Unraveling the Diversity of Haemosporidians in Brazilian Non-Passerine Birds: Insights from Midwestern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Study Area
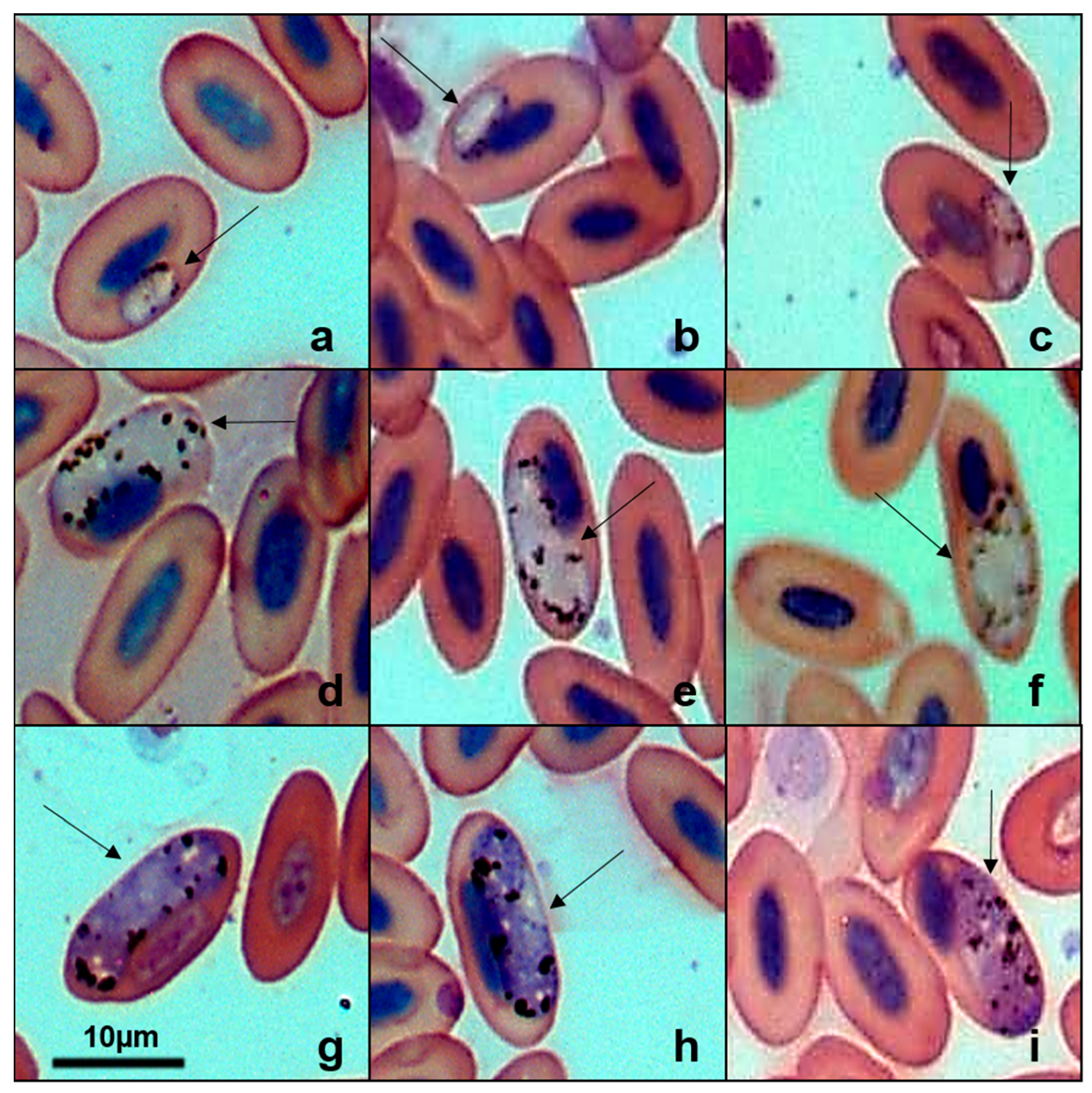
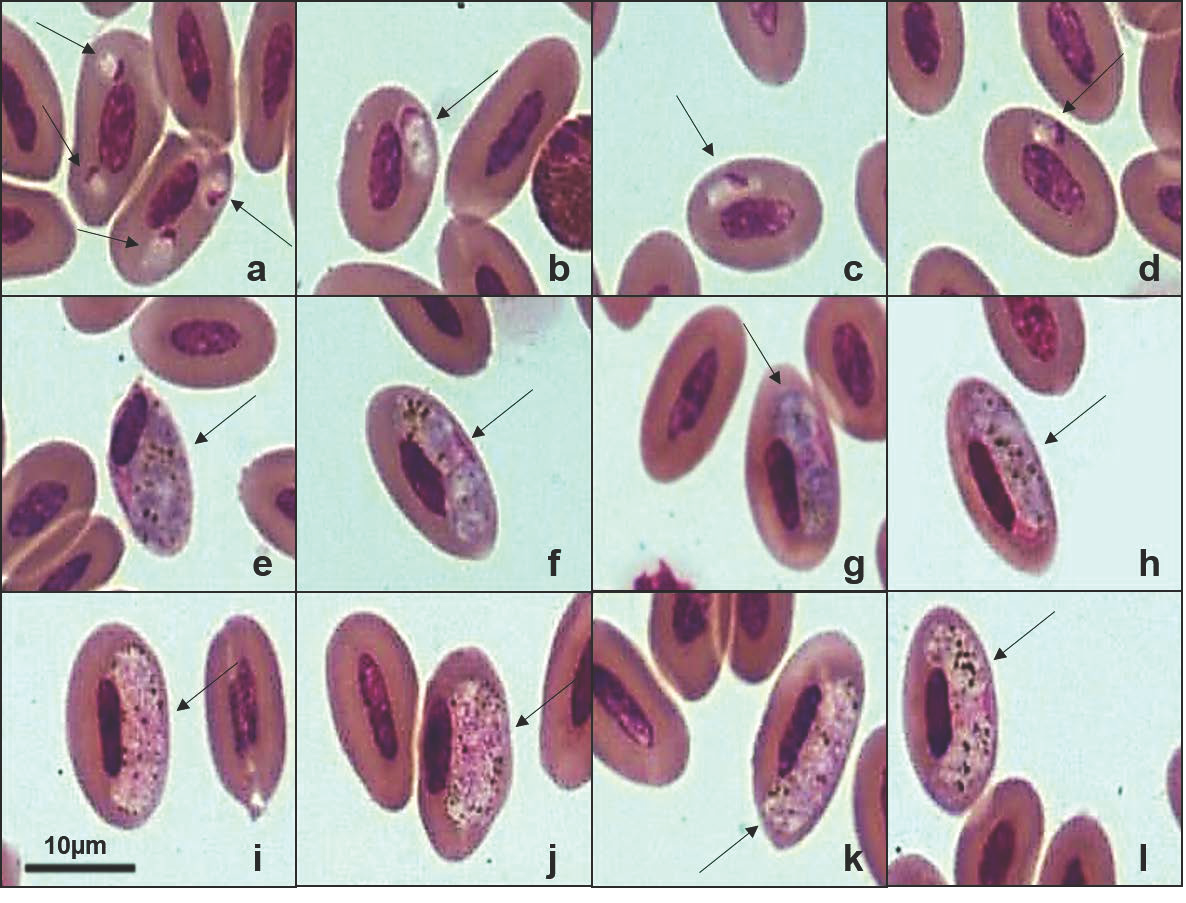
2.2. Microscopic Examination and Morphological Characterization
2.3. Molecular Detection of Plasmodium spp. and Haemoproteus spp.
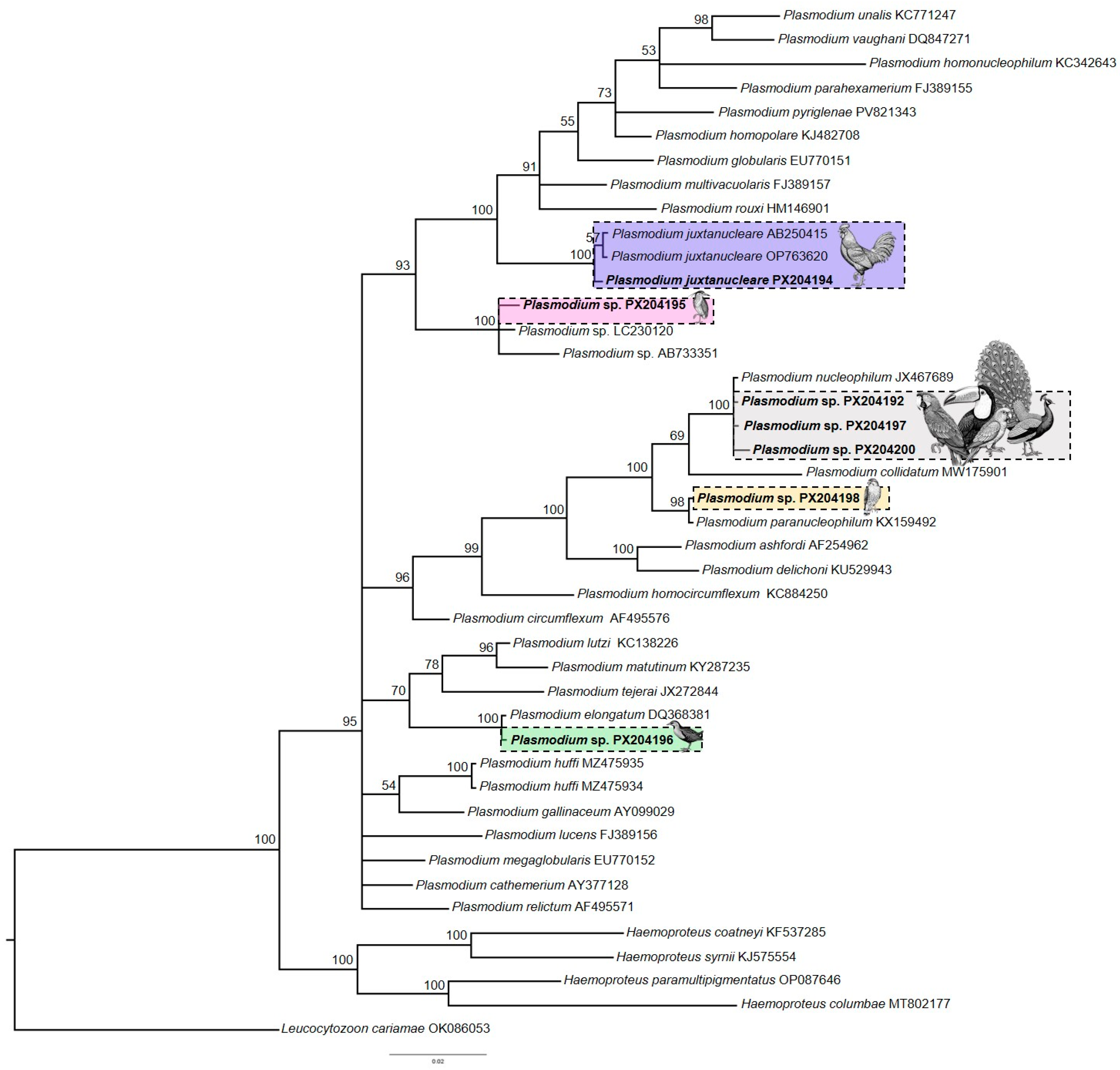
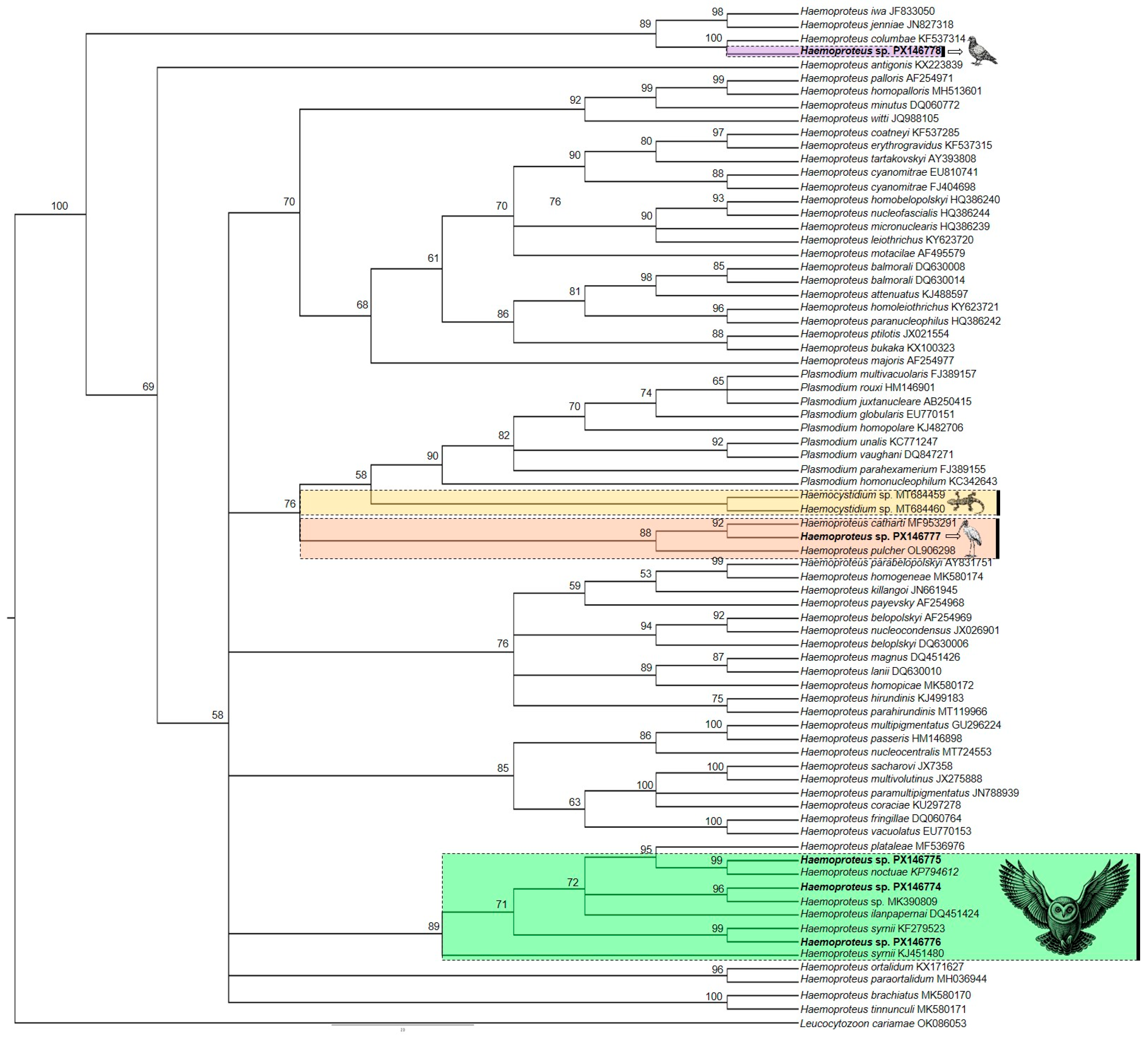
2.4. Phylogenetic Analysis
3. Results
3.1. Occurrence of Haemosporidian Infection
3.2. Molecular and Phylogenetic Analysis of Plasmodium spp.
3.3. Molecular, Phylogenetic, and Morphological Analysis of Haemoproteus spp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Atkinson, C.T.; LaPointe, D.A. Introduced avian diseases, climate change, and the future of Hawaiian honeycreepers. J. Avian Med. Surg. 2009, 23, 53–63. [Google Scholar] [CrossRef]
- Fecchio, A.; Chagas, C.R.; Bell, J.A.; Kirchgatter, K. Evolutionary ecology, taxonomy, and systematics of avian malaria and related parasites. Acta Trop. 2020, 204, 105364. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian malaria parasites. Malar. J. 2018, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Iezhova, T.A. Keys to the avian Haemoproteus parasites (Haemosporida, Haemoproteidae). Malar. J. 2022, 21, 269. