rate of Lethal Effect
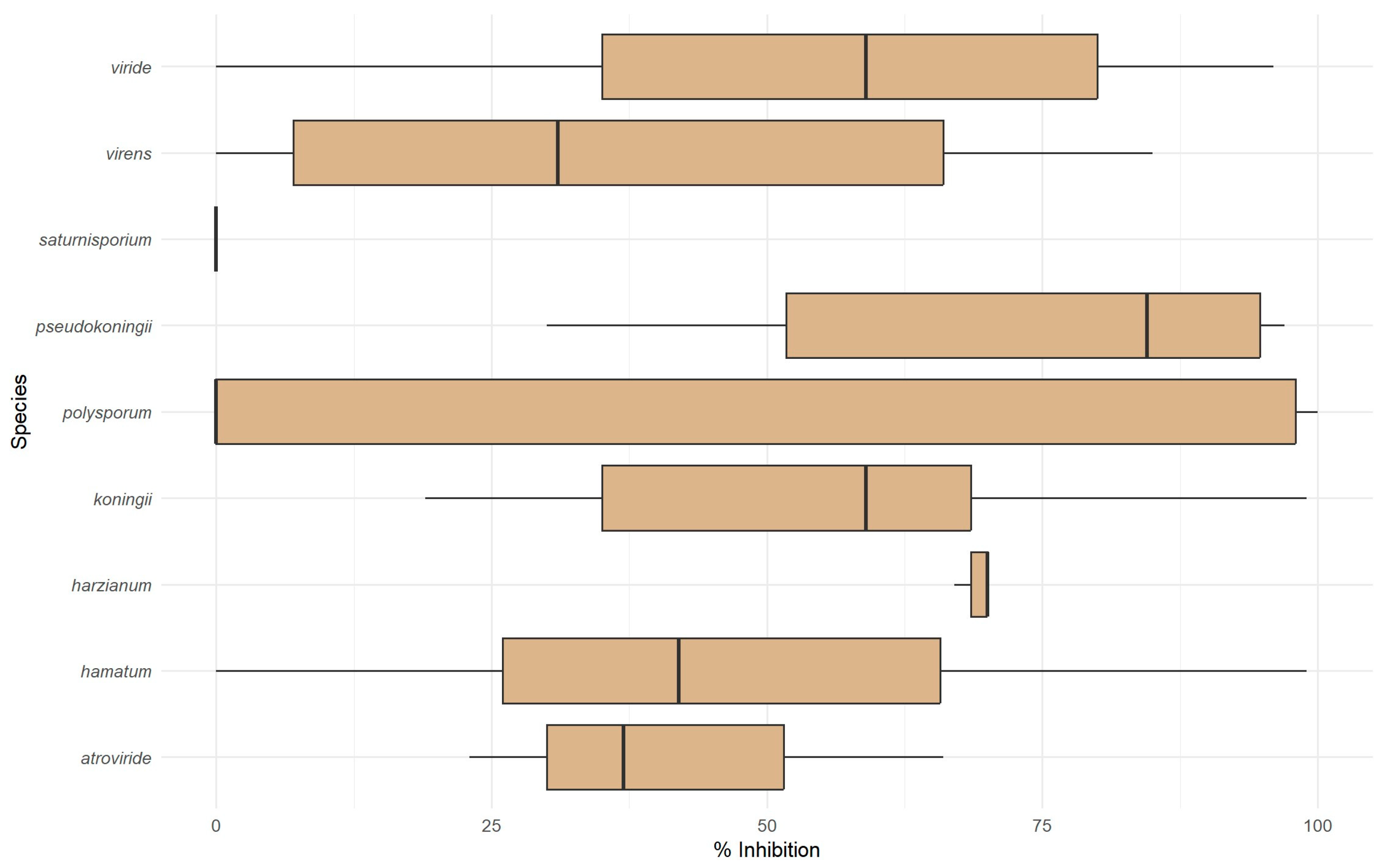
3.4. Antibiosis Microplate Assay for Antagonistic Effect
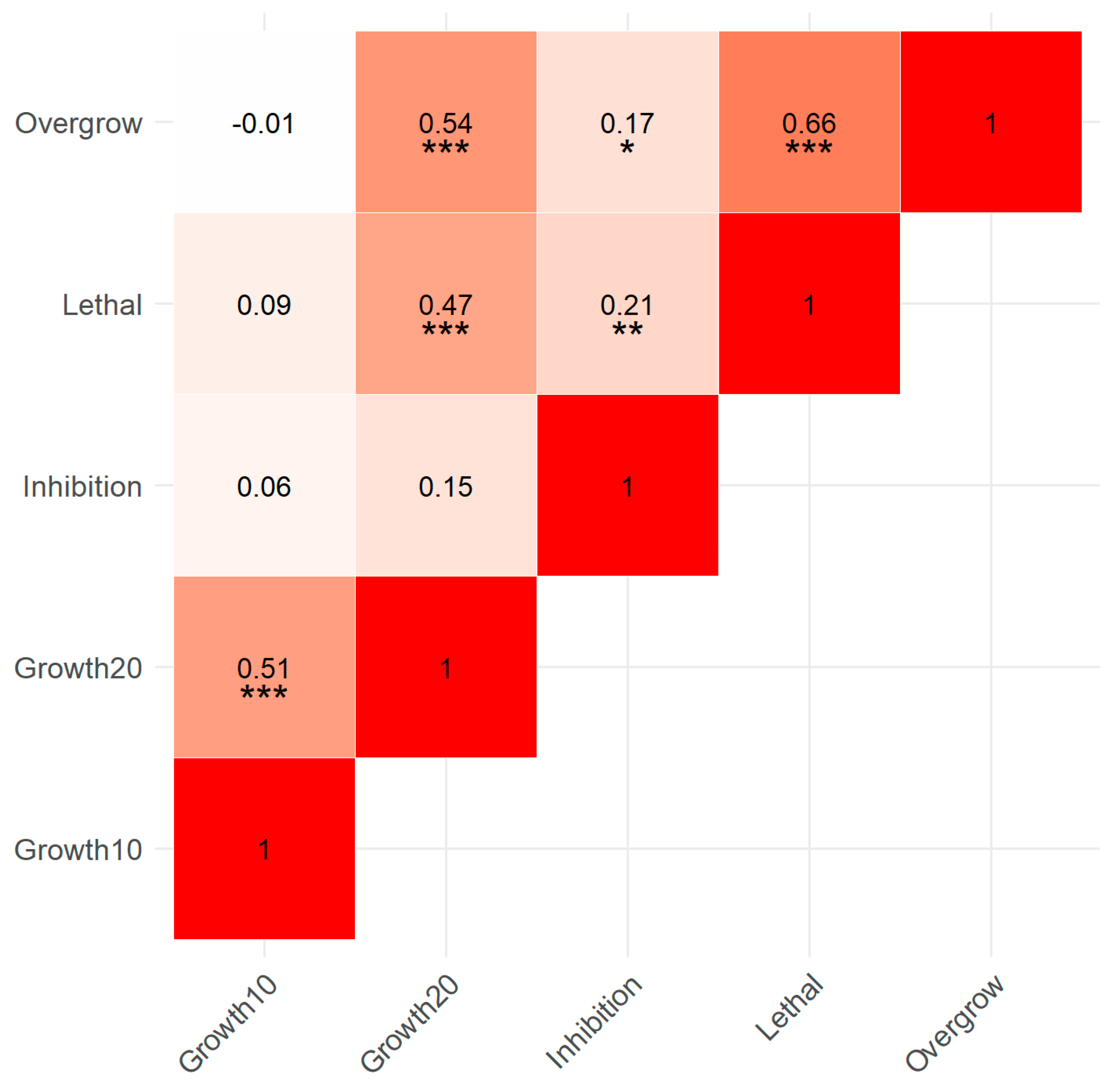
3.5. Correlation of Assay Variables
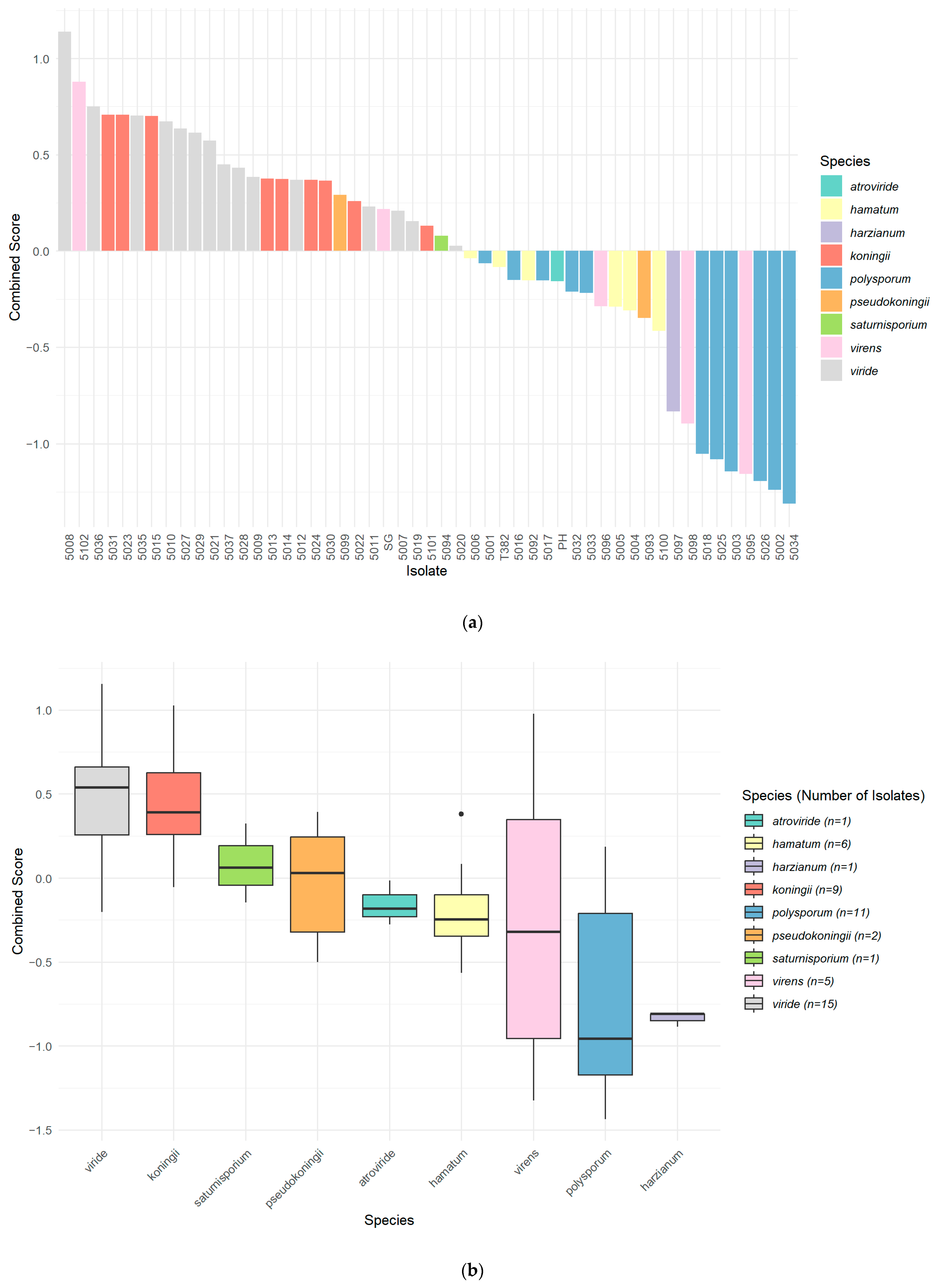
3.6. Combined Biocontrol Variable
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werres, S.; Marwitz, R.; Man In’t veld, W.A.; De Cock, A.W.A.M.; Bonants, P.J.M.; De Weerdt, M.; Themann, K.; Ilieva, E.; Baayen, R.P. Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycol. Res. 2001, 105, 1155–1165. [Google Scholar] [CrossRef]
- Parke, J.L.; Peterson, E.K. Sudden Oak Death, Sudden Larch Death, and Ramorum Blight. Plant Health Instr. 2019, 19. Available online: https://www.apsnet.org/edcenter/pdlessons/Pages/SuddenOakDeath.aspx (accessed on 20 January 2025).
