Abstract
Human Parvovirus B19 (hB19V) is a widespread virus, causing erythema infectiosum in children and several clinical manifestations from acute to persistent infections in adults. In early 2024, ECDC reported an increased human Parvovirus B19 circulation in 14 European countries. A hB19V outbreak was also reported in Tuscany, Italy, prompting a detailed investigation of its genetic characteristics. In this context, through strict monitoring of circulating strains via next-generation sequencing (NGS), we carried out a phylogenetic analysis based on the whole of hB19V genomes. Phylogenetic clustering assigned all isolates to the G1a genotype, although with some mutations in NS1, VP1, and VP2, compared to the reference strains. Further characterization of these variants is necessary to fully assess their potential implications for public health. This study provides valuable insights into the spread of Parvovirus B19 and underlines the importance of continuous genomic surveillance to monitor and respond to possible hB19V epidemics that could impact public health.
1. Introduction
Human parvovirus B19 (hB19V) is a single-stranded DNA virus (ssDNA) belonging to the Parvoviridae family. It is the aetiologic agent of the erythema infectiosum (fifth disease) in children and it can infect adults, particularly those who are immunosuppressed or have chronic hematological diseases, causing a wide range of clinical manifestations [1]. Although most of the patients are asymptomatic or have mild symptoms, when hB19V affects adults, it can lead to more serious conditions, such as arthropathy, aplastic crises, and hydrops fetalis in pregnant women [2,3]. HB19V can be transmitted via respiratory secretions, hand-to-mouth contact, blood transfusion, or transplacental transmission [4]. The incubation period varies from 4 to 14 days after exposure, but it can last up to 3 weeks [5]. HB19V infections usually follow a seasonal pattern, with incidence peaks typically occurring in late spring or early summer, especially in temperate countries [6]. Larger epidemics have been reported every 3–4 years, with fluctuation in the number of cases across different seasons [7]. Since the first few months of the 2024, and especially during late spring-early summer, an unexpected surge in hB19V was noted in a lot of European countries [8,9], including Italy. Indeed, a recent study reported an outbreak of hB19V in pregnant women in Northern Italy [10]. In Tuscany, specifically in Siena area, only three cases were recorded in our laboratory in 2023; a lot of requests arrived at the Virology Unit and various samples were positively diagnosed from January to September 2024.
PCR-based molecular assay is widely employed for hB19V diagnosis; however, many routine clinical laboratories rely solely on serology, using commercial ELISA or immunofluorescence-based assays for anti hB19V IgG and IgM antibodies analysis [11].
HB19V genome is a 5596-nucleotide-long ssDNA molecule, encoding for several structural and non-structural proteins. Its genome is composed of two main regions (Figure 1): the non-coding region, formed by two inverted terminal repeats (ITR) and mainly involved in viral replication and the encapsidation process, and the coding region for four non-structural proteins (NS1, 7.5-, 9-, and 11-kDa) and two structural proteins (VP1 and VP2).
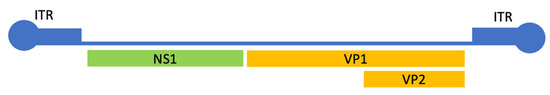
Figure 1.
A representation of the hB19V genome. The 5596nt genome is composed of 5′ and 3′ inverted terminal repeats, and three main genes: the non-structural protein1 (NS1), the viral protein 1 (VP1), and the viral protein 2 (VP2). The two proteins share the whole sequence of VP2, but VP1 has an additional N-terminal domain known as the VP1 unique region (VP1u).
ITRs are 383 nucleotides in length and form imperfect palindromic sequences that can be folded into hairpins expressed in two alternative orientations. They play a crucial role in viral replication processes. Indeed, the ability of palindromic sequences to fold in self-priming, hairpin secondary structures, and the presence of specific cis-recognition sequences acting as origins of replication, allow replication of the viral DNA through a rolling hairpin mechanism [12].