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New insights from molecular data. Int. J. Parasitol. 2014, 44, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.F.; Silveira, L.F.; Aleixo, A.; Agne, C.E.; Bencke, G.A.; Bravo, G.A.; Brito, G.R.R.; Cohn-Haft, M.; Maurício, G.N.; Naka, L.N.; et al. Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee—Second edition. Ornithol. Res. 2021, 29, 94–105. [Google Scholar] [CrossRef]
- Moreno, G.; Higa, T.C.S.; Maitelli, G.T.; Oliveira, A.U.; Vilarinho Neto, C.S.; Leite, C.M.C.; Maitelli, G.T.; Ross, J.; Schwenk, L.M.; Araújo Neto, M.D.; et al. Geografia de Mato Grosso; Entrelinhas Editora: Cuiabá, Brazil, 2005. [Google Scholar]
- Durrant, K.L.; Beadell, J.S.; Ishtiaq, F.; Graves, G.R.; Olson, S.L.; Gering, E.; Peirce, M.A.; Milensky, C.M.; Schmidt, B.K.; Gebhard, C.; et al. Avian hematozoa in South America: A comparison of temperate and tropical zones. Ornithol. Monogr. 2006, 60, 98–111. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Junk, W.J.; Da Cunha, C.N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Marquis, R.J. The Cerrado of Brazil: Ecology and Natural History of a Neotropical Savanna; Columbia University Press: New York, NY, USA, 2002. [Google Scholar]
