Epidemiology of Chewing Lice (Phthiraptera: Mallophaga) Fauna of Poultry in Sub-Saharan Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Article Selection Process and Criteria Used
2.3. Charting the Data and Summarizing the Results
3. Results
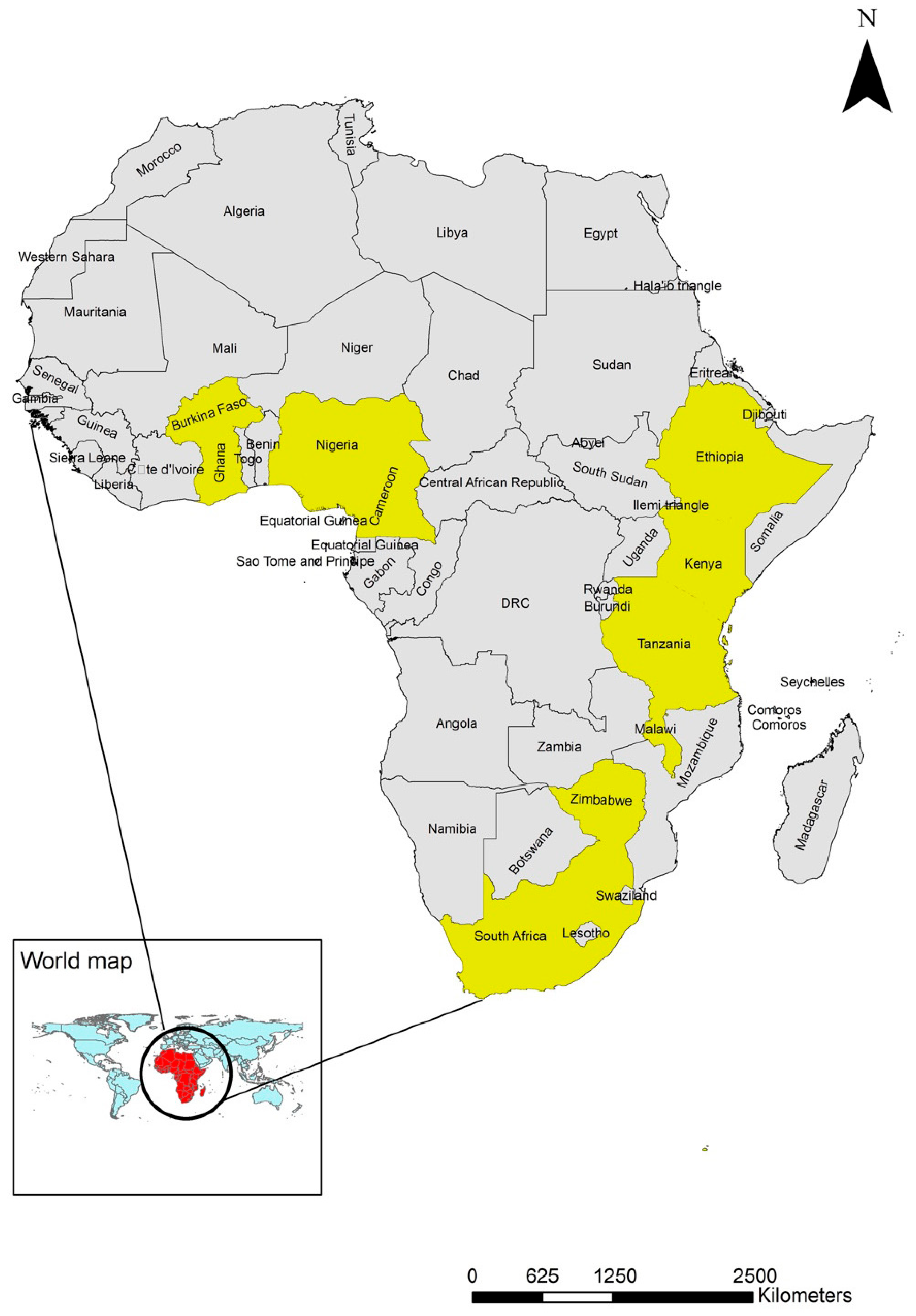
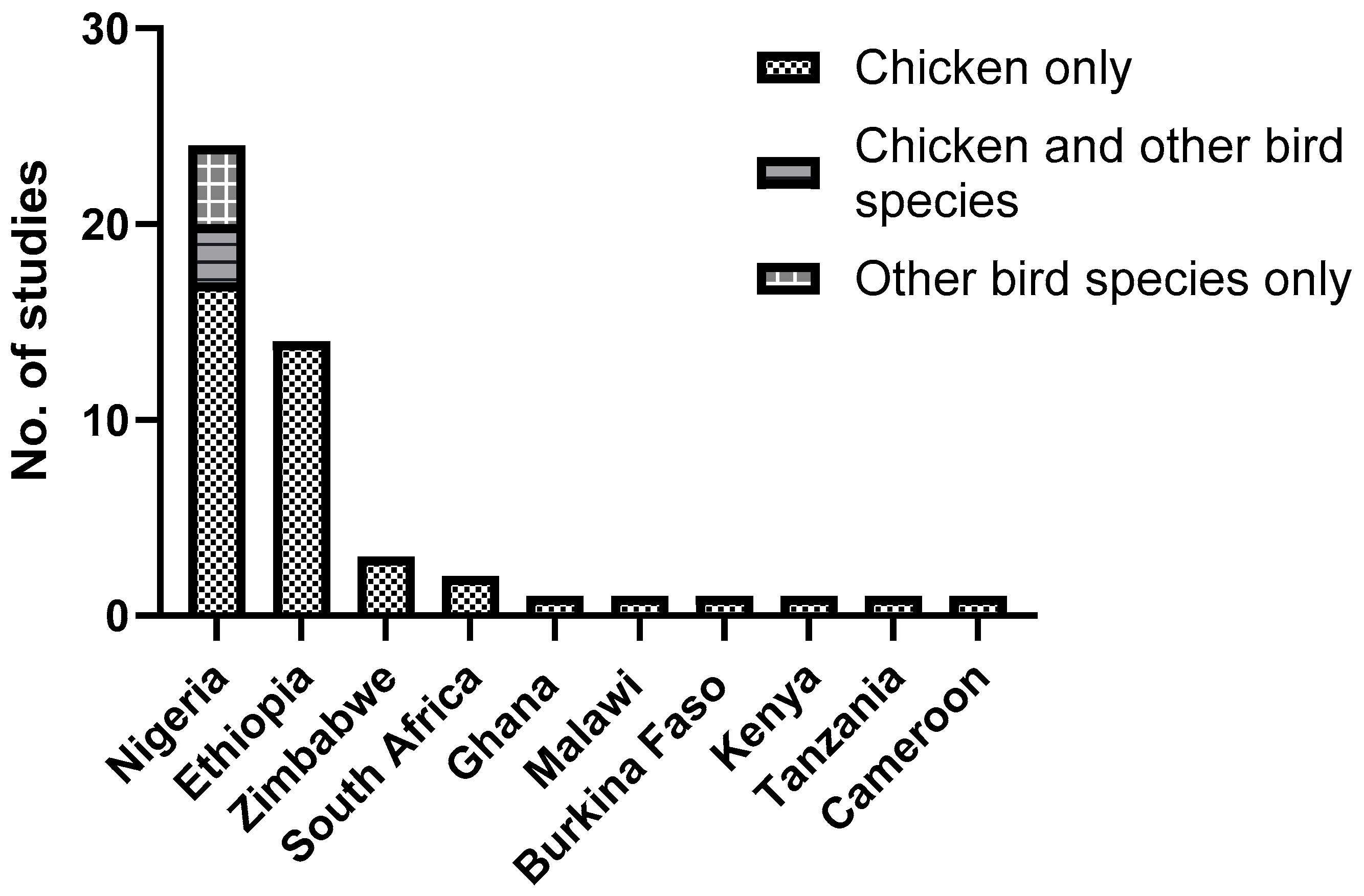
3.1. Geographical Distribution and Species Diversity of Chewing Lice in Poultry
3.2. Prevalence of Chewing Lice
3.3. Risk Factors Influencing the Distribution of Chewing Lice in Poultry
3.3.1. Age of the Birds
3.3.2. Sex of the Birds
3.3.3. Breed of the Birds
3.3.4. Husbandry Practices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shitta, K.B.; James-Rugu, N.N.; Chessed, G.; Ngwamah, J.S. Ectoparasites of chickens in Wukari Local Government Area of Taraba State, North-East Nigeria. Niger. J. Parasitol. 2017, 38, 39–42. [Google Scholar] [CrossRef]
- Tamiru, F.; Dagmawit, A.; Askale, G.; Solomon, S.; Morka, D.; Waktole, T. Prevalence of ectoparasite infestation in chicken in and around Ambo Town. Ethiopia. J. Vet. Sci. Technol. 2014, 5, 10–4172. [Google Scholar]
- Ogada, S.; Lichoti, J.; Oyier, P.A. A survey on disease prevalence, ectoparasite infestation and chick mortality in poultry populations of Kenya. Livest. Res. Rural Dev. 2015, 28, 1–13. [Google Scholar]
- Amede, Y.; Tilahun, K.; Bekele, M. Prevalence of ectoparasites in Haramaya University intensive poultry farm. Glob. Vet. 2011, 7, 264–269. [Google Scholar]
- Aboagye, I.F.; Korang, E.; Offeh, A.; Davis, H.E. Assessment of ectoparasitic infestation in chickens (Gallus gallus domesticus) in the Sunyani west district, Ghana. J. Sci. Technol. 2014, 34, 11–17. [Google Scholar] [CrossRef]
- Mata, W.; Galgalo, W.; Jilo, K. Prevalence of the major ectoparasites of poultry in extensive and intensive farms in Jimma, Southwestern Ethiopia. J. Parasitol. Vector Biol. 2018, 10, 87–96. [Google Scholar]
- Prakashbabu, B.C.; Thenmozhi, V.; Limon, G.; Kundu, K.; Kumar, S.; Garg, R.; Clark, E.L.; Rao, A.S.; Raj, D.G.; Raman, M.; et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet. Parasitol. 2017, 233, 62–72. [Google Scholar] [CrossRef]
- Kebede, A.; Abebe, B.; Zewdie, T. Study on prevalence of ectoparasites of poultry in and around Jimma town. Eur. J. Biol. Sci. 2017, 9, 18–26. [Google Scholar]
- Tompkins, D.M.; Clayton, D. Host resources govern the specificity of swiftlet lice: Size matters. J. Anim. Ecol. 1999, 68, 489–500. [Google Scholar] [CrossRef]
- Johnson, K.P.; Clayton, D.H.; Price, R.D.; Hellenthal, R.A.; Palma, R.L. The Chewing Lice: World Checklist and Biological Overview; Illinois Natural History Survey Special Publication: Champaign, IL, USA, 2003; Volume 24, p. 501. [Google Scholar]
