Systemic Soluble and Cellular Immune Response in Acute Rheumatic Fever and Rheumatic Heart Disease: A Systematic Review of Human Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, Interventions and Comparators
2.2. Systematic Review Protocol and Search Strategy
2.3. Data Sources
2.4. Eligibility Criteria
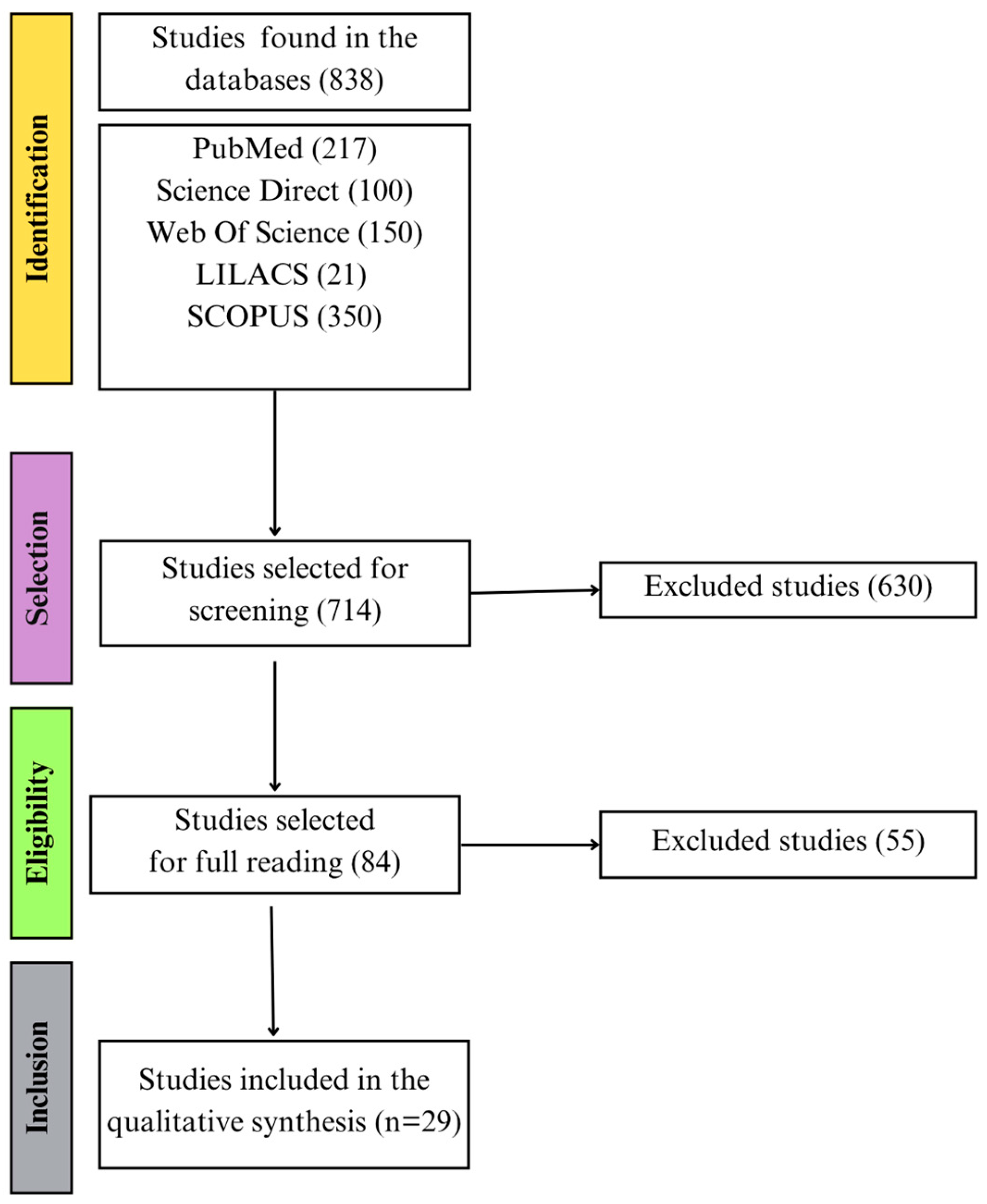
2.5. Selection of Studies and Data Extraction
2.6. Quality of Reporting Human Studies in Acute Rheumatic Fever and Rheumatic Heart Disease
3. Results
3.1. Included Studies
3.2. General Characteristics of Human Studies on Acute Rheumatic Fever and Rheumatic Heart Disease
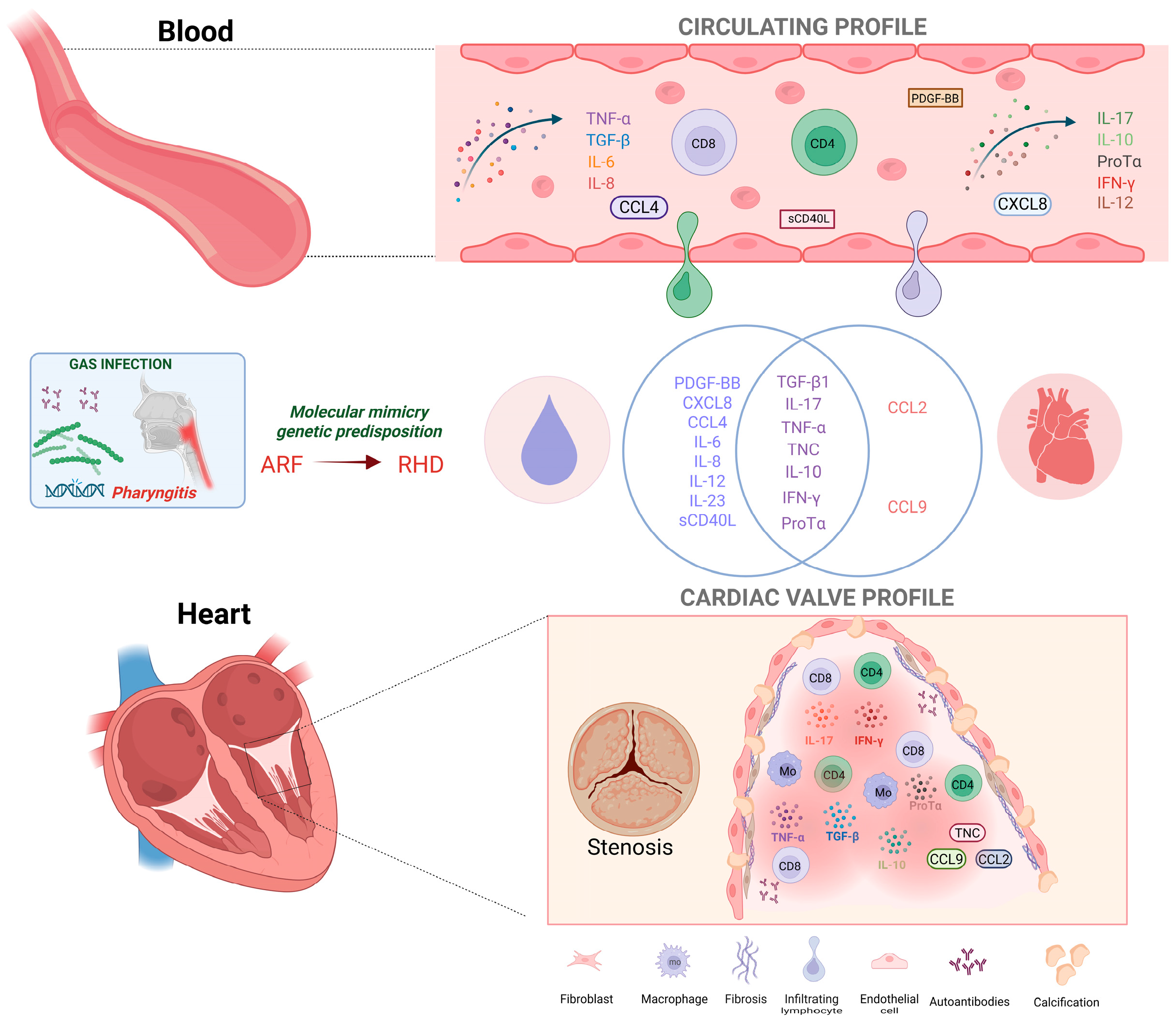
3.3. Characterization of the Immune Response in Patients with Acute Rheumatic Fever
3.4. Characterization of the Cellular Immune Response in Patients with Rheumatic Heart Disease
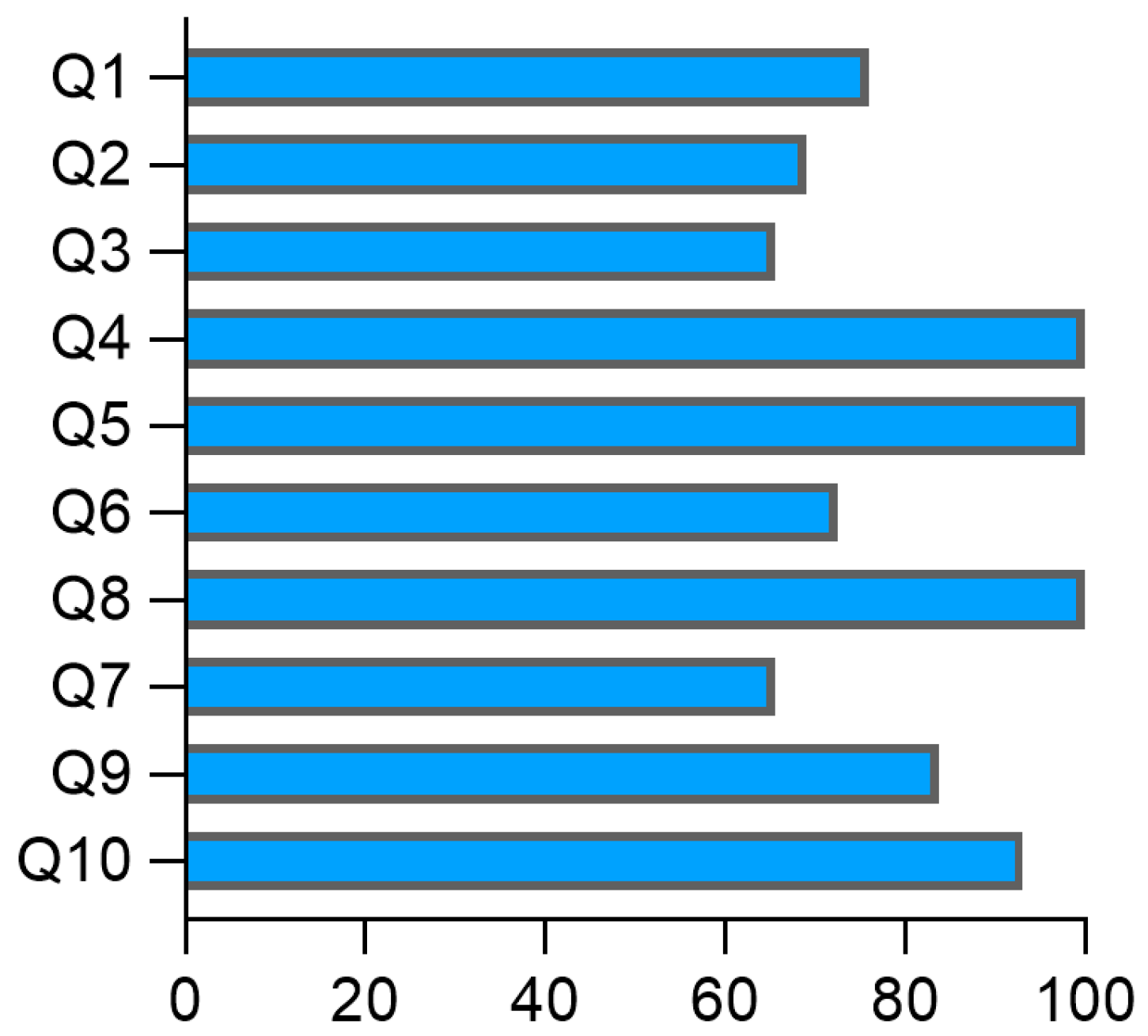
3.5. Assessment of the Risk of Bias of Studies in Acute Rheumatic Fever and Rheumatic Heart Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARF | Acute Rheumatic Fever |
| RHD | Rheumatic Heart Disease |
| PBMC | Peripheral blood mononuclear cell |
References
- Carapetis, J.R.; McDonald, M.; Wilson, N.J. Acute rheumatic fever. Lancet 2005, 366, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Guilherme, L. Acute rheumatic fever. Lancet 2018, 392, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Rothenbühler, M.; O’Sullivan, C.J.; Stortecky, S.; Stefanini, G.G.; Spitzer, E.; Estill, J.; Shrestha, N.R.; Keiser, O.; Jüni, P.; Pilgrim, T. Active surveillance for rheumatic heart disease in endemic regions: A systematic review and meta-analysis of prevalence among children and adolescents. Lancet Glob. Health 2014, 2, e717–e726. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.T.B.C.; Passos, L.S.A.; Guarçoni, F.V.; Aguiar, J.M.D.S.; da Silva, R.B.R.; de Paula, T.M.N.; dos Santos, R.F.; Nassif, M.C.L.; Gomes, N.F.A.; Tan, T.C.; et al. Rheumatic heart disease in the modern era: Recent developments and current challenges. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180041. [Google Scholar] [CrossRef]
- Stollerman, G.H. Rheumatogenic streptococci and autoimmunity. Clin. Immunol. Immunopathol. 1991, 61, 131–142. [Google Scholar] [CrossRef]
- Martins, T.B.; Hoffman, J.I.; Augustine, N.H.; Phansalkar, A.R.; Fischetti, V.A.; Zabriskie, J.B.; Cleary, P.P.; Musser, J.M.; Veasy, L.G.; Hill, H.R. Comprehensive analysis of antibody responses to streptococcal and tissue antigens in patients with acute rheumatic fever. Int. Immunol. 2008, 20, 445–452. [Google Scholar] [CrossRef]
- Raizada, V.; Williams, R.C., Jr.; Chopra, P.; Gopinath, N.; Prakash, K.; Sharma, K.B.; Cherian, K.M.; Panday, S.; Arora, R.; Nigam, M.; et al. Tissue distribution of lymphocytes in rheumatic heart valves as defined by monoclonal anti-T cell antibodies. Am. J. Med. 1983, 74, 90–96. [Google Scholar] [CrossRef]
- Kemeny, E.; Grieve, T.; Marcus, R.; Sareli, P.; Zabriskie, J.B. Identification of mononuclear cells and T cell subsets in rheumatic valvulitis. Clin. Immunol. Immunopathol. 1989, 52, 225–237. [Google Scholar] [CrossRef]