- Rosenfeld, G. Corante pancrômico para hematologia e citologia clínica. Nova combinação dos componentes do May-Grunwald e do Giemsa num só corante de emprego rápido. Mem. Inst. Butantan. 1974, 20, 329–335. [Google Scholar]
- Godfrey, R.D., Jr.; Fedynich, A.M.; Pence, D.B. Quantification of hematozoa in blood smears. J. Wildl. Dis. 1987, 23, 558–565. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Fallon, S.M.; Ricklefs, R.E.; Swanson, B.L.; Bermingham, E. Detecting avian malaria: An improved polymerase chain reaction diagnostic. J. Parasitol. 2003, 89, 1044–1047. [Google Scholar] [CrossRef]
- Roos, F.L.; Belo, N.O.; Silveira, P.; Braga, E.M. Prevalence and diversity of avian malaria parasites in migratory Black Skimmers (Rynchops niger, Laridae, Charadriiformes) from the Brazilian Amazon Basin. Parasitol. Res. 2015, 114, 3903–3911. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Péarez-Tris, J.; Waldenströum, J.; Hellgren, O. Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: Multiple cases of cryptic speciation? Evolution 2004, 58, 1617–1621. [Google Scholar] [CrossRef]
- Minh, B.Q.; Trifinopoulos, J.; Schrempf, D.; Schmidt, H.A.; Lanfear, R. IQ-TREE Version 2.0: Tutorials and Manual. Phylogenomic Software by Maximum Likelihood. 2019. Available online: http://www.iqtree.org (accessed on 9 September 2025).