The non-structural 1 protein (NS1) is involved in viral replication, cytotoxicity, and host immune response. The structural VP proteins include VP1, containing a unique N-terminus (VP1u), and are critical for virus entry via the phospholipase A2 (vPLA2) domain, and VP2, which contributes to the viral capsid and is involved in virus-receptor binding [13,14]. The VP1 and VP2 regions are of particular interest, with up to 3% variability at the amino acid level [15]. Mutations in these genes can impact the virus’ ability to replicate, escape immune detection, or alter its interaction with the host.
Based on the phylogenetic analysis of the NS1-VP1u region, hB19V was classified into three genotypes: genotype 1, genotype 2, and genotype 3 [16,17]. Genotype 1 is prevalent worldwide and can be divided into two subgroups (genotype 1a and 1b); genotype 2 is commonly harbored in tissues of elderly people, but it is only sporadically detected as a circulating virus; while genotype 3 is only found in restricted areas, such as western Africa [18,19]. This last genotype shows a wider genetic variability in comparison with the other genotypes, and for this reason, it may show a more limited geographical distribution.
This research examines the surge of hB19V cases from January 2023 to September 2024, in the Siena area, Tuscany, with the aim to present an overview of genotypic characterization of hB19V positive cases in this area.
2. Materials and Methods
2.1. Clinical Samples
During the 2023–2024 period, 550 samples were processed for presumptive infection with hB19V at the Microbiology and Virology Unit of ‘S. Maria delle Scotte’ Hospital in Siena (Italy).
Among all the samples, 40 out of 181 blood samples were positive for hB19V by molecular assay, and 59 out of 369 serum samples were anti-hB19V IgM and IgG positive by serological investigation. The detection of both IgM and IgG was indicative of acute hB19V infection. Blood and serum samples of the same patient were only available for 24 subjects. Ethical approval was obtained from the local Ethical Committee for clinical trials (Protocol BIOBANCA VIROMICRO-2023, approval no. 25836, 15 January 2024) (Comitato Etico Regionale per la Sperimentazione Clinica della Toscana—sezione AREA VASTA SUD EST) in terms of General Data Protection and Regulation (GDPR) upon written informed consent signed by all subjects prior to participating in this study.
2.2. Serological and Molecular Analysis
The nucleic acid extraction was performed with the automatic extractor EZ1 and the DNA Blood kit (Qiagen GmbH, Hamburg Germany). HB19V viral load of blood samples was detected performing real-time-PCR using the Parvovirus B19 R-GeneTM kit (Biomerieux, Florence, Italy), according to the manufacturer’s instructions. The exact sequences of the primers used in the kit are typically not disclosed publicly, however, they are carefully selected to match highly conserved regions of the NS1 or VP2 genes to ensure specificity and sensitivity. Serological IgM and IgG analysis of patient sera was carried out by using the LIAISONTM Biotrin Parvovirus B19 IgM and IgG kit on the Liaison XL platform (Diasorin S.p.A.; Vercelli, Italy), according to the manufacturer’s instructions.
This test uses chemiluminescence immunoassay (CLIA) technology for the qualitative determination of specific IgG and IgM antibodies for Parvovirus B19. Results are reported in arbitrary units (U/mL). The manufacturer considers samples with values <0.9 U/mL as negative, >1.1 U/mL as positive, and between 0.9 and 1.1 U/mL as equivocal.
