Bovine Clinical E. coli Mastitis in Italian Dairy Herds Is Not Associated with a Specific Pathotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Identification
2.2. DNA Extraction, and Nanopore Sequencing Library Preparation
2.3. Bioinformatic Genome Sequencing Analysis
2.4. Bioinformatic Analysis of Antimicrobial-Resistance Genes and Virulence Factors
2.5. Statistical Analysis
3. Results
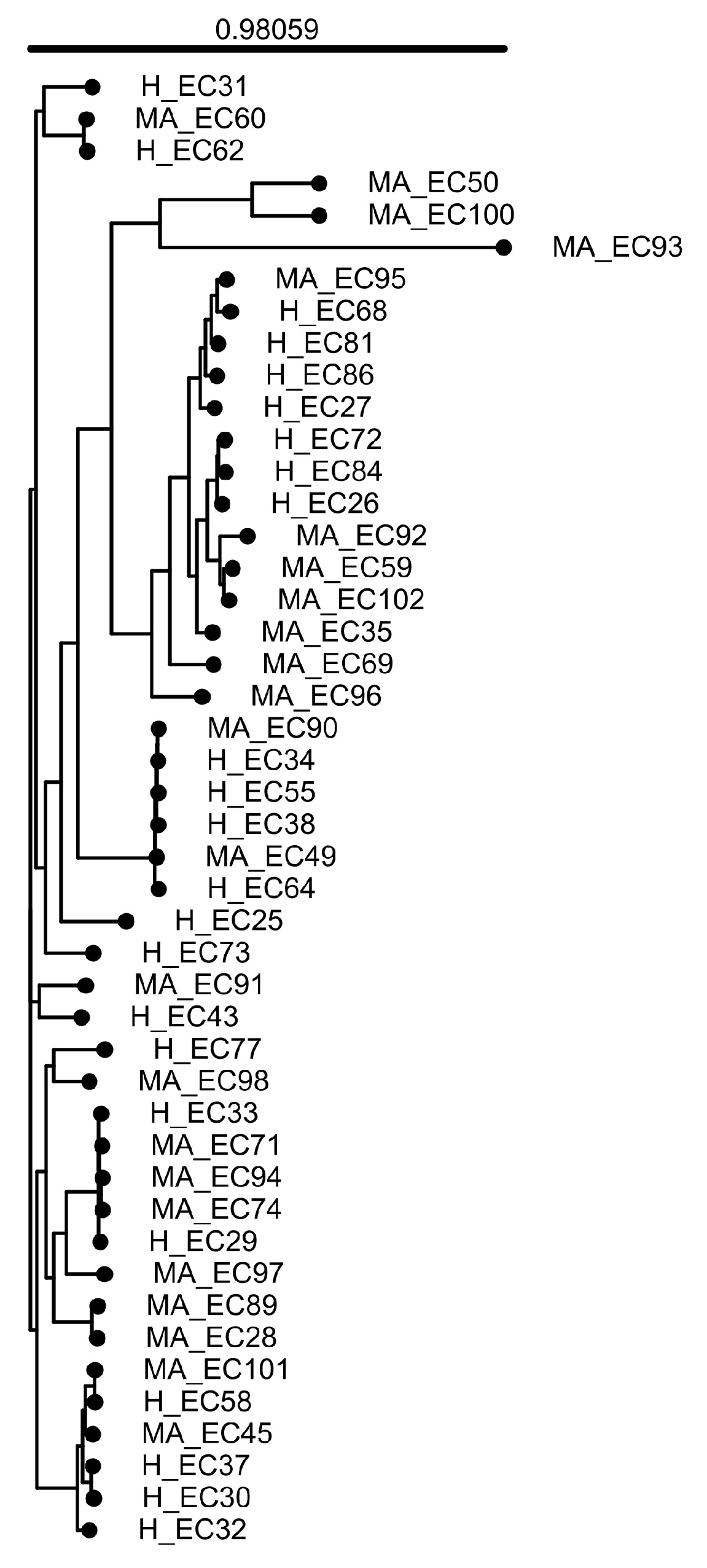
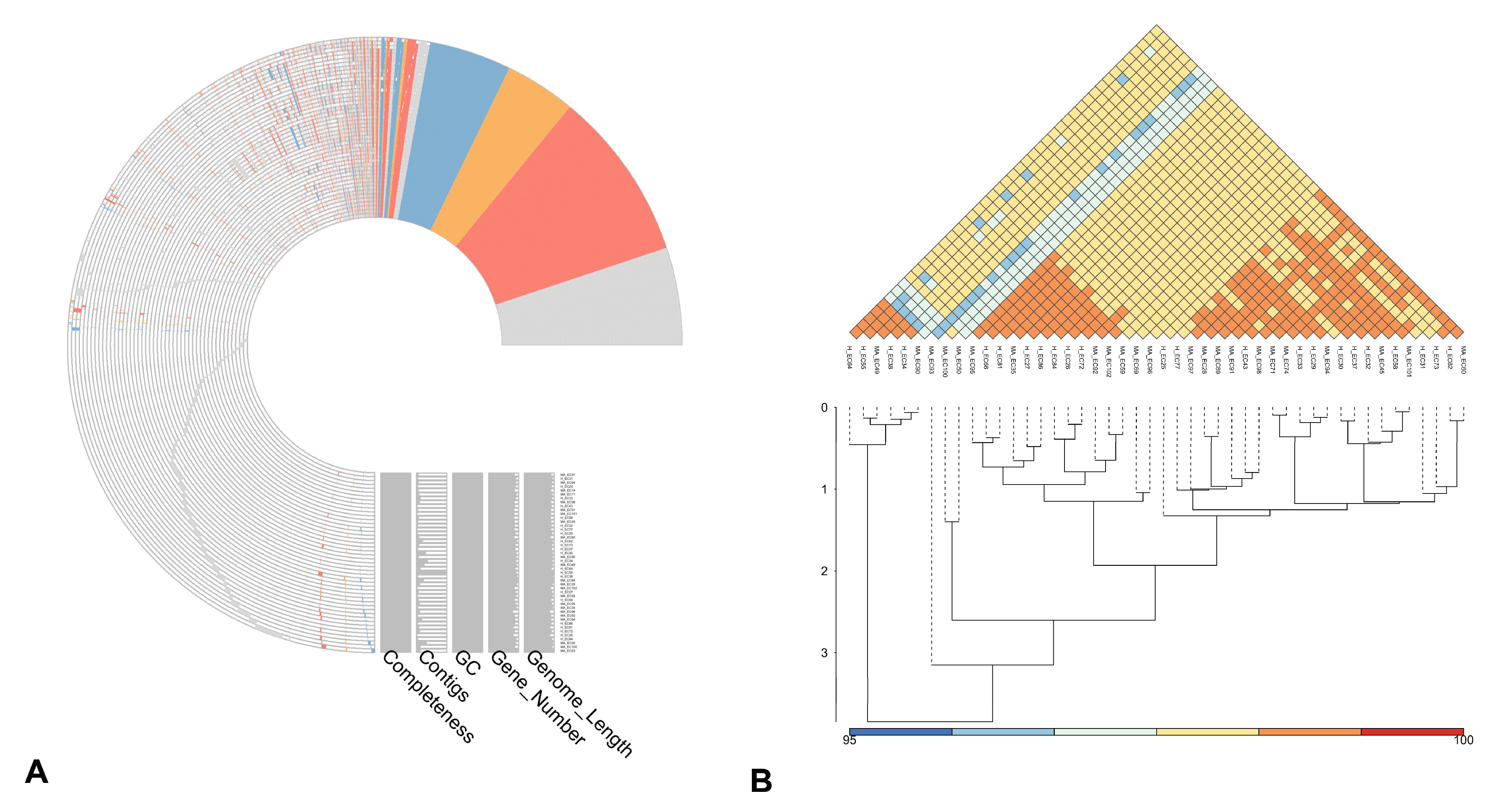
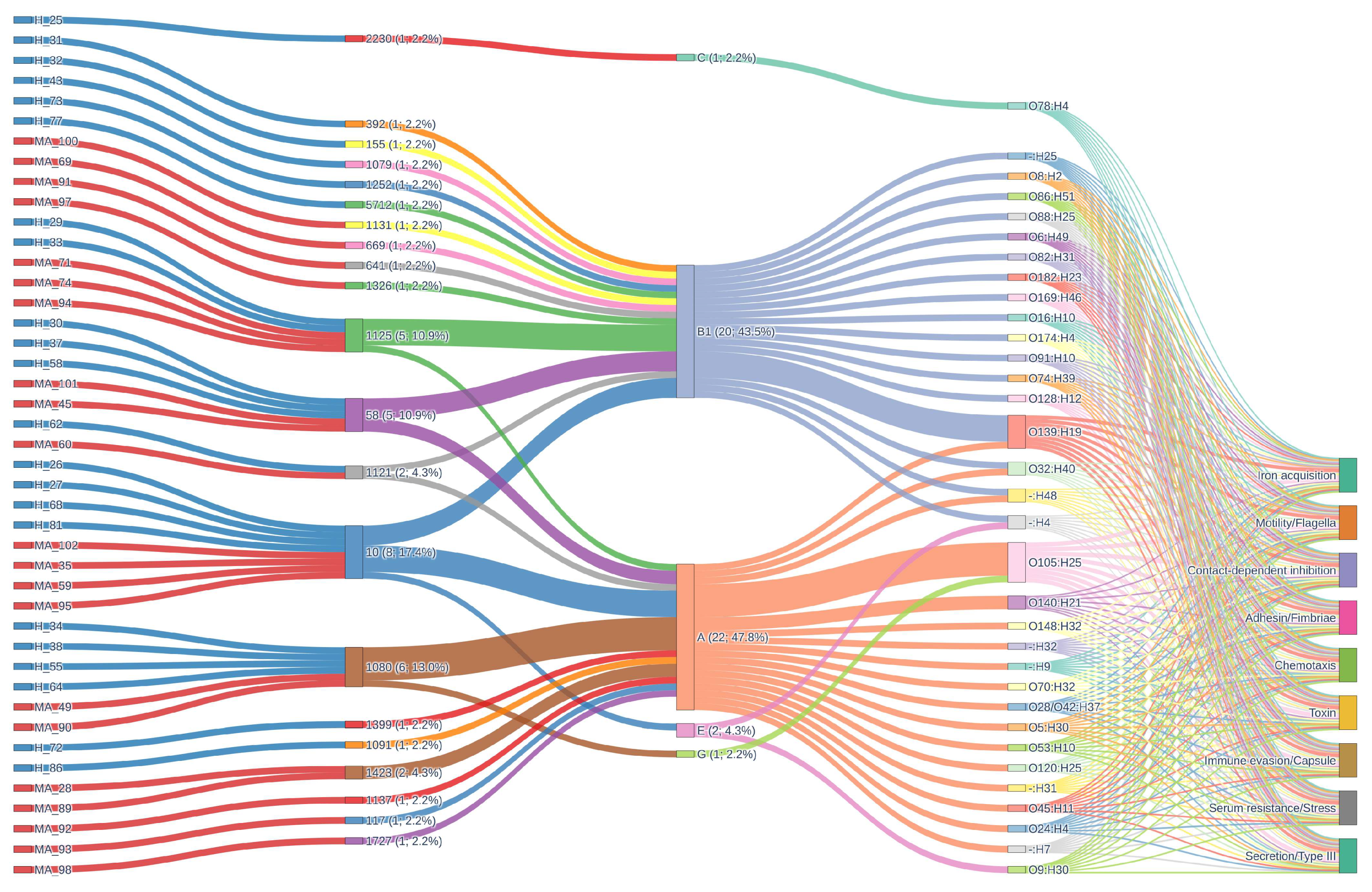