- Grünwald, N.J.; Goss, E.M.; Press, C.M. Phytophthora ramorum: A Pathogen with a Remarkably Wide Host Range Causing Sudden Oak Death on Oaks and Ramorum Blight on Woody Ornamentals. Mol. Plant Pathol. 2008, 9, 729–740. [Google Scholar] [CrossRef]
- Shamoun, S.F.; Rioux, D.; Callan, B.E.; James, D.; Hamelin, R.; Bilodeau, G.J.; Elliott, M.; Lévesque, C.A.; Becker, E.; McKenney, D.; et al. An Overview of Canadian Research Activities on Diseases Caused by Phytophthora ramorum: Results, Progress, and Challenges. Plant Dis. 2018, 102, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M. Phytophthora ramorum: Integrative Research and Management of an Emerging Pathogen in California and Oregon Forests. Annu. Rev. Phytopathol. 2005, 43, 309–350. [Google Scholar] [CrossRef] [PubMed]
- Shishkoff, N. Susceptibility of Some Common Container Weeds to Phytophthora ramorum. Plant Dis. 2004, 96, 1026–1032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Canadian Food Inspection Agency. D-01-01: Phytosanitary Requirements to Prevent the Entry and Spread of Phytophthora ramorum (18th Revision); CFIA Directive D-01-01; Canadian Food Inspection Agency: Ottawa, ON, Canada, 13 June 2013. [Google Scholar]
- Canadian Food Inspection Agency. PI-010: Regulatory Response Protocol for Nurseries Confirmed with Phytophthora ramorum; CFIA—PI-010; Canadian Food Inspection Agency: Ottawa, ON, Canada, 29 April 2019. [Google Scholar]
- Grünwald, N.J.; Goss, E.M. Evolution and Population Genetics of Exotic and Re-emerging Pathogens: Novel Tools and Approaches. Annu. Rev. Phytopathol. 2011, 49, 249–267. [Google Scholar] [CrossRef]
- Saldana-Mendoza, S.A.; Pacios-Michelena, S.; Palacios-Ponce, A.; Chavez-Gonzalez, M.; Aguilar, C.N. Trichoderma as a Biological Control Agent: Mechanisms of Action, Benefits for Crops and Development of Formulation. World J. Microbiol. Biotechnol. 2023, 39, 269. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–Plant–Pathogen Interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Elliott, M.; Shamoun, S.F.; Sumampong, G.; James, D.; Masri, S.; Varga, A. Evaluation of several commercial biocontrol products on European and North American populations of Phytophthora ramorum. Biocontrol Sci. Technol. 2009, 19, 709–721. [Google Scholar] [CrossRef]
- Shamoun, S.F.; Elliott, M. Phytophthora ramorum Werres, de Cock & Man in’t Veld, Sudden Oak Death/Encre des chênes rouges (Peronosporaceae). In Biological Control Programmes in Canada; Vankosky, M.A., Martel, V., Eds.; CABI: Wallingford, UK, 2024; pp. 593–601. [Google Scholar] [CrossRef]
- Kamoun, S.; Lamour, K. Oomycete Genetics and Genomics: Diversity, Interactions, and Research Tools. In Phytophthora: Genomics of Plant-Associated Fungi; Springer: Berlin/Heidelberg, Germany, 2002; pp. 197–213. [Google Scholar]
- Smith, V.L.; Wilcox, W.F.; Harman, G.E. Potential for Biological Control of Phytophthora Root and Crown Rots of Apple by Trichoderma and Gliocladium spp. Phytopathology 1990, 80, 880–885. [Google Scholar] [CrossRef]
- Howell, C.R. The Role of Antibiosis in Biocontrol. In Trichoderma and Gliocladium; Harman, G.E., Kubicek, C.P., Eds.; Taylor and Francis Ltd.: London, UK, 1998; Volume 2, pp. 173–183. [Google Scholar]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol Mechanisms of Trichoderma Strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Gouit, S.; Chair, I.; Belabess, Z.; Legrifi, I.; Goura, K.; Tahiri, A.; Lazraq, A.; Lahlali, R. Harnessing Trichoderma spp.: A promising approach to control apple scab disease. Pathogens 2024, 13, 752. [Google Scholar] [CrossRef]
- Ramzan, U.; Abid, K.; Zafar, M.A.; Anwar, A.M.; Nadeem, M.; Tanveer, U.; Fatima, U. Trichoderma: Multitalented biocontrol agent. Int. J. Agric. Biosci. 2023, 12, 77–82. [Google Scholar] [CrossRef]
- Goldfarb, B.; Nelson, E.E.; Hansen, E.M. Trichoderma spp.: Growth Rates and Antagonism to Phellinus weirii in Vitro. Mycologia 1989, 81, 375–381. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 November 2024).