- Guilherme, L.; Cury, P.; Demarchi, L.M.; Coelho, V.; Abel, L.; Lopez, A.P.; Oshiro, S.E.; Aliotti, S.; Cunha-Neto, E.; Pomerantzeff, P.M.; et al. Rheumatic heart disease: Proinflammatory cytokines play a role in the progression and maintenance of valvular lesions. Am. J. Pathol. 2004, 165, 1583–1591. [Google Scholar] [CrossRef]
- Rosa, V.E.E. Histopathological characterization of mitral valvular lesions in patients with rheumatic heart disease: Is inflammation also to blame for chronic valvular heart disease progression? Arq. Bras. Cardiol. 2021, 116, 413–414. [Google Scholar] [CrossRef]
- Guilherme, L.; Weidebach, W.F.d.S.; Kiss, M.H.; Snitcowsky, R.; Kalil, J. Association of human leukocyte class II antigens with rheumatic fever or rheumatic heart disease in a Brazilian population. Circulation 1991, 83, 1995–1998. [Google Scholar] [CrossRef]
- Tarasoutchi, F.; Montera, M.W.; Grinberg, M.; Barbosa, M.R.; Piñeiro, D.J.; Sánchez, C.R.M.; Barbosa, M.M. Diretriz Brasileira de Valvopatias—SBC 2011/I Diretriz Interamericana de Valvopatias—SIAC 2011. Arq. Bras. Cardiol. 2011, 97 (5 Suppl. S1). [Google Scholar] [CrossRef]
- Guilherme, L.; Oshiro, S.E.; Faé, K.C.; Cunha-Neto, E.; Renesto, G.; Goldberg, A.C.; Tanaka, A.C.; A Pomerantzeff, P.M.; Kiss, M.H.; Silva, C.; et al. T-cell reactivity against streptococcal antigens in the periphery mirrors reactivity of heart-infiltrating T lymphocytes in rheumatic heart disease patients. Infect. Immun. 2001, 69, 5345–5351. [Google Scholar] [CrossRef]
- Braga, A.L.L.; Achutti, A.C.; Ramos, A.I.O.; Weksler, C.; Mota, C.C.C.; dos Santos, C.C.L.; de Sá, D.T.M.; da Silva, D.L.M.; Câmara, E.J.N.; Borges, F.M.S.; et al. Diretrizes Brasileiras para Diagnóstico e Prevenção da Febre Reumática. Arq Bras Cardiol. 2009, 93, 3–18. [Google Scholar] [CrossRef]
- Faé, K.C.; Palacios, S.A.; Nogueira, L.G.; Oshiro, S.E.; Demarchi, L.M.F.; Bilate, A.M.B.; Pomerantzeff, P.M.A.; Brandão, C.; Thomaz, P.G.; dos Reis, M.; et al. CXCL9/MIG mediates T cells recruitment to valvular tissue lesions of chronic rheumatic heart disease patients. Inflammation 2013, 36, 800–811. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ozgen, H.; Ucar, B.; Yildirim, A.; Colak, O.; Bal, C.; Kilic, Z. Plasma adiponectin levels and relations with cytokines in children with acute rheumatic fever. Cardiol. Young 2015, 25, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Kutukquler, N.; Narin, N. Plasma interleukin-7 (IL-7) and IL-8 concentrations in acute rheumatic fever and chronic rheumatic heart disease. Scand J Rheumatol 1995, 24, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Narula, J.; Bhatia, R.; Shallendri, K.; Koicha, M.; Taneja, V.; Jhingan, B.; Pothineni, R.B.; Malaviya, A.N.; Mehra, N.K.; et al. Immunologic and immunogenetic studies in rheumatic fever and rheumatic heart disease. Indian J Pediatr. 1990, 42, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Mohan, C.; Wahi, P.L.; Anand, I.S.; Ganguly, N.K. Increase in activated T cells and reduction in suppressor/cytotoxic T cells in acute rheumatic fever and active rheumatic heart disease: A longitudinal study. J Infect Dis. 1993, 167, 979983. [Google Scholar] [CrossRef]