- Tessema, W. Study on prevalence of Ectoparasites in poultry managed under backyard system in Mareka Woreda of Dawuro zone, Snnpr, Ethiopia. Vet. Anim. Sci. 2019, 5, 1–8. [Google Scholar]
- Permin, A.; Hansen, J.W. Epidemiology, Diagnosis, and Control of Poultry Parasites; Food and Agricultural Organization (FAO): Rome, Italy, 1998; p. 160. [Google Scholar]
- Mullen, G.R.; Durden, L.A. (Eds.) Medical and Veterinary Entomology, 2nd ed.; Academic Press: London, UK, 2009. [Google Scholar]
- Materu, A.E.; Mkhandi, J.W. Ectoparasites of free ranging local chickens in urban and peri-urban areas of Morogoro municipality, Tanzania. Int. J. Vet. Sci. Anim. Husb. 2022, 7, 11–15. [Google Scholar] [CrossRef]
- Adang, K.L.; Oniye, S.J.; Ezealor, A.U.; Abdu, P.A.; Ajanusi, O.J.; Yoriyo, K.P. Ectoparasites and intestinal helminths of speckled pigeon (Columba guinea Hartlaub and Finsch 1870) in Zaria, Nigeria. Sci. World J. 2009, 4, 1–5. [Google Scholar] [CrossRef]
- Mukaratirwa, S.; Hove, T. A survey of ectoparasites, cestodes and management of free-range indigenous chickens in rural Zimbabwe. J. S. Afr. Vet. Assoc. 2009, 80, 188–191. [Google Scholar] [CrossRef]
- Mukaratirwa, S.; Khumalo, M.P. Prevalence of chewing lice in free-range chickens from selected rural localities of KwaZulu-Natal, South Africa. Int. J. Appl. Res. Vet. Med. 2012, 10, 85. [Google Scholar]
- de Roest, C.H. Prevalence of Ectoparasites on Chickens (Gallus gallus domesticus) in the Mnisi Area of Mpumalanga Province, South Africa. Master’s Thesis, Ultretch University, Utrecht, The Netherlands, 2014. [Google Scholar]
- Fabiyi, J.P.; Alayande, M.O.; Akintule, A.O.; Lawal, M.D.; Mahmuda, A.; Usman, M. Prevalence and seasonal fluctuations of ectoparasites infesting backyard turkeys, Meleagris gallopavo, in Sokoto, Northwestern Nigeria. J. Anim. Husb. Vet. Med. Trop. 2017, 70, 21–24. [Google Scholar]
- Zoundi, A.; Sinaré, Y.; Thiombiano, N.G.; Bagayan, M.; Chabi, B.M.A.; Soubeiga, P.; Boungou, M. Diversity of ectoparasites of Gallus domesticus, NGOU 1990 (hens) and Numida meleagris, Linnaeus 1758 (Guinea fowl) reared in extensive system in the commune of Loumbila, Burkina Faso. Int. J. Zool. Appl. Biosci. 2024, 9, 16–23. [Google Scholar] [CrossRef]
- Bala, A.Y.; Anka, S.A.; Waziri, A.; Shehu, H. Preliminary survey of ectoparasites infesting chickens (Gallus domesticus) in four areas of Sokoto Metropolis. Niger. J. Basic Appl. Sci. 2011, 19, 173–180. [Google Scholar]
- Nwadike, C.C.; Ilozumba, P.C.O.; Gaius, C.J. Study on the prevalence of ectoparasitic arthropods on free-range Gallus domesticus in two communities in Awka. Asian J. Res. Zool. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Urquhart, G.; Armour, J.; Duncan, J.L.; Dunn, A.M.; Jennings, F. Veterinary Parasitology; Churchill Livingstone Inc.: New York, NY, USA, 1987; pp. 100–109. [Google Scholar]