2.3. Next Generation Sequencing
Before starting the NGS protocol, the quality of extracted genetic material was assessed using a NanodropTM spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) by evaluating the A260/A280 ratio that provides information about sample contamination. The quantity of the total nucleic acid was then assessed with a Qubit DNA high-sensitivity assay kit and Qubit 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Twenty-three samples were selected for NGS analysis. Library preparation was carried out following the Illumina RNA Prep with Enrichment kits (Illumina S.r.l. Milan, Italy) according to the manufacturer’s instructions. Briefly, 8.5 μL of extracted RNA, by the methodology described above, was denatured, followed by first- and second-strand DNA synthesis. This was followed by tagmentation, which uses enrichment bead-linked transposomes (BLT) to tagment double-stranded cDNA. This process fragments cDNA and adds adapter sequences. After tagmentation, the fragments were purified and amplified to add index adapter sequences for dual indexing. An index set, containing 96 unique, single-use Illumina DNA/RNA UD indexes, was used. Following clean up, 7.5 μL of the library was used for hybridization using oligos from the respiratory viral panel. This was followed by bead-based capture of hybridized probes, amplification, clean up, and quantification of the enriched library. Normalized libraries diluted to an equimolar concentration of 10 pM were then pooled and denatured according to Illumina’s instructions and charged on a V2 micro MiSeq 300 cycles flow cell (Illumina S.r.l. Milan, Italy). FastQ files were analyzed with the BaseSpace™ platform by Illumina with the Dragen targeted microbial app (Illumina S.r.l. Milan, Italy). All obtained sequences were uploaded on Genbank (https://www.ncbi.nlm.nih.gov/nucleotide/, accessed on 29 November 2024) with the following accession numbers: PQ660663; PQ660664; PQ660665; PQ660666; PQ660667; PQ660668, PQ660669; PQ660670; PQ660671; PQ660672; PQ660673; PQ660674; PQ660675; PQ660676; PQ660677; PQ660678; PQ660679; PQ660680; PQ660681; PQ660682; PQ660683; PQ660684; and PQ324621.1.
2.4. Phylogenetic Analysis
Sequences were aligned using the Geneious software (version 2025.0.2) with the MUSCLE algorithm, using the default parameters (three iterations and gap opening penalties). After calculation of pairwise distances to determine how similar or different each sequence is from the others. The phylogenetic tree was generated using the neighbor-joining algorithm using the Jukes–Cantor distance model, which assumes equal and constant mutation rates between all nucleotide pairs. Bootstrapping and reconstitution were carried out with 1000 replicates to obtain the confidence level of the phylogenetic tree. Sequence homology comparison was carried out with reference sequences representing the three main hB19V genotypes from the Gen Bank genetic sequence database: genotype1A: NC_00083.2; AF162273.1; genotype 1B: DQ357065.1; genotype 2: AY044266.2; AY064475.1; genotype 3: AY083234.1; and AX003421.1.
2.5. Evolutionary Rate and Selection Analysis
Selection pressure was analyzed in three proteins of hB19V based on the genome dataset.
All the sequences were aligned using the MUSCLE algorithm on the Geneious software. SLAC methods (www.datamonkey.org) were used to calculate ω values (ω = dN/dS) of B19V, where dN stands for the nonsynonymous substitution rate and the dS is for the synonymous substitution rate.
3. Results and Discussion
From the beginning of 2023 to September 2024, a total of 550 requests for hB19V diagnosis, both by serological and molecular methods, came to the attention of the Microbiology and Virology Unit at ‘S. Maria delle Scotte’ Hospital, based in Siena, Tuscany. Among them, ninety-nine samples were positive for hB19V (18%), of which, only three were collected in 2023 (two in April and one in October 2023).
Our data show an increased number of positive samples since the first months of 2024 (Figure 2), reaching the highest peak in late spring/early summer 2024, according to the reported scientific literature [9]. A higher number of female subjects tested positive (63 out of 99), while the remaining 36 were male subjects. Twenty-four samples were from pediatric patients (0–14 years old), whereas the remaining seventy-five samples were from adults (>15 years old).
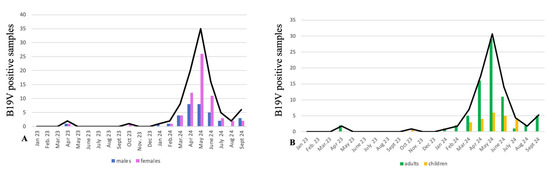
Figure 2.
Parvovirus B19 cases from 2023 to the end of September 2024 in Siena area, Italy. (A) Number of positive samples distributed by gender; blue bars are from males and purple bars from females. (B) Number of positive samples distributed by age; green bars represent adult patients (>15 years old), and yellow bars correspond to children. This study showed that the hB19V infection was more frequent in adult females.