- Oner, T.; Ozdemir, R.; Genc, D.B.; Kucuk, M.; Karadeniz, C.; Demirpence, S.; Yilmazer, M.M.; Mese, T.; Tavli, V.; Genel, F. Parameters indicative of persistence of valvular pathology at initial diagnosis in acute rheumatic carditis: The role of albumin and CD19 expression. J. Pediatr. 2016, 92, 581–587. [Google Scholar] [CrossRef]
- Kim, M.L.; Martin, W.J.; Minigo, G.; Keeble, J.L.; Garnham, A.L.; Pacini, G.; Smyth, G.K.; Speed, T.P.; Carapetis, J.; Wicks, I.P. Dysregulated IL-1β–GM-CSF axis in acute rheumatic fever that is limited by hydroxychloroquine. Circulation 2018, 138, 2648–2651. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Altara, R.; Mallat, Z.; Booz, G.W.; Zouein, F.A. The CXCL10/CXCR3 axis and cardiac inflammation: Implications for immunotherapy to treat infectious and noninfectious diseases of the heart. J. Immunol. Res. 2016, 4396368. [Google Scholar] [CrossRef]
- Ngwenyama, N.; Salvador, A.M.; Velázquez, F.; Nevers, T.; Levy, A.; Aronovitz, M.; Luster, A.D.; Huggins, G.S.; Alcaide, P. CXCR3 regulates CD4+ T cell cardiotropism in pressure overload–induced cardiac dysfunction. JCI Insight 2019, 4, e125214. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, M.; Baykan, A.; Cakir, M.; Vural, C.; Sunkak, S.; Unal, E.; Eken, A. The number and activity of CD3+TCR Vα7.2+CD161+ cells are increased in children with acute rheumatic fever. Int. J. Cardiol. 2021, 333, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Yegin, O.; Coşkun, M.; Ertuğ, H. Cytokines in acute rheumatic fever. Eur. J. Pediatr. 1996, 156, 25–29. [Google Scholar] [CrossRef]
- Bilik, M.Z.; Kaplan, I.; Polat, N.; Akil, M.A.; Akyüz, A.; Acet, H.; Yüksel, M.; Inci, Ü.; Kayan, F.; Toprak, N. Serum levels of IL-17 and IL-23 in patients with rheumatic mitral stenosis. Medicine 2016, 95, e3562. [Google Scholar] [CrossRef]
- Neves, E.G.A.; Koh, C.C.; Padilha da Silva, J.L.; Passos, L.S.A.; Villani, F.N.A.; dos Santos, J.S.C.; Menezes, C.A.S.; Silva, V.R.; Tormin, J.P.A.S.; Evangelista, G.F.B.; et al. Systemic cytokines, chemokines and growth factors reveal specific and shared immunological characteristics in infectious cardiomyopathies. Cytokine 2021, 148, 155744. [Google Scholar] [CrossRef]
- Sharma, G.; Shetkar, S.; Bhasin, A.; Ramakrishnan, L.; Juneja, R.; Naik, N.; Roy, A.; Ramakrishnan, S.; Bhargava, B.; Bahl, V.K. High sensitive C-reactive protein and interleukin 6 in atrial fibrillation with rheumatic mitral stenosis from Indian cohort. Indian Heart J. 2017, 69, 505–511. [Google Scholar] [CrossRef]
- Cagli, K.E.; Aras, D.; Topaloglu, S.; Geyik, B.; Ayaz, S.; Cagirci, G.; Kisacik, H.L.; Korkmaz, S. Plasma levels of tumor necrosis factor-α and its receptors in patients with mitral stenosis and sinus rhythm undergoing percutaneous balloon valvuloplasty. Heart Vessel. 2010, 25, 131–137. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, X.F.; Yi, D.H.; Xu, P.; Liu, H.; Chang, Q.; Yang, S.M.; Li, Z.F.; Gao, H.B.; Hao, G.J. Synergistic effects of cyclic strain and Th1-like cytokines on tenascin-C production by rheumatic aortic valve interstitial cells. Clin. Exp. Immunol. 2009, 155, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Diamantino Soares, A.C.; Araújo Passos, L.S.; Sable, C.; Beaton, A.; Ribeiro, V.T.; Gollob, K.J.; Dutra, W.O.; Nunes, M.C.P. Circulating cytokines predict severity of rheumatic heart disease. Int. J. Cardiol. 2019, 289, 107–109. [Google Scholar] [CrossRef]