- RStudio Team. RStudio: Integrated Development Environment for R; Version 2023.03.0; RStudio, PBC: Boston, MA, USA, 2023; Available online: https://www.rstudio.com/ (accessed on 20 November 2024).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data. R Package Version 1.1.2. 2024. Available online: https://github.com/tidyverse/tidyr (accessed on 20 November 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R Package Version 1.1.0; 2024. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 20 November 2024).
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research; R Package Version 2.3.0; Northwestern University: Evanston, IL, USA, 2024; Available online: https://CRAN.R-project.org/package=psych (accessed on 20 November 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; R Package Version 3.4.0; Springer-Verlag New York: New York, NY, USA, 2024; ISBN 978-3-319-24277-4. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 20 November 2024).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes, R Package Version 1.1-3; 2024. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 20 November 2024).
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma spp. as Inducers of Plant Growth and Systemic Resistance. Microb. Biotechnol. 2009, 2, 509–517. [Google Scholar] [CrossRef][Green Version]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Chet, I.; Inbar, J. Biological Control of Fungal Pathogens. Appl. Biochem. Biotechnol. 1994, 48, 37–43. [Google Scholar] [CrossRef]
- Pokhrel, A.; Adhikari, A.; Oli, D.; Paudel, B.; Pandit, S.; GC, B.; Tharu, B.R.R. Biocontrol Potential and Mode of Action of Trichoderma Against Fungal Plant Diseases. Acta Sci. Agric. 2022, 6, 10–21. [Google Scholar] [CrossRef]
- Diby, P.; Saju, K.A.; Jisha, P.J.; Sarma, Y.R.; Kumar, A.; Anandaraj, M. Mycolytic Enzymes Produced by Pseudomonas fluorescens and Trichoderma spp. Against Phytophthora capsici, the Foot Rot Pathogen of Black Pepper (Piper nigrum L.); Division of Crop Protection, Indian Institute of Spices Research, Marikkunnu P.O.: Calicut, India, 2005. [Google Scholar]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Rodrigues, A.O.; May De Mio, L.L.; Soccol, C.R. Trichoderma as a Powerful Fungal Disease Control Agent for a More Sustainable and Healthy Agriculture: Recent Studies and Molecular Insights. Planta 2023, 257, 31. [Google Scholar] [CrossRef]
- Hoitink, H.A.J.; Madden, L.V.; Dorrance, A.E. Systemic Resistance Induced by Trichoderma spp.: Interactions Between the Host, the Pathogen, the Biocontrol Agent, and Soil Organic Matter Quality. Phytopathology 2006, 96, 186–189. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J. Antagonistic Fungi, Trichoderma spp.: Panoply of Biological Control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
| Accession | Species 1 | Host | Location | Collected by/Source |
|---|---|---|---|---|
| PFC 5001 | polysporum | Black spruce | Comox, BC | T. Osono |
| PFC 5002 | polysporum | Douglas fir | Comox, BC | T. Osono |
| PFC 5003 | polysporum | Black spruce | Comox, BC | T. Osono |
| PFC 5004 | hamatum | Black spruce | Comox, BC | T. Osono |
| PFC 5005 | hamatum | Black spruce | Comox, BC | T. Osono |
| PFC 5006 | hamatum | Douglas fir | Comox, BC | T. Osono |
| PFC 5007 | viride | Black spruce | Comox, BC | T. Osono |
| PFC 5008 | viride | Black spruce | Comox, BC | T. Osono |
| PFC 5009 | viride | Black spruce | Comox, BC | T. Osono |
| PFC 5010 | viride | Douglas fir | Berms, SK | T. Osono |