3.1. General Features of the Genomes
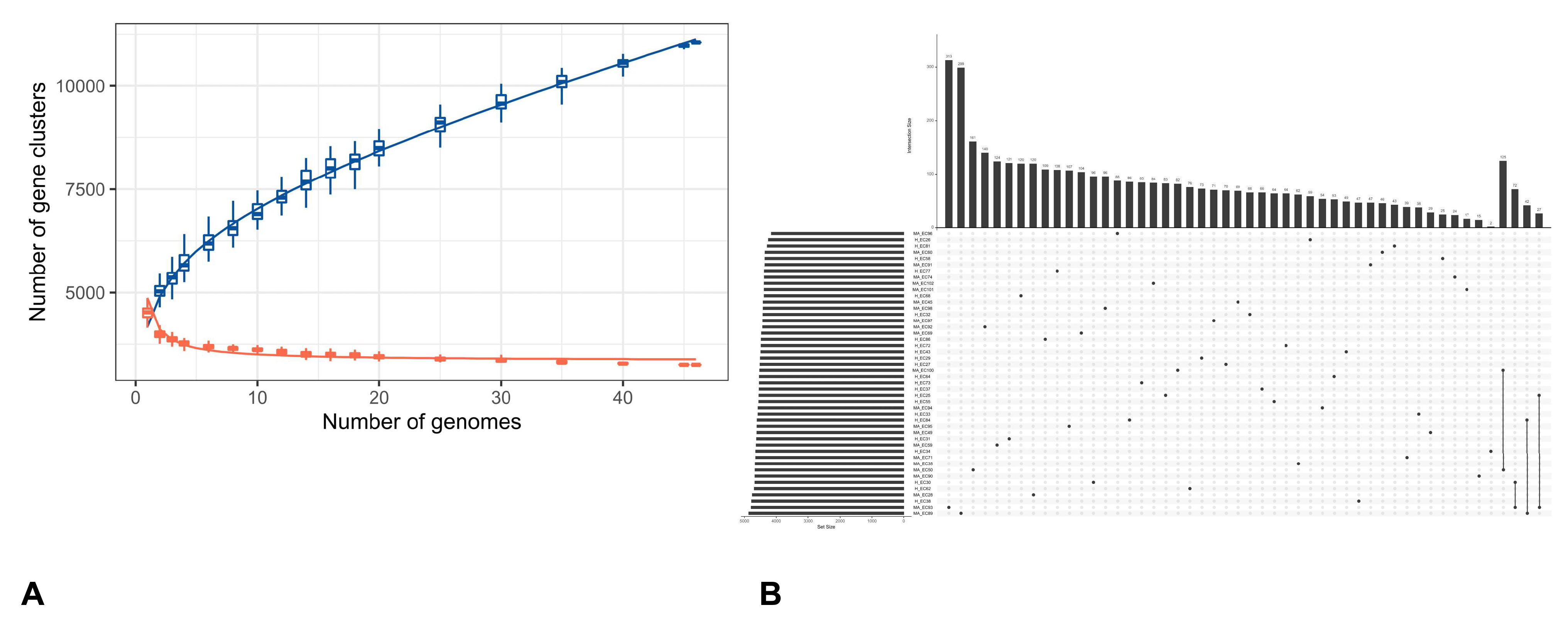