- Chen, M.C.; Chang, H.W.; Wu, C.J.; Yang, C.H.; Hung, W.C.; Yeh, K.H.; Fu, M. Percutaneous transluminal mitral valvuloplasty reduces circulating soluble CD40 ligand in rheumatic mitral stenosis. Chest 2005, 128, 36–41. [Google Scholar] [CrossRef]
- Henn, V.; Steinbach, S.; Büchner, K.; Presek, P.; Kroczek, R.A. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood 2001, 98, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.Y.; Clark, E.A. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 2009, 21, 265–272. [Google Scholar] [CrossRef] [PubMed]
- van Os, B.W.; Vos, W.G.; Bosmans, L.A.; van Tiel, C.M.; den Toom, M.; Beckers, L.; Admiraal, M.; Hoeksema, M.A.; de Winther, M.P.; Lutgens, E. CD40L modulates CD4+ T-cell activation through receptor for activated C kinase 1. Eur. J. Immunol. 2023, 53, 2350520. [Google Scholar] [CrossRef]
- Angeli, F.; Verdecchia, P.; Savonitto, S.; Cavallini, S.; Santucci, A.; Coiro, S.; Sclafani, R.; Riccini, C.; De Servi, S.; Cavallini, C. Soluble CD40 ligand and outcome in patients with coronary artery disease undergoing percutaneous coronary intervention. Clin. Chem. Lab. Med. 2022, 60, 118–126. [Google Scholar] [CrossRef]
- Bosmans, L.A.; Bosch, L.; Kusters, P.J.H.; Lutgens, E.; Seijkens, T.T.P. The CD40-CD40L dyad as immunotherapeutic target in cardiovascular disease. J. Cardiovasc. Transl. Res. 2020, 14, 13–22. [Google Scholar] [CrossRef]
- Shami, A.; Edsfeldt, A.; Bengtsson, E.; Nilsson, J.; Shore, A.C.; Natali, A.; Khan, F.; Lutgens, E.; Gonçalves, I. Soluble CD40 levels in plasma are associated with cardiovascular disease and in carotid plaques with a vulnerable phenotype. J. Stroke 2021, 23, 367–376. [Google Scholar] [CrossRef]
- Tormin, J.P.A.S.; Nascimento, B.R.; Sable, C.A.; da Silva, J.L.P.; Brandao-de-Resende, C.; Rocha, L.P.C.; Pinto, C.H.; Neves, E.G.A.; Macedo, F.V.; Fraga, C.L.; et al. Cytokine gene functional polymorphisms and phenotypic expression as predictors of evolution from latent to clinical rheumatic heart disease. Cytokine 2021, 138, 155370. [Google Scholar] [CrossRef] [PubMed]
- Bas, D.H.; Baser, K.; Yavuz, E.; Bolayir, H.A.; Yaman, B.; Unlu, S.; Cengel, A.; Bagriacik, E.U.; Yalcin, R. A shift in the balance of regulatory T and T helper 17 cells in rheumatic heart disease. Int. J. Cardiol. 2014, 168, 2825–2830. [Google Scholar] [CrossRef]
- Leão, S.C.; Lima, M.R.M.; do Nascimento, H.M.; Octacilio-Silva, S.; de Andrade Rodrigues, T.M. IL-10 e ET-1 como biomarcadores de doença valvar reumática. Braz. J. Cardiovasc. Surg. 2014, 29, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Lei, H.; Qin, S.; Ma, K.; Wang, X. TGF-β1 expression and atrial myocardium fibrosis increase in atrial fibrillation secondary to rheumatic heart disease. Clin. Cardiol. 2010, 33, 149–156. [Google Scholar] [CrossRef]