| PFC 5011 | viride | Black spruce | Berms, SK | T. Osono |
| PFC 5012 | viride | Jack pine | Berms, SK | T. Osono |
| PFC 5013 | koningii | Douglas fir | Groundhog, ON | T. Osono |
| PFC 5014 | koningii | Black spruce | Groundhog, ON | T. Osono |
| PFC 5015 | koningii | Douglas fir | Groundhog, ON | T. Osono |
| PFC 5016 | polysporum | Douglas fir | Groundhog, ON | T. Osono |
| PFC 5017 | polysporum | Douglas fir | Groundhog, ON | T. Osono |
| PFC 5018 | polysporum | Black spruce | Groundhog, ON | T. Osono |
| PFC 5019 | viride | Black spruce | Groundhog, ON | T. Osono |
| PFC 5020 | viride | Douglas fir | Groundhog, ON | T. Osono |
| PFC 5021 | viride | Black spruce | Groundhog, ON | T. Osono |
| PFC 5022 | koningii | Black spruce | CPRS, QC | T. Osono |
| PFC 5023 | koningii | Black spruce | CPRS, QC | T. Osono |
| PFC 5024 | koningii | Douglas fir | CPRS, QC | T. Osono |
| PFC 5025 | polysporum | Douglas fir | CPRS, QC | T. Osono |
| PFC 5026 | polysporum | Black spruce | CPRS, QC | T. Osono |
| PFC 5027 | viride | Black spruce | CPRS, QC | T. Osono |
| PFC 5028 | viride | Black spruce | CPRS, QC | T. Osono |
| PFC 5029 | viride | Black spruce | CPRS, QC | T. Osono |
| PFC 5030 | koningii | Douglas fir | Nashwaak, NB | T. Osono |
| PFC 5031 | koningii | Balsam fir | Nashwaak, NB | T. Osono |
| PFC 5032 | polysporum | Douglas fir | Nashwaak, NB | T. Osono |
| PFC 5033 | polysporum | Douglas fir | Nashwaak, NB | T. Osono |
| PFC 5034 | polysporum | Black spruce | Nashwaak, NB | T. Osono |
| PFC 5035 | viride | Douglas fir | Nashwaak, NB | T. Osono |
| PFC 5036 | viride | Black spruce | Nashwaak, NB | T. Osono |
| PFC 5037 | viride | Douglas fir | Nashwaak, NB | T. Osono |
| PFC 5092 | hamatum | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5093 | pseudokoningii | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5094 | saturnisporum | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5095 | virens | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5096 | virens | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5097 | harzianum | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5098 | virens | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5099 | pseudokoningii | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5100 | hamatum | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5101 | koningii | Douglas fir | Oregon, USA | M. Elliott |
| PFC 5102 | virens | Douglas fir | Oregon, USA | M. Elliott |
| PlantHelper™ | atroviride | AmPac Biotech | ||
| T382 | hamatum | Sylvan Bioproducts | ||
| SoilGard™ | virens | Certis Biologicals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, E.; Rajakulendran, N.; Shamoun, S.F. Biocontrol Potential of Trichoderma spp. Against Phytophthora ramorum. Pathogens 2025, 14, 136. https://doi.org/10.3390/pathogens14020136
Becker E, Rajakulendran N, Shamoun SF. Biocontrol Potential of Trichoderma spp. Against Phytophthora ramorum. Pathogens. 2025; 14(2):136. https://doi.org/10.3390/pathogens14020136
Chicago/Turabian StyleBecker, Elisa, Nirusan Rajakulendran, and Simon Francis Shamoun. 2025. "Biocontrol Potential of Trichoderma spp. Against Phytophthora ramorum" Pathogens 14, no. 2: 136. https://doi.org/10.3390/pathogens14020136
APA StyleBecker, E., Rajakulendran, N., & Shamoun, S. F. (2025). Biocontrol Potential of Trichoderma spp. Against Phytophthora ramorum. Pathogens, 14(2), 136. https://doi.org/10.3390/pathogens14020136