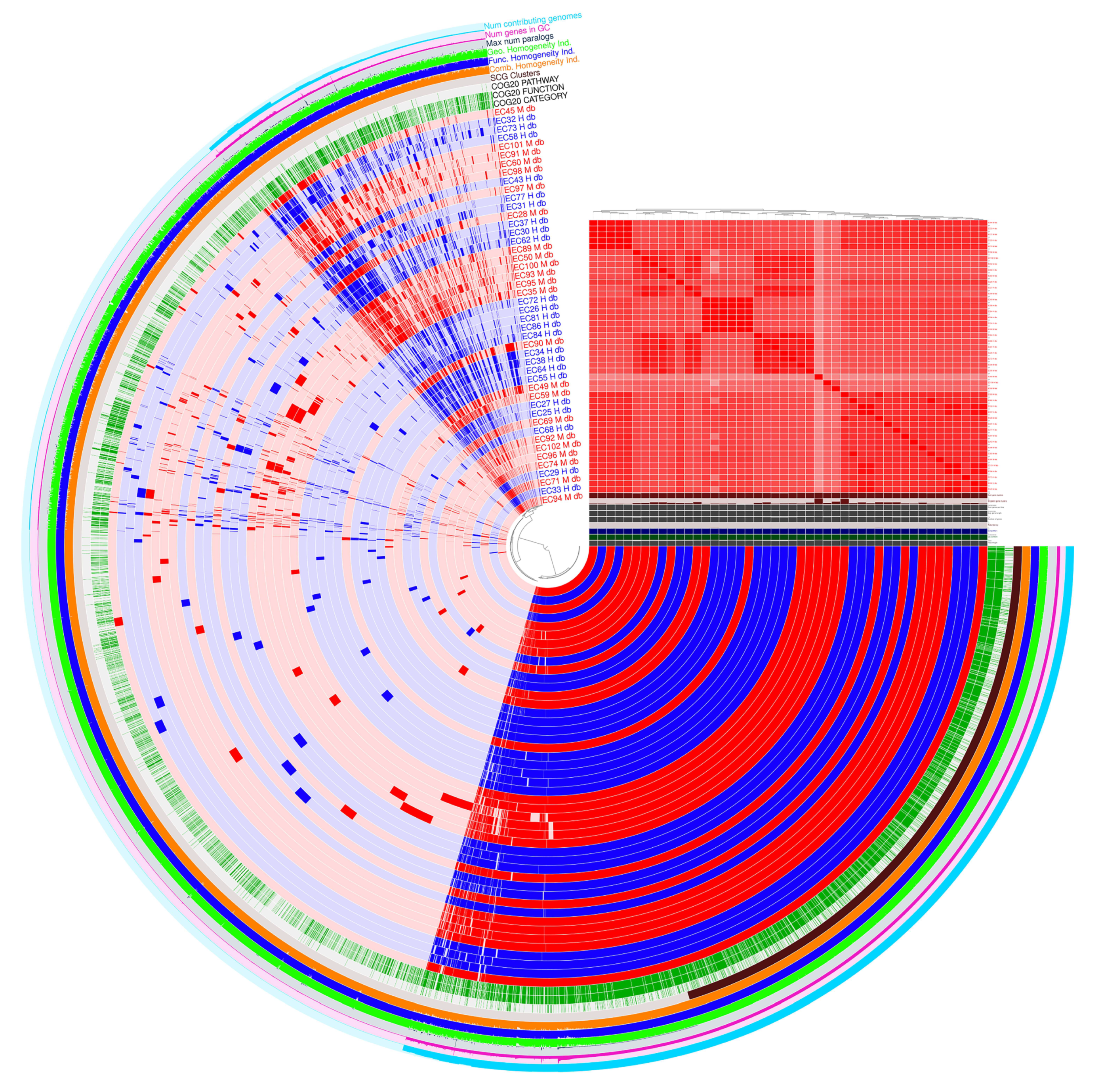
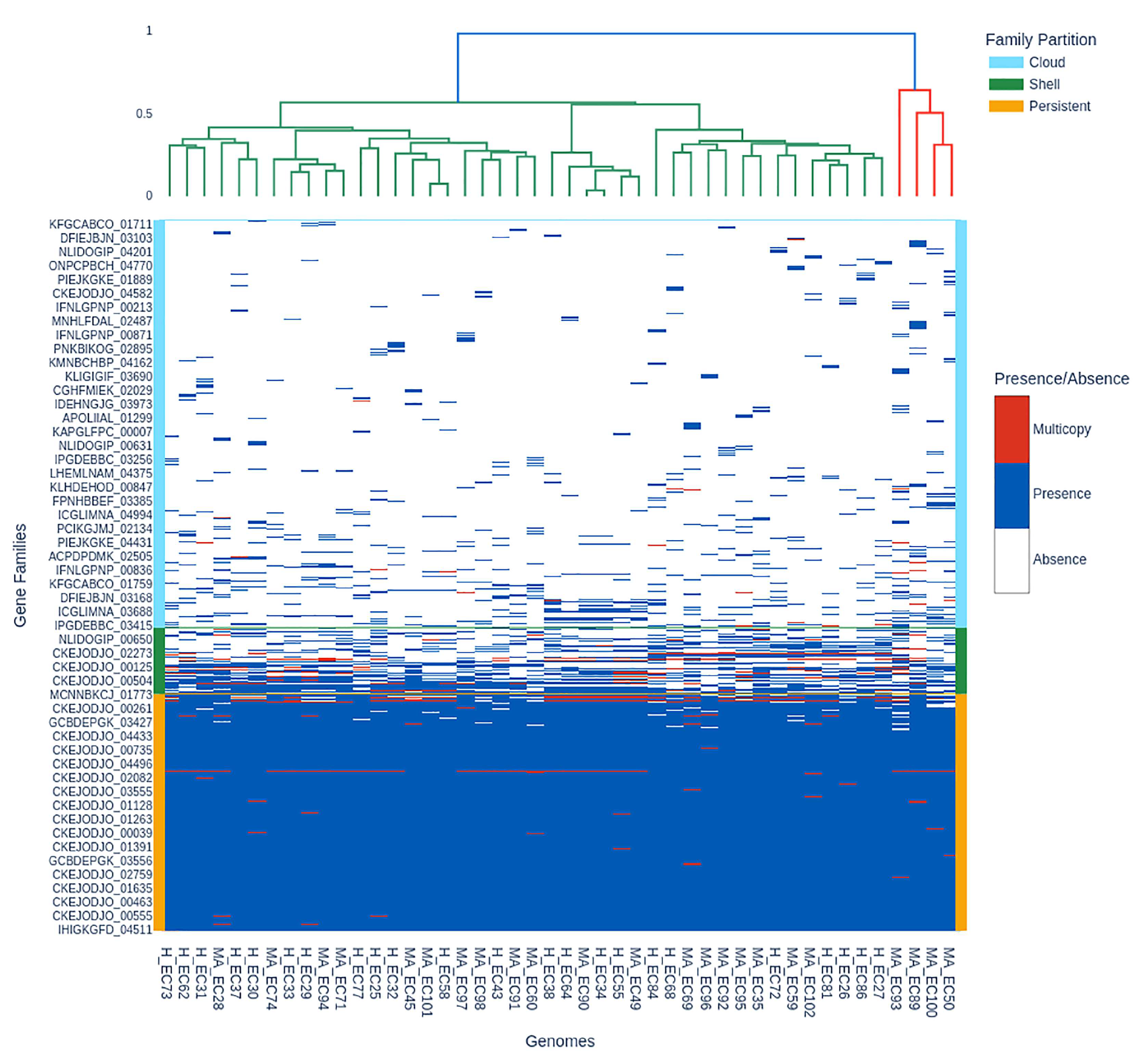
3.2. Pangenome Visualization with