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Vieira, L.M.D.C.; Pereira, P.H.O.; da Rocha Vilela, D.A.; Landau, I.; Pacheco, M.A.; Escalante, A.A.; Ferreira, F.C., Jr.; Braga, É.M. Leucocytozoon cariamae n. sp. and Haemoproteus pulcher coinfection in Cariama cristata (Aves: Cariamiformes): First mitochondrial genome analysis and morphological description of a leucocytozoid in Brazil. Parasitology 2023, 150, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Pornpanom, P.; Chagas, C.R.F.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Valkiūnas, G.; Salakij, C. Molecular prevalence and phylogenetic relationship of Haemoproteus and Plasmodium parasites of owls in Thailand: Data from a rehabilitation centre. Int. J. Parasitol. Parasites Wildl. 2019, 9, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Karadjian, G.; Martinsen, E.; Duval, L.; Chavatte, J.M.; Landau, I. Haemoproteus ilanpapernai n. sp. (Apicomplexa, Haemoproteidae) in Strix seloputo from Singapore: Morphological description and reassignment of molecular data. Parasite 2014, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, A.; Palinauskas, V.; Platonova, E.; Kobylkov, D.; Vakoliuk, I.; Valkiūnas, G. The strategy to survive primary malaria infection: An experimental study on behavioral changes in parasitized birds. PLoS ONE 2016, 11, e0159216. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Guimarães, L.d.O.; Monteiro, E.F.; Valkiūnas, G.; Katayama, M.V.; Santos, S.V.; Guida, F.J.V.; Simões, R.F.; Kirchgatter, K. Hemosporidian parasites of free-living birds in the São Paulo Zoo, Brazil. Parasitol. Res. 2016, 115, 1443–1452. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Valkiūnas, G.; de Oliveira Guimarães, L.; Monteiro, E.F.; Guida, F.J.V.; Simões, R.F.; Rodrigues, P.T.; de Albuquerque, L.E.J.; Kirchgatter, K. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar. J. 2017, 16, 83. [Google Scholar] [CrossRef]
- Belo, N.O.; Passos, L.F.; Júnior, L.M.C.; Goulart, C.E.; Sherlock, T.M.; Braga, E.M. Avian malaria in captive psittacine birds: Detection by microscopy and 18S rRNA gene amplification. Prev. Vet. Med. 2009, 88, 220–224. [Google Scholar] [CrossRef]
- Morel, A.P.; Webster, A.; Prusch, F.; Anicet, M.; Marsicano, G.; Trainini, G.; Stocker, J.; Giani, D.; Bandarra, P.M.; da Rocha, M.I.S.; et al. Molecular detection and phylogenetic relationship of Haemosporida parasites in free-ranging wild raptors from Brazil. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100521. [Google Scholar] [CrossRef]
- Vanstreels, R.E.T.; Kolesnikovas, C.K.; Sandri, S.; Silveira, P.; Belo, N.O.; Ferreira, F.C., Jr.; Epiphanio, S.; Steindel, M.; Braga, E.M.; Catao-Dias, J.L. Outbreak of avian malaria associated to multiple species of Plasmodium in magellanic penguins undergoing rehabilitation in southern Brazil. PLoS ONE 2014, 9, e94994. [Google Scholar] [CrossRef]
- Anjos, C.C.; Chagas, C.R.; Fecchio, A.; Schunck, F.; Costa-Nascimento, M.J.; Monteiro, E.F.; Mathias, B.S.; Bell, J.A.; Guimarães, L.O.; Comiche, K.J.M.; et al. Avian malaria and related parasites from resident and migratory birds in the Brazilian Atlantic Forest, with description of a new Haemoproteus species. Pathogens 2021, 10, 103. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 3 December 2025).