- Sudiana, E.; Santoso, S.; Yani, E. Prevalence and diversity of ectoparasites in scavenging chickens (Gallus domesticus) and their association to body weight. Biodiversitas 2020, 21, 3163–3169. [Google Scholar]
- Ekpo, U.F.; Ogbooye, A.A.; Oluwole, A.S.; Takeet, M. A preliminary survey on the parasites of free-range chicken in Abeokuta, Ogun State, Nigeria. J. Nat. Sci. Eng. Technol. 2011, 9, 123–130. [Google Scholar]
- Lawal, J.R.; Yusuf, Z.B.; Dauda, J.; Gazali, Y.A.; Biu, A.A. Ectoparasites infestation and its associated risk factors in village chickens (Gallus gallus domesticus) in and around Potiskum, Yobe State, Nigeria. J. Anim. Husb. Dairy Sci. 2017, 1, 8–19. [Google Scholar] [CrossRef]
- Bassey, S.E.; Marroh, R. A survey of ectoparasites in the free-range domestic fowl, Gallus gallus domesticus. Amassoma, Bayelsa State, Nigeria. J. Environ. Bio Res. 2018, 2, 1–3. [Google Scholar]
- Luka, J.; Peter, A.M.; Zango, M.K.; Musa, J.; Malgwi, E.A.; Pindar, H.M.; Alfred, C.M.; Medugu, Y.D. Ectoparasitic fauna of poultry species in Maiduguri, Borno State, Nigeria. Sokoto J. Vet. Sci. 2022, 20, 232–239. [Google Scholar] [CrossRef]
- Zeryehun, T.; Yohannes, Y. Ectoparasite infestation of free scavenging chickens reared under traditional backyard production system in Wolayita Zone, southern Ethiopia. Ethiop. Vet. J. 2015, 19, 55–66. [Google Scholar] [CrossRef]
- Rabana, J.; Adamu, L.; Dauda, J.; Abubakar, A. Ectoparasitosis in domesticated turkeys (Meleagris gallopavo) in Jere Area, Borno State, Nigeria. Int. J. Vet. Sci. Res. 2019, 5, 11–22. [Google Scholar]
- Kouam, M.K.; Fokeng, A.N.; Biekop, H.F.; Touko, A.B.H.; Tebug, T.T. Prevalence and clinical signs of chewing lice in local chickens (Gallus gallus domesticus) in Menoua Division, Western highlands of Cameroon. Vet. Parasitol. Reg. Stud. Rep. 2022, 34, 100772. [Google Scholar]
- Abubakar, B.S.; Aliyu, A.A. Survey on the Prevalence of Ectoparasite Infestation on Domestic Fowl (Gallus gallus domesticus Linnaeus, 1758) Sold in Keffi Market, Nasarawa State, Nigeria. J. Health Wellness Saf. Res. 2024, 4, 69–76. [Google Scholar]
- Malau, M.B.; Rugu, N.J. The prevalence of lice and fleas of chicken in Bokkos local government area of plateau State, Nigeria. Glob. J. Pure Appl. Sci. 2001, 7, 433–436. [Google Scholar] [CrossRef]
- Ahaotu, E.O.; Akinfemi, A.; Okorie, K.C. Economic importance and widespread of ectoparasites infestation in indigenous chickens (Gallus gallus domesticus). A study from selected local government councils and states in Nigeria. Sustain. Agri. Food Environ. Res. 2019, 72, 17–31. [Google Scholar] [CrossRef]
- Ashenafi, H.; Yimer, E. Ectoparasites of local scavenging chickens of central Ethiopia. Ethiop. J. Sci. 2005, 28, 69–74. [Google Scholar]