- Zhao, Z.; He, D.; Ling, F.; Chu, T.; Huang, D.; Wu, H.; Ge, J. CD4+ T cells and TGFβ1/MAPK signal pathway involved in the valvular hyperblastosis and fibrosis in patients with rheumatic heart disease. Exp. Mol. Pathol. 2020, 114, 104429. [Google Scholar] [CrossRef]
- Sapru, R.P.; Ganguly, N.K.; Sharma, S.; Chandani, R.E.; Gupta, B.A.K. Cellular reaction to group A beta-haemolytic streptococcal membrane antigen and its relation to complement levels in patients with rheumatic heart disease. Med. J. 1977, 2, 422–424. [Google Scholar] [CrossRef]
- Zedan, M.M.; El-Shennawy, F.A.; Abou-Bakr, H.M.; Al-Basousy, A.M.; Almahdy, Z.M. Interleukin-2 in relation to T cell sub-populations in rheumatic heart disease. Arch. Dis. Child. 1992, 67, 731–734. [Google Scholar] [CrossRef]
- Carrión, F.; Fernandez, M.; Iruretagoyena, M.; Coelho Andrade, L.E.; Odete-Hilário, M.; Figueroa, F. Selective depletion of Vβ2+CD8+ T cells in peripheral blood from rheumatic heart disease patients. J. Autoimmun. 2003, 20, 183–190. [Google Scholar] [CrossRef]
- Toor, D.; Vohra, H. Immune responsiveness during disease progression from acute rheumatic fever to chronic rheumatic heart disease. J. Microbes Infect. 2012, 217, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Kirvan, C.A.; Canini, H.; Swedo, S.E.; Hill, H.; Veasy, G.; Jankelow, D.; Kosanke, S.; Ward, K.; Zhao, Y.D.; Alvarez, K.; et al. IgG2 rules: N-acetyl-β-D-glucosamine-specific IgG2 and Th17/Th1 cooperation may promote the pathogenesis of acute rheumatic heart disease and be a biomarker of the autoimmune sequelae of Streptococcus pyogenes. Front. Cardiovasc. Med. 2023, 9, 919700. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, X.; Liu, C.; Cai, X.; Xiang, M.; Liu, S.; Li, R.; Lin, Z.; Liu, D.; Dong, M.; et al. New insight into the role of lipid metabolism-related proteins in rheumatic heart valve disease. Lipids Health Dis. 2022, 21, 110. [Google Scholar] [CrossRef]
- Kim, L.; Kim, D.K.; Yang, W.I.; Shin, D.H.; Jung, I.M.; Park, H.K.; Chang, B.C. Overexpression of transforming growth factor-β1 in the valvular fibrosis of chronic rheumatic heart disease. J. Korean Med. Sci. 2008, 23, 41–48. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Miao, L.; Zhao, R.; Wu, Y.; Kong, X. CC-chemokine receptor 7 and its ligand CCL19 promote mitral valve interstitial cell migration and repair. J. Biomed. Res. 2015, 29, 456–464. [Google Scholar] [CrossRef]
- Passos, L.S.A.; Jha, P.K.; Becker-Greene, D.; Blaser, M.C.; Romero, D.; Lupieri, A.; Sukhova, G.K.; Libby, P.; Singh, S.A.; Dutra, W.O.; et al. Prothymosin alpha: A novel contributor to estradiol receptor alpha-mediated CD8+ T-cell pathogenic responses and recognition of type 1 collagen in rheumatic heart valve disease. Circulation 2022, 145, 531–548. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Primers 2016, 2, 15084. [Google Scholar] [CrossRef]
- Zhuang, S.; Guo, D.; Yu, D. A mini review of the pathogenesis of acute rheumatic fever and rheumatic heart disease. Front. Cell. Infect. Microbiol. 2025, 15, 1447149. [Google Scholar] [CrossRef]