- Marzal, A.; Magallanes, S.; Salas-Rengifo, T.; Muriel, J.; Navarro, C.; Vecco, D.; Guerra-Saldaña, C.; Mendo, L.; Paredes, V.; González-Blázquez, M.; et al. Prevalence and diversity of avian malaria parasites in illegally traded white-winged parakeets in Peruvian Amazonas. Anim. Conserv. 2024, 27, 364–373. [Google Scholar] [CrossRef]
- Ortiz-Catedral, L.; Brunton, D.; Stidworthy, M.F.; Elsheikha, H.M.; Pennycott, T.; Schulze, C.; Braun, M.; Wink, M.; Gerlach, H.; Pendl, H.; et al. Haemoproteus minutus is highly virulent for Australasian and South American parrots. Parasites Vectors 2019, 12, 40. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Pendl, H.; Olias, P. New Haemoproteus parasite of parrots, with remarks on the virulence of haemoproteids in naive avian hosts. Acta Trop. 2017, 176, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.E. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 1968, 70, 101–120. [Google Scholar] [CrossRef]
- Collar, N.; Boesman, P.F.D.; Sharpe, C.J. Red-and-green Macaw (Ara chloropterus), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Collar, N.; Boesman, P.F.D.; Sharpe, C.J. White-eyed Parakeet (Psittacara leucophthalmus), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Sánchez-Mercado, A.; Asmüssen, M.; Rodríguez, J.P.; Moran, L.; Cardozo-Urdaneta, A.; Morales, L.I. Illegal trade of the Psittacidae in Venezuela. Oryx 2020, 54, 77–83. [Google Scholar] [CrossRef]
- Ramesh, K.; McGowan, P. On the current status of Indian peafowl Pavo cristatus (Aves: Galliformes: Phasianidae): Keeping the common species common. J. Threat. Taxa 2009, 1, 106–108. [Google Scholar] [CrossRef]
- Tostes, R.; Dias, R.J.P.; Martinele, I.; Senra, M.V.X.; D’Agosto, M.; Massard, C.L. Multidisciplinary re-description of Plasmodium (Novyella) paranucleophilum in Brazilian wild birds of the Atlantic Forest kept in captivity. Parasitol. Res. 2017, 116, 1887–1897. [Google Scholar] [CrossRef]
- Ferreira, F.C., Jr.; de Angeli Dutra, D.; Silveira, P.; Pacheco, R.C.; Witter, R.; de Souza Ramos, D.G.; Pacheco, M.A.; Escalante, A.A.; Braga, É.M. A new pathogen spillover from domestic to wild animals: Plasmodium juxtanucleare infects free-living passerines in Brazil. Parasitology 2018, 145, 1949–1958. [Google Scholar] [CrossRef]
- Bishop, M.A.; Bennett, G.F. The haemoproteids of the avian order Strigiformes. Can. J. Zool. 1989, 67, 2676–2684. [Google Scholar] [CrossRef]
- Karadjian, G.; Puech, M.P.; Duval, L.; Chavatte, J.M.; Snounou, G.; Landau, I. Haemoproteus syrnii in Strix aluco from France: Morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite 2013, 20, 32. [Google Scholar] [CrossRef]
- Mayer, M.A. Über sein Entwicklung von Halteridium. Arch. Fur Schiffs Und Tropenhygiene 1910, 14, 197–202. [Google Scholar]
- Barino, G.T.M.; Rossi, M.F.; De Oliveira, L.; Reis, J.L., Jr.; D’Agosto, M.; Dias, R.J.P. Haemoproteus syrnii (Haemosporida: Haemoproteidae) in owls from Brazil: Morphological and molecular characterization, potential cryptic species, and exo-erythrocytic stages. Parasitol. Res. 2021, 120, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Giorgiadis, M.; Guillot, J.; Duval, L.; Landau, I.; Quintard, B. Haemosporidian parasites from captive Strigiformes in France. Parasitol. Res. 2020, 119, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Bukauskaitė, D.; Žiegytė, R.; Palinauskas, V.; Iezhova, T.A.; Dimitrov, D.; Ilgūnas, M.; Bernotienė, R.; Markovet, M.Y.; Valkiūnas, G. Biting midges (Culicoides, Diptera) transmit Haemoproteus parasites of owls: Evidence from sporogony and molecular phylogeny. Parasites Vectors 2015, 8, 303. [Google Scholar] [CrossRef]
- Kruse, W. Ueber blutparasiten. Arch. Für Pathol. Anat. Und Physiol. Und Für Klin. Med. 1890, 120, 541–560. [Google Scholar]