- Belihu, K.; Mamo, A.; Lobago, F.; Ayana, D. Prevalence of ectoparasites in backyard local chickens in three agroecologic zones of East Shoa, Ethiopia. J. Vet. Med. 2009, 160, 537–541. [Google Scholar]
- Mekuria, S.; Gezahegn, E. Prevalence of External parasite of poultry in intensive and backyard chicken farm at Wolayta Soddo town, Southern Ethiopia. Vet. World 2010, 3, 533–538. [Google Scholar]
- Dube, S.; Zindi, P.; Mbanga, J.; Dube, C. A study of scavenging poultry gastrointestinal and ecto-parasites in rural areas of Matebeleland Province, Zimbabwe. Int. J. Poult. Sci. 2010, 9, 911–915. [Google Scholar] [CrossRef]
- Banda, Z. Ectoparasites of indigenous Malawi chickens. Aust. J. Basic Appl. Sci. 2011, 5, 1454–1460. [Google Scholar]
- Edosomwan, E.U.; Olumese, E.R.; Igetei, E.J. A study of ecto-and endo parasites of domestic birds in Etsako Municipality, Edo North of Nigeria. Niger. Soc. Exp. Biol. J. 2011, 11, 209–216. [Google Scholar]
- Mulugeta, A.; Chanie, M.; Bogale, B. Major constraints of village poultry production in Demba Gofa District of Southern Region, Ethiopia. Br. J. Poult. Sci. 2013, 2, 01–06. [Google Scholar]
- Alemu, N.; Muktar, Y.; Kassaye, D.; Hiko, A. Prevalence of lice and fleas in backyard chickens of Bishoftu Town, Ethiopia. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 2136–2142. [Google Scholar]
- Lawal, J.R.; Bello, A.M.; Balami, S.Y.; Wakil, Y.; Yusuf, Z.B.; Dauda, J.; Mshelia, E.S.; Mana, H.P.; Adam, M.K.; Biu, A.A. Prevalence and economic significance of ectoparasites infestation in village chickens (Gallus gallus domesticus) in Gombe, Northeastern Nigeria. Direct Res. J. Agric. Food Sci. 2016, 4, 94–103. [Google Scholar]
- Love, O.; Johnny, R.; Valentine, I.C. A study of the prevalence and abundance of chewing lice (Phthiraptera) in selected poultry farms in Benin City, Edo State. Int. J. Anim. Sci. Technol. 2017, 1, 35–42. [Google Scholar]
- Edosomwan, E.U.; Igetei, E.J. Ecto-and endo-parasites of domestic birds in Owan west, east and Akoko-Edo in Edo state of Nigeria. Int. J. Zool. Stud. 2018, 3, 28–35. [Google Scholar]
- Okechukwu, P.C.; Ikpeze, O.O. Ectoparasites found on intensively-reared chickens at semi-urban Emene in south-Eastern Nigeria. Biomed. Diagn. 2020, 4, 92–101. [Google Scholar]
- Onyekachi, O. Prevalence of ectoparasites infestation of chicken in three poultry farms in Awka. Asian Basic Appl. Res. J. 2022, 3, 41–53. [Google Scholar]
- Nwadike, C.C.; Agbata, D.O.; Okeke, J.J.; Okeke, O.A.; Nnatuanya, I.O.; Afoemezie, P.I.; Udeh, N.P.; Irikannu, K.F. Ectoparasites and gastrointestinal helminth of domestic pigeons in Awka, southeastern Nigeria. Biosci. J. 2023, 11, 1–7. [Google Scholar]
- Endale, H.; Aliye, S.; Mathewos, M.; Adimasu, W. Identification and estimation of the prevalence of ectoparasites of backyard chicken in Boloso Sore District, Wolaita zone, Southern Ethiopia. Vet. Parasitol. Reg. Stud. 2023, 42, 100884. [Google Scholar] [CrossRef]