- Faé, K.C.; Diefenbach da Silva, D.; Oshiro, S.E.; Tanaka, A.C.; Pomerantzeff, A.P.; Douay, C.; Charron, D.; Toubert, A.; Cunningham, M.W.; Kalil, J.; et al. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J. Immunol. 2006, 176, 5662–5670. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Kosanke, S.; Dunn, S.T.; Jankelow, D.; Duran, C.M.G.; Cunningham, M.W. Pathogenic mechanisms in rheumatic carditis: Focus on valvular endothelium. J. Infect. Dis. 2001, 183, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Sheppard, R. Fibrosis in heart disease: Understanding the role of transforming growth factor-β1 in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006, 118, 10–24. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Varma, S.; Mohan Kumar, H.N.; Yusaf, J.; Goyal, M.; Mehta, V.; Tyagi, S. Circulating level of regulatory T cells in rheumatic heart disease: An observational study. Indian Heart J. 2016, 68, 342–348. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Nealis, T.B.; Subramaniam, M.; Pandya, S.; Stock, S.R.; Ignatiev, C.I.; Sebo, T.J.; Rosengart, T.K.; Edwards, W.D.; McCarthy, P.M.; et al. Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation 2005, 111, 3296–3301. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.V. Evidence for lack of myocardial injury in children with acute rheumatic carditis. Cardiol Young 2002, 12, 519–523. [Google Scholar] [CrossRef] [PubMed]
| Molecules Evaluated | Main Results | References |
|---|---|---|
| Serum | ||
| TNF-a, IL-6, and IL-8, Adiponectin, | IL-6 presented higher sensitivity and specificity for segregating ARF patients from controls; adiponectin levels were higher in the carditis groups than in the control group. | [18] |
| Plasma | ||
| IL-7, IL-8 | Patients with ARF exhibited significantly higher IL-8 levels than children with chronic RHD, those with streptococcal pharyngitis, and healthy controls. | [19] |
| Blood | ||
| CD4+ and CD8+ T-cells | ARF patients presented an increased CD4/CD8 ratio compared to healthy controls. | [20] |
| CD8+ cells | A lower frequency of CD8+ T cells was found in patients with acute rheumatic carditis and regurgitation compared to those with regurgitation regression. | [22] |
| Peripheral blood mononuclear cells | ||
| CD4+ cells | CD4+ cells were significantly higher in the peripheral blood of ARF and RHD patients than in healthy donors | [21] |
| Cytokines, chemokines, growth factors. Th1, Th2, and Th17 cells | The levels of TNF, IL-17F, and GM-CSF were significantly higher in PBMCs from ARF patients, compared to those from healthy donors. increased frequency of CD4+ T cells (CXCR3+, CCR4−, CCR6-CRTH2) in ARF patients compared to healthy controls. | [23] |
| CD3+TCR+ MAIT cells | Acute and recovered ARF patients have an elevated number of circulating CD3+TCR Vα7.2+CD161+ cells than the control group; circulating CD3+TCR Vα7.2+CD161+ cells in acute and recovered ARF patients produce more IFN-γ and TNF-a. | [27] |
| Molecules Evaluated | Main Results | References |
|---|---|---|
| Serum | ||
| IL-17, Il-23 | Higher IL-17 and IL-23 levels were observed in RHD patients compared with healthy controls. | [29] |
| Protein C reactive, IL-6 | hs-CRP and IL-6 showed a statistically significant increase in RHD patients compared to the control group. | [31] |