This increase in the number of hB19V-positive samples was partially attributed to the impact of COVID-19 restrictions both on viral circulation and immunity patterns. Studies and reports highlight that strict public health measures during the pandemic, such as social distancing, school closures, and reduced social interactions, significantly disrupted the transmission of various infectious diseases, including hB19V [20,21,22,23]. These measures led to a decline in population level immunity, particularly among children, who missed typical exposures to common pathogens [24,25]. Our study showed females were more affected than males. The higher prevalence in women might be attributed, in part, to their greater likelihood of caring for young children, potentially enhancing viral exposure. This may represent a problem, since non-immune pregnant women are at risk for fetal infections by hB19V, leading to greater complications, especially if the infection occurs in the first or second trimester. It has been widely documented that Parvovirus infection during pregnancy can be passed to the fetus in 30–50% of cases, with a high risk of developing anemia, fetal hydrops, and in some cases pre-birth death [26]. It is not known if pregnant women were present in our cohort, since clinical data were not available.
This high number of positive cases allowed us to characterize the circulating B19V genotype circulating in this outbreak.
As mentioned before, three different hB19V genotypes were defined: genotype 1, divided in two subgroups (1a and 1b), genotype 2, and genotype 3. Although genotype 1a is the most frequent worldwide, it appears to have been partially substituted by genotype 2 in the last 50 years; but nowadays, it is sporadically detected only in rare cases, and usually in elderly people. Genotype 3 is less frequent, and it is generally associated with peculiar, restricted areas [14,27].
For this purpose, 23 samples with PCR-Ct between 25 and 36 were selected for a next generation sequencing (NGS) analysis. The following bioinformatic analysis with the Dragen Microbial Enrichment Plus software (ver 1.1.0) (IlluminaTM) identified hB19V in 100% of sequenced samples. The mean coverage was 98.5% in all samples with a mean depth of 115X. The consensus sequences were generated and aligned with the CLUSTAL and MUSCLE algorithm with a minimum GC content of 50%. All aligned sequences were used to conduct a phylogenetic analysis; the three genotype references were taken from Genbank (gen1a: AF162273.1; gen 1b: DQ357065.1; gen2: AY064475.1; AY044266.2; gen3: AX003421.1; and AY083234.1). The phylogenetic tree was constructed based on the neighbor-joining method with 1,000 bootstrap repetitions (cutoff 50). The resulting phylogenetic tree (Figure 3) shows that all samples were clustered under genotype 1a and were closely related with each other (d = 0.008; d.s = 0.0062) with a distance of 0.147 against genotype 3, 0.128 against genotype 2, and 0.058 against genotype 1b; 0.2% of nucleotide divergence and 3% of amino acid divergence.
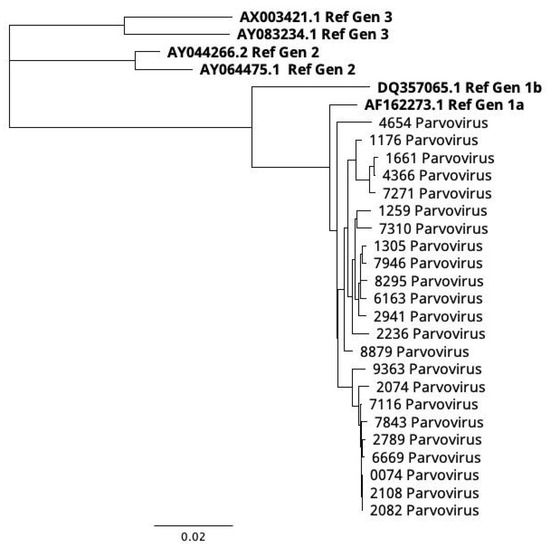
Figure 3.
Phylogenetic tree of NGS samples. The phylogenetic tree shows that all samples were clustered under the genotype 1a. No samples were clustered under the genotype 1b, genotype 2, and genotype 3. The used references are highlighted in bold, all of them are accessible in the Genbank database. Genotype 1A: NC_00083.2; AF162273.1; genotype 1B:DQ357065.1; genotype 2: AY044266.2; AY064475.1; and genotype 3: AY083234.1; AX003421.1.