- Maru, M.; Alemu, N.Y.; Mulaw, A. Prevalence of ectoparasites in poultry in Ebinat District, Northwest Ethiopia. Daagu Int. J. Basic Appl. Res. 2023, 5, 240–251. [Google Scholar]
- Permin, A.; Esmann, J.B.; Hoj, C.H.; Hove, T.; Mukaratirwa, S. Ecto-, endo-and haemoparasites in free-range chickens in the Goromonzi District in Zimbabwe. Prev. Vet. Med. 2002, 54, 213–224. [Google Scholar] [CrossRef]
- Odenu, R.A.; Mohammed, B.R.; Simon, M.K.; Agbede, R.I.S. Ecto-parasites of Domestic Chickens (Gallus gallus domesticus) in Gwagwalada Area Council, Abuja, Nigeria-West Africa. Alex. J. Vet. Sci. 2016, 51, 140–146. [Google Scholar]
- Usman, M.; Fabiyi, J.P.; Mohammed, A.A.; Mera, U.M.; Mahmuda, A.; Alayande, M.O.; Lawal, M.D.; Danmaigoro, A. Ectoparasites and haemoparasites of chickens in Sokoto, Northewestern Nigeria. Sci. J. Zool. 2012, 1, 74–78. [Google Scholar]
- Audi, A.H.; Asmau, A.M. Prevalence of Bird Louse, Menacanthus cornutus (Pthiraptera: Amblycera) In Four Selected Poultry Farms in Kano State, Nigeria. Bayero J. Pure Appl. Sci. 2014, 7, 142–146. [Google Scholar] [CrossRef]
- Mungube, E.O.; Bauni, S.M.; Tenhagen, B.A.; Wamae, L.W.; Nzioka, S.M.; Muhammed, L.; Nginyi, J.M. Prevalence of parasites of the local scavenging chickens in a selected semi-arid zone of Eastern Kenya. Trop. Anim. Health Prod. 2008, 40, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nahal, A.; Righi, S.; Boucheikhchoukh, M.; Benakhla, A. Prevalence of ectoparasites in free-range backyard chicken flocks in northeast Algeria. Vet. Stanica 2021, 52, 693–702. [Google Scholar] [CrossRef]
- Mohammed, N.H.; Mohammed, R.G.; Khalaf, W.K. Incidence and morphological study of lice infested chicken (Gallus gallus domesticus) in Nineveh governorate, Iraq. Iraqi J. Vet. Sci. 2025, 39, 87–93. [Google Scholar] [CrossRef]
- Al-Nasser, A.; Al-Khalaifa, H.; Al-Saffar, A.; Khalil, F.; Albahouh, M.; Ragheb, G.; Al-Haddad, A.; Mashaly, M. Overview of chicken taxonomy and domestication. J. World’s Poult. Sci. 2007, 63, 285–300. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Selaledi, L.; Hassan, Z.M.; Mabelebele, M. Local chicken breeds of Africa: Their description, uses and conservation methods. Animals 2020, 10, 2257. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, S.; Amin, E.; Shano, S.; Samad, M.A.; Shirin, T.; Hassan, M.M.; Flora, M.S. Assessment of poultry rearing practices and risk factors of H5N1 and H9N2 virus circulating among backyard chickens and ducks in rural communities. PLoS ONE 2022, 17, e0275852. [Google Scholar] [CrossRef] [PubMed]