| IFN-γ, TNF-a, Tenascin-C | The levels of IFN-γ, TNF-a and Tenascin-C were significantly higher in RHD patients compared with healthy controls. | [33] |
| IL-17, TGβ1, IL-10 | The levels of IL-17 and TGFβ1 were markedly increased in the RHD group compared with those in the healthy control donors. | [43] |
| IL-10, TNF-a, IL-4 | The levels of IL-10 were higher in RHD patients who had replaced the native mitral valve and in patients without surgical treatment than in those in the control group. | [44] |
| TGFβ1 | The levels of TGF-β1 were increased in RHD patients with atrial fibrillation compared to those with sinus rhythm | [45] |
| Plasma | ||
| IL-8, IL-1α, IL-6, TNF-a | TNF-a and IL-8 levels were significantly higher in patients with RHD and cardiac failure when compared with those in the ARF group. | [28] |
| Cytokines, chemokines and growth factors | The levels of IL-12, IFN-γ, IL-17, IL-4, IL-1Ra, CCL4, and PDGF-BB are increased in the RHD group compared to the control. | [30] |
| TNF-a, sTNF-R | TNF-a and sTNF-R levels were found to be significantly higher in rheumatic mitral stenosis than in the healthy control group | [32] |
| IFN-γ, TNF-a, IL-17A, IL-10, IL-6, IL-4, IL-2 | IL-6 and TNF-a were positively correlated in patients with severe but not in stable RHD. | [34] |
| sCD40L | Patients with moderate-to-severe mitral stenosis had higher venous plasma levels of soluble CD40L than healthy volunteers. | [35] |
| Cytokines, chemokines and growth factors | CCL5, CXCL8, IL-1ra, IL-4, IL-9, and PDGF-BB levels distinguished clinical RHD from latent disease with 100% sensitivity and specificity; CXCL8, G-CSF, IL-15, IL-1ra, IL-4, and IL-7 levels predicted clinical RHD with 100% sensitivity and specificity compared to healthy controls. | [42] |
| ProTα | ProTα levels were significantly higher in RHD patients than in healthy controls. | [55] |
| Blood | ||
| CD4+ cells; TGF-β1 | The percentage of CD4+ T cells of RHD patients was significantly higher than that in the RHD negative group. | [46] |
| IL-2, T-cells | There was a significant increase in IL-2 in active RHD patients compared with RHD patients without heart failure and rheumatic activity and healthy controls. An increased CD4/CD8 ratio was also observed in the peripheral blood of RHD patients, accompanied by a reduction in circulating CD8+ T cells compared with the control group. | [48] |
| TCR Vβ2 repertoire of CD3+, CD4+ and CD8+ peripheral blood T cells | The percentage of CD3+ T cells was significantly higher in RHD patients than in healthy controls; the expression of the Vβ2 on the CD8+ subset of the RHD patients was significantly decreased compared with healthy controls or ARF patients. | [49] |
| Peripheral blood mononuclear cells | ||
| T helper 17 (TH17) cells, Treg cells | T helper 17/Treg ratio was significantly higher in patients with RHD compared with healthy control subjects. | [43] |
| T cells | The T-cell population in patients with RHD was reduced compared to those healthy donors. | [47] |
| CD4+ T-cells, CD8+ T-cells | The frequency of CD4+ T cells was significantly increased in ARF cases compared to newly diagnosed RHD cases and chronic rheumatics; the CD4/CD8 ratio declined count with the progression of the disease. | [50] |