These results enhance the close relationship in the evolutionary history of all those isolates, showing few genetic differences.
All detected mutations were point mutations, and the analysis revealed a broad polymorphic distribution, with frequencies ranging from 13% to 100%. Of these, 40.4% (59/146) were located in the NS1 gene, 8.9% (13/146) were exclusive to the VP1 gene, and the remaining 50.7% (74/146) were in the VP2 gene, which shares its sequence with VP1, but differs by 227 amino acids (Supplementary Material Figure S1). These data (Table 1) demonstrate a greater variability in the VP1/VP2 genes according to the published literature [28]. The mutation rate per base length was calculated and estimated to be µ = 0.24 for the NS1 gene, µ = 0.34 for the VP1, and µ = 0.29 for the VP2.

Table 1.
The nucleotide mutations shared by all samples analyzed with the NGS approach.
In addition, the dN/dS ratio was calculated for NS1, VP1, and VP2 genes, obtaining for all of them a value which is less than 1: ω = 0.023 for NS1, ω = 0.040 for VP1, and ω = 0.050 for VP2 (dev st for: NS1 = 0.01, VP1 = 0.01; and Vp2 = 0.02), respectively.
The low ω values observed for the three examined proteins suggest a strong negative selection which can act to preserve the critical functions of the proteins which are vital for the virus life cycle. Similar results have been reported also in other studies [29,30,31].
Further studies are urgently needed to assess the distribution of these variants over time. The cohort must certainly be expanded to better understand the distribution and role of these variants in the long term.
The translation of the sequences revealed the presence of non-synonymous mutations (listed in Table 2) within our cohort of samples. Among these changes, five were present in NS1 protein, three in VP1, and three in VP1/VP2. The mutation rates per base for the non-synonymous mutations in the proteins NS1, VP1, and VP2 were calculated to understand the evolutionary pressures acting on these viral components. The results show a significantly higher mutation rate for NS1 (0.3333 mutations per base) compared to VP1 (0.1265 mutations per base) and VP2 (0.0121 mutations per base). These findings suggest that while VP2 is under stronger negative selection to maintain its structural and functional integrity as a capsid protein, NS1 may tolerate more mutations, possibly due to its involvement in immune evasion [32]. VP1 shows an intermediate mutation rate, indicating a balance between evolutionary flexibility and the need to preserve viral function.

Table 2.
List of amino acid mutations and their frequency in the hB19V sequences.
It has been documented that variations in the VP1/VP2 capsid proteins may influence the virus interaction with host immune responses and cellular receptors, potentially enhancing its ability to evade immunity and spread more efficiently within the population [32]. These mutations were randomly dispersed among the analyzed genomes and never present in all samples. However, while most of the changes occurred among amino acids similar for physicochemical properties, the replacement of positively charged lysine with negatively charged (K14E) aspartic acid in VP1u could affect the protein. This mutation could have significant consequences for the virus, including changes in capsid proteins, immune evasion, and infectivity.
Indeed, this mutation is located in the N-terminal 1-80 aa of VP1u, which is rich in neutralizing epitopes [33]. In this regard, a deficient immune response to VP1u has been associated with persistent infections, emphasizing the important role of the immune response against VP1u in clearing the virus [34,35]. However, in this study, it was not possible to correlate this mutation with a more severe or prolonged disease, because samples were collected for diagnosis without knowing the subjects’ clinical status. All of the mutations found in hB19V isolates and summarized in Table 2 have already been reported in scientific literature, such as the F554S amino acid change, localized in the carboxy-terminal part of NS1 gene, and detected in 9/23 subjects [36]. This region, being highly polymorphic, was identified as exposed to positive selective pressure [30,31].