- Lay, D.C., Jr.; Fulton, R.M.; Hester, P.Y.; Karcher, D.M.; Kjaer, J.B.; Mench, J.A.; Mullens, B.A.; Newberry, R.C.; Nicol, C.J.; O’Sullivan, N.P.; et al. Hen welfare in different housing systems. Poult. Sci. J. 2011, 90, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Waliaula, P.K.; Kiarie, E.G.; Diarra, M.S. Predisposition factors and control strategies of avian pathogenic Escherichia coli in laying hens. Front. Vet. Sci. 2024, 11, 474549. [Google Scholar] [CrossRef]
- Nair, S.S.; Gouge, D.H.; Murillo, A.C. Backyard Chickens and Ectoparasites: Introduction and Management; The University of Arizona: Tucson, AZ, USA, 2021. [Google Scholar]

| Chewing Lice Species | Country of the Study | Host Studied | Authors |
|---|---|---|---|
| Menacanthus stramineus | Nigeria, Ethiopia, Kenya, South Africa, Ghana, Malawi, Burkina Faso, Zimbabwe, Cameroon | Chickens, turkeys | [2,5,16,18,21,25,26,27,28,29,30,31,32] |
| Menacanthus pallidulus | Nigeria | Chickens | [1] |
| Menopon gallinae | Nigeria, Ethiopia, Tanzania, South Africa, Ghana, Burkina Faso, Zimbabwe, Cameroon | Chickens, pigeons, ducks | [4,5,6,8,14,15,16,17,18,20,21,22,26,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] |
| Lipeurus caponis | Nigeria, Ethiopia, South Africa, Malawi, Burkina Faso, Zimbabwe | Chickens, pigeons, ducks, turkeys, guinea fowls | [2,4,6,8,11,17,18,20,21,22,25,26,28,34,35,36,39,40,41,43,44,45,46,47,50,51,52] |
| Gallacanthus cornutus | South Africa | Chickens | [18] |
| Goniocotes gigas | Ethiopia, South Africa | Chickens | [11,17,39,41,49,52] |
| Goniodes gigas | Nigeria, Ethiopia, Tanzania, South Africa, Burkina Faso, Zimbabwe | Chickens, pigeons, ducks, turkeys, guinea fowls | [14,16,17,20,28,34,35,36,43,45,51,53] |
| Goniodes meleagridis | Nigeria | Chickens | [52] |
| Goniodes gallinae | Nigeria | Chickens, pigeons, ducks | [40,48] |
| Goniocotes gallinae | Nigeria, Ethiopia, Burkina Faso, Zimbabwe, Cameroon | Chickens, pigeons, ducks | [21,29,38,40,46] |
| Goniocotes hologester | Zimbabwe, Nigeria | Chickens | [38,45] |
| Cuclotogaster heterographus | Nigeria, Ethiopia, South Africa | Chickens, pigeons, ducks | [1,2,6,8,17,29,37,45,50] |
| Stenocrotaphus gigas | South Africa | Chickens | [18] |
| Goniodes dissimilis | Nigeria, Ethiopia | Chickens, pigeons, ducks | [35,40,45] |
| Columbicola columbae | Nigeria, Burkina Faso | Pigeons, chickens, ducks, turkeys, guinea fowls | [15,20,28,40,45,48] |
| Lipeurus tropicalis | Nigeria | Chickens, turkeys | [30,53] |
| Chelopistes meleagridis | Nigeria | Chickens, pigeons, ducks, turkeys | [30,40,45] |
| Amyrsidea powelli | Nigeria | Chickens | [33,53] |
| Menacanthus cornutus | Nigeria | Chickens | [1,33,53,54] |
| Country of Study | Chewing Lice Species | Host Studied | Type of Husbandry | Total Examined | Total Infected | Prevalence (%) | Identification Method | Author(s) |
|---|---|---|---|---|---|---|---|---|
| Ethiopia | Menacanthus stramineus | Chickens | Intensive | 384 | 30 | 7.8 | Morphology | [4] |