| ProTα, estrogen receptor alpha | Circulating CD4+ and CD8 T+ cells showed a higher median intensity of fluorescence (MFI) of estrogen receptor alpha (ERα) in RHD patients than in healthy controls; expression of (ProTα) and ERα correlated strongly in circulating CD8+ T cells from RHD patients. | [55] |
| Tissue | ||
| Tenascin-C | Tenascin-C abundance was much higher in RHD aortic valves than in the control group. | [33] |
| CD4+/TGF-β1 | The CD4+ T cell number in the valve tissue from RHD patients was significantly higher than in the RHD-negative group; TGF-β1 levels in the tissue valve from the RHD group were higher compared with the control group. | [46] |
| Il-17, IFN-γ | RHD tissues were found to have elevated levels of IFN-γ in comparison to control non-RHD heart tissue; IL-17 was seen throughout in all RHD valvular tissues; No IL-17 was observed in control non-RHD tissue. | [51] |
| TNF-a, IL-10, CCL2 | Mitral valve from RHD patients presented strong expression of TNF-a, IL-10, and CCL2 compared to ischemic mitral valve from the control group | [52] |
| TGF-β1 | High TGFβ1 expression was identified in the rheumatic mitral valve than in the control group; high TGFβ1 expression correlated with severe valvular fibrosis, inflammatory cell infiltration, neovascularization, and calcification in the valves of RHD patients. | [53] |
| CCL19 | CCL19 was expressed in the RHD valve, but not in control valves. | [54] |
| ProTα, CD8+ T cells | ProTα was more highly expressed in aortic and mitral valves from RHD patients than in those from the control group. ProTα+ cells were localized in areas rich in inflammatory infiltrates, which exhibited a higher frequency of CD4+ and CD8+ cells compared to aortic valves from the control group, and a higher frequency of CD68+ cells compared to both aortic and mitral valves from the control group | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, A.L.d.S.; Neves, E.G.A.; Cavalcante, B.M.; Dutra, W.O. Systemic Soluble and Cellular Immune Response in Acute Rheumatic Fever and Rheumatic Heart Disease: A Systematic Review of Human Studies. Pathogens 2025, 14, 1185. https://doi.org/10.3390/pathogens14111185
Resende ALdS, Neves EGA, Cavalcante BM, Dutra WO. Systemic Soluble and Cellular Immune Response in Acute Rheumatic Fever and Rheumatic Heart Disease: A Systematic Review of Human Studies. Pathogens. 2025; 14(11):1185. https://doi.org/10.3390/pathogens14111185
Chicago/Turabian StyleResende, Ana Luiza da Silva, Eula Graciele Amorim Neves, Brenda Martins Cavalcante, and Walderez Ornelas Dutra. 2025. "Systemic Soluble and Cellular Immune Response in Acute Rheumatic Fever and Rheumatic Heart Disease: A Systematic Review of Human Studies" Pathogens 14, no. 11: 1185. https://doi.org/10.3390/pathogens14111185
APA StyleResende, A. L. d. S., Neves, E. G. A., Cavalcante, B. M., & Dutra, W. O. (2025). Systemic Soluble and Cellular Immune Response in Acute Rheumatic Fever and Rheumatic Heart Disease: A Systematic Review of Human Studies. Pathogens, 14(11), 1185. https://doi.org/10.3390/pathogens14111185