In conclusion, this is the first report on hB19V genotypes circulating during the 2024 outbreak in Tuscany, Italy. This recent surge in hB19V cases across different European countries, coupled with increased viral circulation and shifting epidemiological patterns, may signify a heightened exposure risk for a larger part of the population, including those traditionally considered at low risk, such as immunocompetent adults. Although often perceived as a benign childhood illness, this study reported an increase in infections, particularly in the adults. This underscores the need for a reassessment of risk stratification, even among immune competent adults and adolescents. Although the genotyping of the current circulating strain does not seem to show particular changes in hB19V genotypes, further studies, both on a larger court and on the protein conformation variability, could be needed to understand the possible impact of these mutations on proteins and their level of involvement in virus virulence and transmissibility.
Moreover, the recording and continuing observation of mutations, especially in limited areas, is important to inform health authorities and put in place recommendations and preventive measures. The use of existing non-specific surveillance systems and their adaptation to new health events is essential for effective epidemiological monitoring and control.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14020121/s1, Figure S1: Illustration of the nucleotide variation frequencies detected in NS1 (a), VP1 (b) and VP2 (c) genes.
Author Contributions
Methodology, writing-original draft preparation, formal analysis, G.B.; writing review and editing, G.A.; conceptualization, validation, supervision, writing-review and editing, funding acquisition, M.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Italian Ministry of Health-CUP B93C2200121000, CCM 2022 ‘SURVEID’.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (Comitato Etico Regionale per la Sperimentazione Clinica della Toscana—sezione AREA VASTA SUD EST) (Protocol BIOBANCA VIROMICRO-2023, approval no. 25836, 15 January 2024) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Datasets analyzed or generated during the study can be provided, upon reasonable request, by contacting the corresponding author (mariagrazia.cusi@unisi.it).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Servey, J.T.; Reamy, B.V.; Hodge, J. Clinical presentations of parvovirus B19 infection. Am. Fam. Physician 2007, 75, 373–376. [Google Scholar] [PubMed]
- Badrinath, A.; Gardere, A.; Samantha, L.; Palermo, S.L.; Campbell, K.S.; Kloc, A. Analysis of Parvovirus B19 persistence and reactivation in human heart layers. Front. Virol. 2024, 4, 1304779. [Google Scholar] [CrossRef]
- Lehmann, T.; von Landenberg, P.; Modrow, S. Parvovirus B19 and Autoimmunity: A Review of Current Findings in Adult Infections. Autoimmun. Rev. 2003, 2, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, F.P.; De Moura Guimarães, C.; Alberto Borges Peixoto, A.; Monezi Pontes, K.F.; Bonasoni, M.P.; Tonni, G.; Araujo Júnior, E. Parvovirus B19 Infection and Pregnancy: Review of the Current Knowledge. J. Pers. Med. 2024, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- De Cnc Garcia, R.; Leon, L.A. Human Parvovirus B19: A Review of Clinical and Epidemiologial Aspects in Brazil. Future Microbiol. 2021, 16, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Gallinella, G. Parvoviridae. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 259–277. [Google Scholar]
- Enders, M.; Weidner, A.; Enders, G. Current epidemiological aspects of human parvovirus B19 infection during pregnancy and childhood in the western part of Germany. Epidemiol. Infect. 2007, 135, 563–569. [Google Scholar] [CrossRef]