| Ethiopia | Menopon gallinae | Chickens | Intensive | 384 | 20 | 5.2 | Morphology | [4] |
| Ethiopia | Lipeurus caponis | Chickens | Intensive | 384 | 75 | 19.5 | Morphology | [4] |
| Ethiopia | Menopon gallinae | Chickens | Extensive | 450 | 40 | 8.9 | Morphology | [29] |
| Ethiopia | Cuclotogaster heterographus | Chickens | Extensive | 450 | 40 | 8.9 | Morphology | [29] |
| Ethiopia | Menacanthus stramineus | Chickens | Extensive | 450 | 37 | 8.3 | Morphology | [29] |
| Ethiopia | Menopon gallinae | Chickens | Extensive | 70 | 38 | 54.29 | Morphology | [49] |
| Ethiopia | Menacanthus stramineus | Chickens | Extensive | 70 | 20 | 28.57 | Morphology | [49] |
| Ethiopia | Goniodes gigas | Chickens | Extensive | 70 | 8 | 11.43 | Morphology | [49] |
| Ethiopia | Goniocotes gallinae | Chickens | Extensive | 70 | 4 | 5.71 | Morphology | [49] |
| Ethiopia | Cuclotogaster heterographus | Chickens | Extensive | 390 | 195 | 50 | Morphology | [2] |
| Ethiopia | Lipeurus caponis | Chickens | Extensive | 390 | 24 | 6.15 | Morphology | [2] |
| Ethiopia | Menacanthus stramineus | Chickens | Extensive | 390 | 5 | 1.28 | Morphology | [2] |
| Nigeria | Menopon gallinae | Pigeons | Extensive | 30 | 17 | 56.7 | Morphology | [15] |
| Nigeria | Columbicola columbae | Pigeons | Extensive | 30 | 18 | 60 | Morphology | [15] |
| Nigeria | Lipeurus tropicalis | Turkeys | Extensive | 265 | 207 | 78 | Morphology | [19] |
| Nigeria | Menacanthus stramineus | Turkeys | Extensive | 265 | 126 | 48 | Morphology | [19] |
| Nigeria | Chelopistes meleagridis | Turkeys | Extensive | 265 | 87 | 33 | Morphology | [19] |
| Nigeria | Menopon gallinae | Ducks | Extensive | 6 | 5 | 83.3 | Morphology | [45] |
| Nigeria | Gonoicotes hologaster | Chickens | Extensive | 13 | 13 | 100 | Morphology | [45] |
| Nigeria | Menopon gallinae | Chickens | Extensive | 1025 | 513 | 50.0 | Morphology | [43] |
| Nigeria | Goniodes gigas | Chickens | Extensive | 1025 | 139 | 13.6 | Morphology | [43] |
| Nigeria | Lipeurus caponis | Chickens | Extensive | 1025 | 227 | 22.1 | Morphology | [43] |
| Nigeria | Menopon gallinae | Chickens | Extensive | 200 | 9 | 4.5 | Morphology | [32] |
| Nigeria | Menacanthus stramineus | Chickens | Extensive | 200 | 3 | 1.5 | Morphology | [32] |
| Nigeria | Amyrsidea powelli | Chickens | Extensive | 100 | 50 | 50 | Morphology | [53] |
| Nigeria | Goniocotes gallinae | Chickens | Extensive | 100 | 74 | 74 | Morphology | [53] |
| Nigeria | Goniodes gigas | Chickens | Extensive | 100 | 56 | 56 | Morphology | [53] |
| Nigeria | Lipeurus tropicalis | Chickens | Extensive | 100 | 94 | 94 | Morphology | [53] |
| Nigeria | Menacanthus cornutus | Chickens | Extensive | 100 | 100 | 100 | Morphology | [53] |
| Nigeria | Menacanthus cornutus | Chickens | Extensive | 240 | 204 | 85 | Morphology | [54] |