- Baker, J.R. Epizootiology of some haematozoic protozoa of English birds. J. Nat. Hist. 1975, 9, 601–609. [Google Scholar] [CrossRef]
- Adriano, E.A.; Cordeiro, N.S. Prevalence and intensity of Haemoproteus columbae in three species of wild doves from Brazil. Mem. Do Inst. Oswaldo Cruz. 2001, 96, 175–178. [Google Scholar] [CrossRef]
- Villar, C.M.; Bryan, A.L., Jr.; Lance, S.L.; Braga, É.M.; Congrains, C.; Del Lama, S.N. Blood parasites in nestlings of wood stork populations from three regions of the American continent. J. Parasitol. 2013, 99, 522–527. [Google Scholar] [CrossRef]
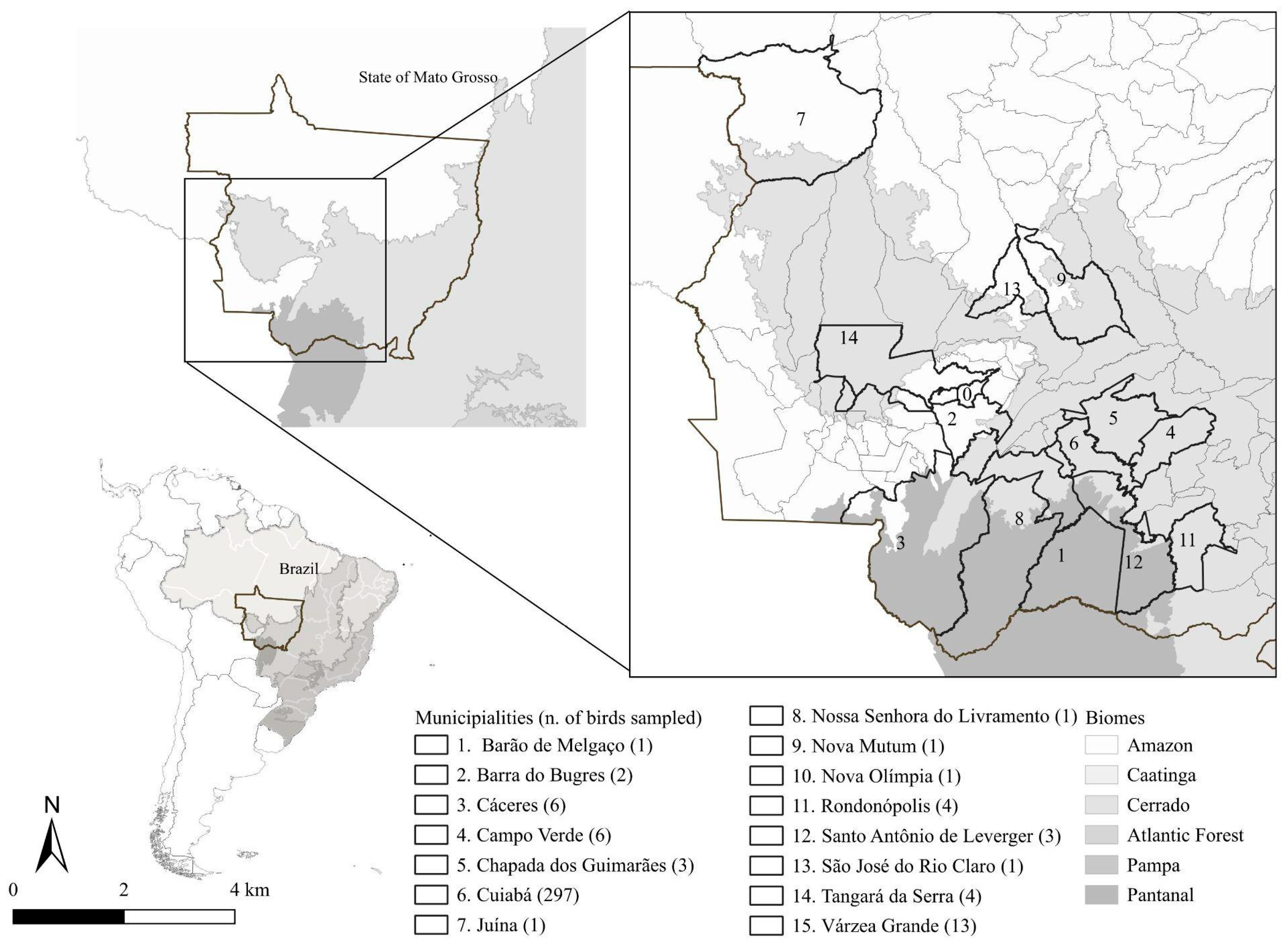
| Order (No. of Birds) Species (No. of Birds) | Common Name (Degree of Threat a) | No. of Birds Tested in Blood Smear | No. of Birds Tested in cPCR and nPCR | Origin b (No. of Birds) | Municipality c (No. of Birds) |
|---|---|---|---|---|---|
| Accipitriformes (41) | |||||
| Gampsonyx swainsonii (1) | Pearl Kite (LC) | 1 | ne d | FL (1) | 6 (1) |
| Harpia harpija (1) | Harpy Eagle (NT) | 1 | ne | FL (1) | 7 (1) |
| Heterospizias meridionalis (2) | Savanna Hawk (LC) | 1 | 2 | FL (2) | 3 (2) |
| Ictinia plumbea (3) | Plumbeous Kite (LC) | 3 | ne | FL (3) | 6 (2), 13 (1) |
| Pandion haliaetus (1) | Osprey (LC) | 1 | ne | FL (1) | 6 (1) |
| Rupornis magnirostris (32) | Roadside Hawk (LC) | 31 | 14 | FL (18), C (14) | 1 (1), 4 (1), 6 (28), 15 (2) |
| Urubitinga urubutinga (1) | Great Black Hawk (LC) | 1 | ne | FL (1) | 6 (1) |
| Anseriformes (6) | |||||
| Anser cygnoides (1) | Swan Goose (EN) | 1 | 1 | C (1) | 6 (1) |
| Cairina moschata (3) | Muscovy Duck (LC) | ne | 3 | C (3) | 6 (3) |
| Chauna torquata (2) | Southern Screamer (LC) | ne | 2 | C (2) | 6 (2) |
| Cariamiformes (9) | |||||
| Cariama cristata (9) | Red-legged Seriema (LC) | 4 | 8 | FL (7), C (2) | 6 (7), 8 (1), 11 (1) |
| Cathartiformes (1) | |||||
| Coragyps atratus (1) | Black Vulture (LC) | 1 | 1 | FL (1) | 6 (1) |
| Ciconiiformes (5) | |||||
| Jabiru mycteria (4) | Jabiru (LC) | 1 | 4 | FL (4) | 6 (4) |
| Mycteria americana (1) | Wood Stork (LC) | 1 | 1 | FL (1) | 10 (1) |
| Columbiformes (3) | |||||
| Columba livia (1) | Rock Pigeon (LC) | ne | 1 | FL (1) | 6 (1) |
| Geotrygon montana (1) | Ruddy Quail-Dove (LC) | ne | 1 | FL (1) | 6 (1) |
| Patagioenas picazuro (1) | Picazuro Pigeon (LC) | 1 | 1 | FL (1) | 14 (1) |
| Cuculiformes (1) | |||||
| Piaya cayana (1) | Squirrel Cuckoo (LC) | 1 | 1 | FL (1) | 6 (1) |
| Falconiformes (18) | |||||
| Caracara Plancus (12) | Southern Caracara (LC) | 12 | 3 | FL (5), C (7) | 6 (12) |
| Falco femoralis (2) | Aplomado Falcon (LC) | 1 | 1 | C (2) | 6 (2) |
| Falco sparverius (1) | American Kestrel (LC) | 1 | ne | C (1) | 6 (1) |
| Herpetotheres cachinnans (1) | Laughing Falcon (LC) | 1 | 1 | FL (1) | 6 (1) |
| Milvago chimachima (1) | Yellow-headed Caracara (LC) | 1 | 1 | FL (1) | 6 (1) |
| Micrastur semitorquatus (1) | Collared Forest-Falcon (LC) | 1 | 1 | FL (1) | 3 (1) |
| Galiformes (9) | |||||
| Gallus gallus (7) | Domestic chicken (LC) | 7 | 4 | C (7) | 6 (7) |
| Ortalis conicollis (1) | Chaco chachalaca (LC) | 1 | 1 | FL (1) | 15 (1) |
| Pavo cristatus (1) | Indian Peafowl (LC) | 1 | 1 | C (1) | 6 (1) |
| Gruiformes (2) | |||||
| Mustelirallus albicollis (1) | Ash-throated Crake (LC) | 1 | 1 | FL (1) | 6 (1) |
| Porphyrio martinica (1) | Purple Gallinule (LC) | ne | 2 | FL (1) | 6 (1) |
| Nyctibiiformes (5) | |||||
| Nyctibius grandis (1) | Great Potoo (LC) | 1 | 1 | FL (1) | 6 (1) |
| Nyctibius griseus (4) | Common Potoo (LC) | 4 | 4 | FL (4) | 6 (4) |
| Pelecaniformes (3) | |||||
| Bulbucus ibis (1) | Cattle Egret (LC) | 1 | 1 | FL (1) | 6 (1) |
| Egretta thula (1) | Snowy Egret (LC) | 1 | 1 | FL (1) | 6 (1) |
| Nycticorax nycticorax (1) | Black-crowned Night-Heron (LC) | 1 | 1 | FL (1) | 6 (1) |
| Piciformes (13) | |||||
| Pteroglossus castanotis (1) | Chestnut-eared Aracari (LC) | 1 | ne | FL (1) | 5 (1) |