- Vyse, A.J.; Andrews, N.J.; Hesketh, L.M.; Pebody, R. The burden of parvovirus B19 infection in women of childbearing age in England and Wales. Epidemiol. Infect. 2007, 135, 1354–1362. [Google Scholar] [CrossRef]
- d’Humières, C.; Fouillet, A.; Verdurme, L.; Lakoussan, S.-B.; Gallien, Y.; Coignard, C.; Hervo, M.; Ebel, A.; Visseaux, B.; Maire, B.; et al. An unusual outbreak of parvovirus B19 infections, France, 2023 to 2024. Euro Surveill. 2024, 29, 2400339. [Google Scholar] [CrossRef]
- Tassis, B.; Testa, L.; Pampo, F.; Boito, S.; Tiso, G.; Accurti, V.; Cetin, I.; Persico, N. The 2024 Outbreak of Parvovirus B19 as a Global Obstetrical Threat Insights From an Obstetrics Referral Center in Northern Italy. J. Med. Virol. 2024, 11, e70046. [Google Scholar] [CrossRef]
- Makhlouf, M.M.; Elwakil, S.G.; Ibrahim, N. Molecular and serological assessment of parvovirus B-19 infection in Egyptian children with sickle cell disease. J. Microbiol. Immunol. Infect. 2017, 50, 565–569. [Google Scholar] [CrossRef]
- Zou, W.; Wang, Z.; Xiong, M.; Chen, A.Y.; Xu, P.; Ganaie, S.S.; Badawi, Y.; Kleiboeker, S.; Nishimune, H.; Ye, S.Q.; et al. Human Parvovirus B19 Utilizes Cellular DNA Replication Machinery for Viral DNA Replication. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ganaie Safder, S.; Qiu, J. Recent Advances in Replication and Infection of Human Parvovirus B19. Front. Cell. Infect. Microbiol. 2018, 8, 166. [Google Scholar]
- Luo, Y.; Qiu, J. Human parvovirus B19: A mechanistic overview of infection and DNA replication. Future Virol. 2015, 10, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Branda, F.; Ciccozzi, A.; Romano, C.; Sanna, D.; Casu, M.; Albanese, M.; Alessandri, F.; d’Ettorre, G.; Ciccozzi, M.; et al. Reassessing the Risk of Severe Parvovirus B19 Infection in the Immunocompetent Population: A Call for Vigilance in the Wake of Resurgence. Viruses 2024, 16, 1352. [Google Scholar] [CrossRef]
- Servant, A.; Laperche, S.; Lallemand, F.; Marinho, V.; De Saint Maur, G.; Meritet, J.F.; Garbarg-Chenon, A. Genetic diversity within human erythroviruses: Identification of three genotypes. J. Virol. 2002, 76, 9124–9134. [Google Scholar] [CrossRef]
- Huübschen, J.M.; Mihneva, Z.; Mentis, A.F.; Schneider, F.; Aboudy, Y.; Grossman, Z.; Rudich, H.; Kasymbekova, K.; Sarv, I.; Nedeljkovic, J.; et al. Phylogenetic analysis of human parvovirus B19 sequences from eleven different countries confirms the predominance of genotype 1 and suggests the spread of genotype 3b. J. Clin. Microbiol. 2009, 47, 3735–3738. [Google Scholar] [CrossRef]
- Gallinella, G.; Venturoli, S.; Manaresi, E.; Musiani, M.; Zerbini, M. B19 virus genome diversity: Epidemiological and clinical correlations. J. Clin. Virol. 2003, 28, 1–13. [Google Scholar] [CrossRef]
- Eis-Hübinger, A.M.; Reber, U.; Edelmann, A.; Kalus, U.; Hofmann, J. Parvovirus B19 genotype 2 in blood donations. Transfusion 2014, 54, 1682–1684. [Google Scholar] [CrossRef]
- Cong, B.; Koç, U.; Bandeira, T.; Bassat, Q.; Bont, L.; Chakhunashvili, G.; Cohen, C.; Desnoyers, C.; Hammitt, L.L.; Heikkinen, T.; et al. Changes in the global hospitalisation burden of respiratory syncytial virus in young children during the COVID-19 pandemic: A systematic analysis. Lancet Infect. Dis. 2024, 46, 361–374. [Google Scholar] [CrossRef]
- Gates, S.; Andreani, J.; Dewar, R.; Smith, D.B.; Templeton, K.; Child, H.T.; Bruer, J.; Golubchik, T.; Wade, M.J.; Jeffries, A.R.; et al. Postpandemic rebound of adeno-associated virus type 2 (AAV2) infections temporally associated with an outbreak of unexplained severe acute hepatitis in children in the United Kingdom. J. Med. Virol. 2023, 95, e28921. [Google Scholar] [CrossRef]