| South Africa | Menopon gallinae | Chickens | Extensive | 18 | 18 | 100 | Morphology | [17] |
| South Africa | Goniocotes gallinae | Chickens | Extensive | 18 | 10 | 55.6 | Morphology | [17] |
| South Africa | Lipeurus caponis | Chickens | Extensive | 18 | 3 | 16.7 | Morphology | [17] |
| South Africa | Goniodes gigas | Chickens | Extensive | 18 | 6 | 33.3 | Morphology | [17] |
| Zimbabwe | Menacanthus stramineus | Chickens | Extensive | 50 | 50 | 100 | Morphology | [16] |
| Zimbabwe | Menopon gallinae | Chickens | Extensive | 50 | 33 | 66 | Morphology | [17] |
| Zimbabwe | Lipeurus caponis | Chickens | Extensive | 50 | 1 | 2 | Morphology | [51] |
| Zimbabwe | Goniocotes gallinae | Chickens | Extensive | 50 | 11 | 22 | Morphology | [51] |
| Zimbabwe | Menacanthus stramineus | Chickens | Extensive | 50 | 44 | 88 | Morphology | [51] |
| Zimbabwe | Menopon gallinae | Chickens | Extensive | 50 | 33 | 66 | Morphology | [51] |
| Cameroon | Menacanthus stramineus | Chickens | Extensive | 400 | 64 | 16 | Morphology | [31] |
| Cameroon | Menopon gallinae | Chickens | Extensive | 400 | 105 | 26.3 | Morphology | [31] |
| Cameroon | Goniocotes gallinae | Chickens | Extensive | 400 | 18 | 4.5 | Morphology | [31] |
| Kenya | Menacanthus stramineus | Chickens | Extensive | 360 | 257 | 71.4 | Morphology | [55] |
| Malawi | Menopon gallinae | Chickens | Extensive | 291 | 99 | 34 | Morphology | [39] |
| Malawi | Menacanthus stramineus | Chickens | Extensive | 291 | 93 | 32 | Morphology | [39] |
| Malawi | Lipeurus caponis | Chickens | Extensive | 286 | 4 | 1.4 | Morphology | [39] |
| Tanzania | Menopon gallinae | Chickens | Extensive | 144 | 70 | 48.6 | Morphology | [14] |
| Tanzania | Goniodes gigas | Chickens | Extensive | 144 | 8 | 5.8 | Morphology | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlondo, S.; Tembe, D.; Malatji, M.P.; Mukaratirwa, S. Epidemiology of Chewing Lice (Phthiraptera: Mallophaga) Fauna of Poultry in Sub-Saharan Africa. Pathogens 2025, 14, 1192. https://doi.org/10.3390/pathogens14121192
Mlondo S, Tembe D, Malatji MP, Mukaratirwa S. Epidemiology of Chewing Lice (Phthiraptera: Mallophaga) Fauna of Poultry in Sub-Saharan Africa. Pathogens. 2025; 14(12):1192. https://doi.org/10.3390/pathogens14121192
Chicago/Turabian StyleMlondo, Silindokuhle, Danisile Tembe, Mokgadi Pulane Malatji, and Samson Mukaratirwa. 2025. "Epidemiology of Chewing Lice (Phthiraptera: Mallophaga) Fauna of Poultry in Sub-Saharan Africa" Pathogens 14, no. 12: 1192. https://doi.org/10.3390/pathogens14121192
APA StyleMlondo, S., Tembe, D., Malatji, M. P., & Mukaratirwa, S. (2025). Epidemiology of Chewing Lice (Phthiraptera: Mallophaga) Fauna of Poultry in Sub-Saharan Africa. Pathogens, 14(12), 1192. https://doi.org/10.3390/pathogens14121192