| Ramphastos toco (11) | Toco toucan (LC) | ne | 11 | FL (3), C (8) | 6 (11) |
| Veniliornis affinis (1) | Red-stained Woodpecker (LC) | 1 | 1 | FL (1) | 6 (1) |
| Psittaciformes (186) | |||||
| Agapornis roseicolis (2) | Agapornis (LC) | ne | 2 | C (2) | 6 (2) |
| Alipiopsitta xanthops (3) | Yellow-faced Parrot (NT) | 1 | 3 | FL (1), C (2) | 6 (3) |
| Amazona aestiva (9) | Turquoise-fronted Parrot (LC) | 4 | 8 | FL (4), C (5) | 6 (9) |
| Amazona amazonica (17) | Orange-winged Parrot (LC) | 5 | 16 | FL (4), C (13) | 6 (17) |
| Amazona ochrocephala (2) | Yellow-crowned Parrot (LC) | 1 | 1 | FL (1), C (1) | 6 (2) |
| Anodorhynchus hyacinthinus (3) | Hyacinth Macaw (VU) | 1 | 3 | FL (3) | 4 (1), 6 (2) |
| Ara ararauna (95) | Blue-and-yellow Macaw (LC) | 73 | 56 | FL (86), C (9) | 3 (2), 4 (4), 5 (1), 6 (82), 11(3), 14 (1), 15 (2) |
| Ara chloropterus (9) | Red-and-green Macaw (LC) | 4 | 9 | FL (6), C (3) | 2 (1), 6 (5), 15 (3) |
| Ara macao (2) | Scarlet Macaw (LC) | ne | 2 | FL (2) | 6 (2) |
| Ara severus (1) | Chestnut-fronted Macaw (LC) | 1 | 1 | C (1) | 6 (1) |
| Brotogeris chiriri (8) | Yellow-chevroned Parakeet (LC) | 4 | 7 | FL (7), C (1) | 6 (7), 15 (1) |
| Diopsittaca nobilis (5) | Red-shouldered Macaw (LC) | 2 | 4 | FL (5) | 6 (3), 15 (2) |
| Eupsittula aurea (9) | Peach-fronted Parakeet (LC) | 4 | 8 | FL (7), C (2) | 5 (1), 6 (8) |
| Nymphicus hollandicus (15) | Cockatiel (LC) | 14 | 15 | C (15) | 6 (14), 15 (1) |
| Primolius auricolis (2) | Yellow-collared Macaw (LC) | 1 | 2 | FL (1), C (1) | 6 (2) |
| Primolius maracana (2) | Blue-winged Macaw Kite (NT) | 1 | 1 | FL (1), C (1) | 6 (2) |
| Psittacara leucophtalmus (2) | White-eyed Parakeet (LC) | 2 | 2 | FL (1), C (1) | 6 (2) |
| Rheiformes (2) | |||||
| Rhea americana (2) | Greater Rhea (NT) | ne | 2 | C (2) | 6 (2) |
| Strigiformes (40) | |||||
| Asio clamator (5) | Spided Owl (LC) | 4 | 3 | FL (2), C (3) | 6 (5) |
| Athene cunicularia (10) | Burrowing Owl (LC) | 9 | 6 | FL (6), C (4) | 6 (8), 12 (2) |
| Glaucidium brasilianum (4) | Ferruginous Pygmy-Owl (LC) | 4 | 1 | FL (1), C (3) | 6 (2), 14 (2) |
| Megascops choliba (7) | Tropical Screech-Owl (LC) | 7 | 4 | FL (2), C (5) | 2 (1), 6 (5), 12 (1) |
| Tyto furcata (14) | American Barn Owl (LC) | 13 | 9 | FL (4), C (10) | 3 (1), 6 (11), 9 (1), 15 (1) |
| Total (344) | 241 | 240 | FL (212), C (132) | 1 (1), 2 (2), 3 (6), 4 (6), 5 (3), 6 (297), 7 (1), 8 (1), 9 (1), 10 (1), 11 (4), 12 (3), 13 (1), 14 (4), 15 (13) |
| Order Species (No. of Birds) | No. of Birds with Positive Blood Smears/Total Blood Smears Tested (%) (Parasitemia per Individual %) | No. of Birds Testing Positive in cPCR/Total n. of Birds Tested in cPCR (%) | No. of Birds Testing Positive in nPCR/Total No. of Birds Tested in nPCR (%) | No. of Sequences Obtained | % Similarity of Genus/Species of Hemosporid (GenBank Access Code)/Lineage—per Individual | Origin/City of Origin of Birds with Sequences Recovered—per Individual ab |
|---|---|---|---|---|---|---|
| Accipitriformes | ||||||
| Heterospizias meridionalis (2) | 0/1 | 1/2 (50) | 0/2 (0) | 0 | ||
| Rupornis magnirostris (32) | 2/31 (6.4) (0.005; 0.015) | 5/14 (35.7) | 3/14 (21.4) | 2 | 100% Plasmodium paranucleophilum (PX204193; PX204198)/CPCT5 7 d,e | FL/6; FL/6 |
| Cariamiformes | ||||||
| Cariama cristata (9) | 1/4 (25) (0.12) | 2/8 (25) | 2/8 (25) | 0 | ||
| Ciconiiformes | ||||||
| Jabiru mycteria (4) | 0/1 | 2/4 (50) | 2/4 (50) | 0 | ||
| Mycteria americana (1) | 1/1 (100) (0.16) | 1/1 (100) | 1/1 (100) | 1 | 100% Haemoproteus sp. (PX146777)/BULIBH1 | FL/10 |
| Columbiformes | ||||||
| Patagioenas picazuro (1) | 1/1 (100) (0.12) | 1/1 (0) | 1/1 (100) | 1 | 98.54% Haemoproteus columbae (PX146778)/PATPIC1061328 e | FL/14 |
| Falconiformes | ||||||