- Forero, E.L.; Knoester, M.; Gard, L.; Ott, A.; Brandenburg, A.H.; McCall, M.B.; Niesters, H.G.; Van Leer-Buter, C. Changes in enterovirus epidemiology after easing of lockdown measures. J. Clin. Virol. 2023, 169, 105617. [Google Scholar] [CrossRef] [PubMed]
- Molenaar-de Backer, M.W.; Hogema, B.M.; Koppelman, M.H.; van de Laar, T.J.; Slot, E.; Zaaijer, H.L. Lower Incidence of Parvovirus-B19 Infections in Dutch Blood Donors during SARS-CoV-2 Pandemic. Microbiol. Spectr. 2021, 9, e00253-21. [Google Scholar] [CrossRef] [PubMed]
- Russcher, A.; van Boven, M.; Benincà, E.; Verweij, E.J.T.; Molenaar-de Backer, M.W.; Zaaijer, H.L.; Vossen, A.C.T.M.; Kroes, A.C.M. Changing epidemiology of parvovirus B19 in the Netherlands since 1990, including its re-emergence after the COVID-19 pandemic. Sci Rep. 2024, 14, 9630. [Google Scholar] [CrossRef] [PubMed]
- Fourgeaud, J.; Allali, S.; Toubiana, J.; Pinhas, Y.; Frange, P.; Leruez-Ville, M.; Cohen, J.F. Post-COVID-19 pandemic outbreak of severe Parvovirus B19 primary infections in Paris, France: 10-year interrupted time-series analysis (2012-2023). J. Clin. Virol. 2023, 167, 105576. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.P.; Walther, F.J.; Kroes, A.C.M.; Oepkes, D. Parvovirus B19 infection in pregnancy: New insights and management. Prenat. Diagn. 2011, 31, 419–425. [Google Scholar] [CrossRef]
- Jain, A.; Kant, R. Genotypes of erythrovirus B19, their geographical distribution & circulation in cases with various clinical manifestations. Indian J. Med. Res. 2018, 147, 239–247. [Google Scholar]
- Karlin, D.G. Parvovirus B19 and Human Parvovirus 4 Encode Similar Proteins in a Reading Frame Overlapping the VP1 Capsid Gene. Viruses 2024, 16, 191. [Google Scholar] [CrossRef]
- Shackelton, L.A.; Holmes, E.C. Phylogenetic evidence for the rapid evolution of human B19 erythrovirus. J. Virol. 2006, 80, 3666–3669. [Google Scholar] [CrossRef]
- Stamenković, G.; Ćirković, V.; Šiljić, M.M.; Blagojević, J.V.; Knežević, A.M.; Joksić, I.D.; Stanojević, M.P. Substitution rate and natural selection in parvovirus B19. Sci. Rep. 2016, 6, 35759. [Google Scholar] [CrossRef]
- Jiang, H.; Qiu, Q.; Zhou, Y.; Xu, W.; Cui, A.; Li, X. The epidemiological and genetic characteristics of human parvovirus B19 in patients with febrile rash illnesses in China. Sci. Rep. 2023, 13, 15913. [Google Scholar] [CrossRef]
- Saikawa, T.; Anderson, S.; Momoeda, M.; Kajigaya, S.; Young, N.S. Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J. Virol. 1993, 67, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Momoeda, M.; Kawase, M.; Kajigaya, S.; Young, N.S. Peptides derived from the unique region of B19 parvovirus minor capsid protein elicit neutralizing antibodies in rabbits. Virology 1995, 206, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, G.J.; Cohen, B.J.; Field, A.M.; Oseas, R.; Blaese, R.M.; Young, N.S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J. Clin. Investig. 1989, 84, 1114–1123. [Google Scholar] [CrossRef]
- Kurtzman, G.; Frickhofen, N.; Kimball, J.; Jenkins, D.W.; Nienhuis, A.W.; Young, N.S. Pure Red-Cell Aplasia of 10 Years’ Duration Due to Persistent Parvovirus B19 Infection and Its Cure with Immunoglobulin Therapy. N. Engl. J. Med. 1989, 321, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; De Coninck, L.; Swinnen, J.; Lazrek, M.; Hober, D.; Matthijnssens, J. Four complete genomes of human parvovirus B19 from amniotic fluid specimens. Microbiol. Resour. Announc. 2023, 12, e0055623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).