| Caracara plancus (12) | 3/12 (25) (0.005; 0.085; 0.15) | 1/3 (33.3) | 0/3 (0) | 0 | ||
| Galliformes | ||||||
| Gallus gallus (7) | 1/7 (14.3) (0.005) | 1/4 (25) | 1/4 (25) | 1 | 100% Plasmodium juxtanucleare (PX204194)/Pj221 | C/6 |
| Pavo cristatus (1) | ne c | 1/1 (100) | 1/1 (100) | 1 | 99.79% Plasmodium nucleophilum (PX204197)/PAVCRI1088056 e | |
| Gruiformes | ||||||
| Mustelirallus albicollis (1) | 1/1 (100) (0.02) | 1/1 (100) | 1/1 (100) | 1 | 100% Plasmodium elongatum (PX204196)/MF101820 | FL/6 |
| Porphyrio martinica (1) | ne | 1/1 (100) | 1/1 (100) | |||
| Pelecaniformes | ||||||
| Nycticorax nycticorax (1) | 1/1 (100) (0.12) | 1/1 (100) | 1/1 (100) | 1 | 100% Plasmodium sp. (PX204195)/CETASFLO-R0263 | FL/6 |
| Piciformes | ||||||
| Ramphastos toco (11) | ne | 4/11 (36.4) | 4/11 (36.4) | 1 | 100% Plasmodium sp. (PX204199)/GAL-2012 | C/6 |
| Psittaciformes | ||||||
| Agapornis roseicolis (2) | ne | 0/2 | 2/2 (100) | 0 | ||
| Alipiopsitta xanthops (3) | 0/1 | 2/3 (66.7) | 2/3 (66.7) | 0 | ||
| Amazona aestiva (9) | 0/4 | 3/8 (37.5) | 0/8 | 0 | ||
| Amazona amazonica (17) | 0/5 | 1/16 (6.2) | 0/16 | 0 | ||
| Ara ararauna (95) | 1/73 (1.4) (0.045) | 7/55 (12.7) | 3/55 (5.4) | 0 | ||
| Ara chloropterus (9) | 0/4 | 1/9 (11.1) | 1/9 (11.1) | 1 | 99.37% Plasmodium nucleophilum (PX204200)/ARACLOS-1657 e | FL/2 |
| Brotogeris chiriri (8) | 0/4) | 2/7 (28.6) | 0/7 | 0 | ||
| Diopsittaca nobilis (5) | 0/2 | 1/4 (25) | 0/4 | 0 | ||
| Eupsittula aurea (9) | 0/4 | 1/8 (12.5) | 0/8 | 0 | ||
| Nymphicus hollandicus (15) | 0/14 | 3/15 (20) | 0/15 | 0 | ||
| Psittacara leucophtalmus (2) | 1/2 (50) (0.005) | 1/2 (50) | 1/2 (50) | 1 | 100% Plasmodium nucleophilum (PX204192)/RVCT27 | C/6 |
| Strigiformes | ||||||
| Asio clamator (5) | 4/4 (100) (0.525; 0.270; 0.180; 0.085) | 2/3 (66.7) | 2/3 (66.7) | 1 | 99.16% Haemoproteus syrnii (PX146776)/ASICLAS-1682 e | FL/6 |
| Athene cunicularia (10) | 1/9 (11.1) (0.015) | 4/6 (66.7) | 1/6 (16.7) | 1 | 99.58% Haemoproteus noctuae (PX146775)/ATHCUNS-1386 e | FL/6 |
| Glaucidium brasilianum (4) | 0/4 (0) | 1/1 (100) | 0/1 | 0 | ||
| Megascops choliba (7) | 4/7 (57.1) (0.01; 0.105; 0.140; 0.105) | 0/4 | 0/4 | 0 | ||
| Tyto furcata (14) | 3/13 (23.1) (0.2; 0.125; 0.36) | 3/9 (33.3) | 1/9 (11.1) | 1 | 98.12% Haemoproteus plataleae (PX146774)/TYTFURCS-1384 e | FL/9 |
| Total (%) | 25/241 (10.37) | 53/240 (22.08) | 30/240 (12.5) | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennig, M.M.; Alves, L.G.M.; Rocha, M.N.A.; Silva, V.L.d.B.; Rosa, B.M.; Ferraz, R.H.d.S.; Corrêa, S.H.R.; Braga, É.M.; Pacheco, R.d.C. Unraveling the Diversity of Haemosporidians in Brazilian Non-Passerine Birds: Insights from Midwestern Brazil. Pathogens 2025, 14, 1286. https://doi.org/10.3390/pathogens14121286
Hennig MM, Alves LGM, Rocha MNA, Silva VLdB, Rosa BM, Ferraz RHdS, Corrêa SHR, Braga ÉM, Pacheco RdC. Unraveling the Diversity of Haemosporidians in Brazilian Non-Passerine Birds: Insights from Midwestern Brazil. Pathogens. 2025; 14(12):1286. https://doi.org/10.3390/pathogens14121286
Chicago/Turabian StyleHennig, Morgana Maira, Luiz Gustavo Magalhães Alves, Marcela Natacha Aparecida Rocha, Victória Luiza de Barros Silva, Brenda Madruga Rosa, Rosa Helena dos Santos Ferraz, Sandra Helena Ramiro Corrêa, Érika Martins Braga, and Richard de Campos Pacheco. 2025. "Unraveling the Diversity of Haemosporidians in Brazilian Non-Passerine Birds: Insights from Midwestern Brazil" Pathogens 14, no. 12: 1286. https://doi.org/10.3390/pathogens14121286
APA StyleHennig, M. M., Alves, L. G. M., Rocha, M. N. A., Silva, V. L. d. B., Rosa, B. M., Ferraz, R. H. d. S., Corrêa, S. H. R., Braga, É. M., & Pacheco, R. d. C. (2025). Unraveling the Diversity of Haemosporidians in Brazilian Non-Passerine Birds: Insights from Midwestern Brazil. Pathogens, 14(12), 1286. https://doi.org/10.3390/pathogens14121286