PPANGGOLIN Pipeline
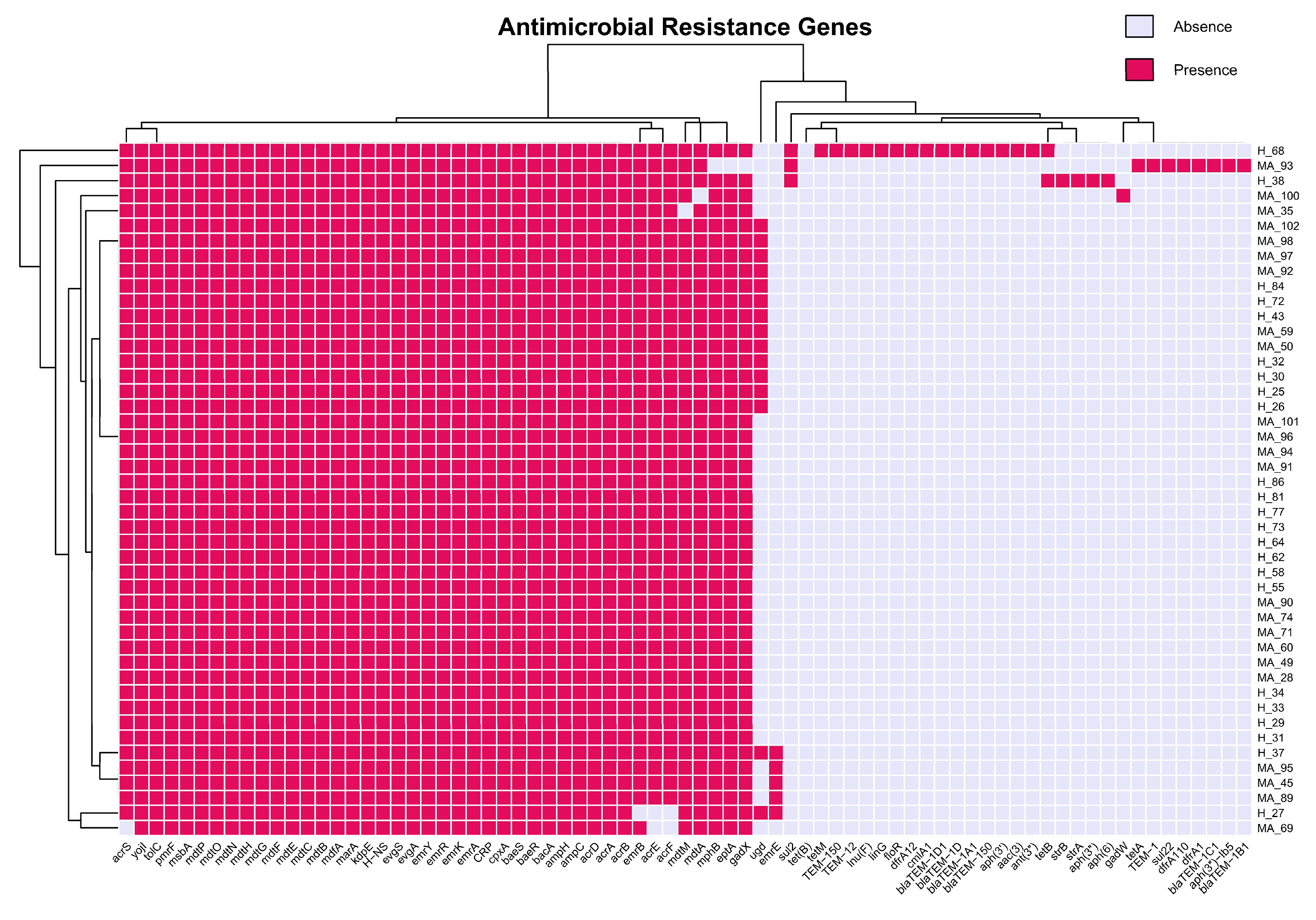
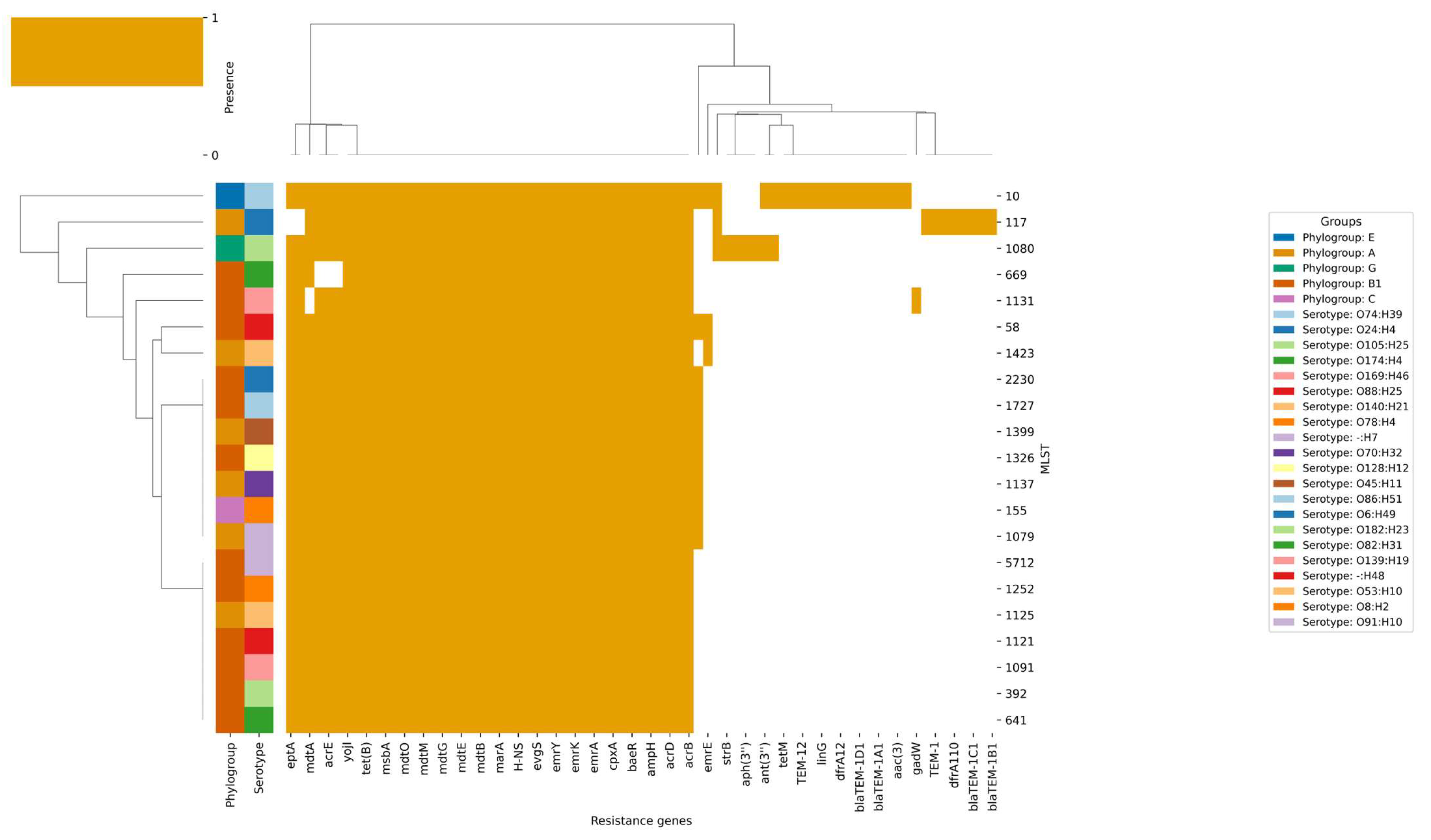
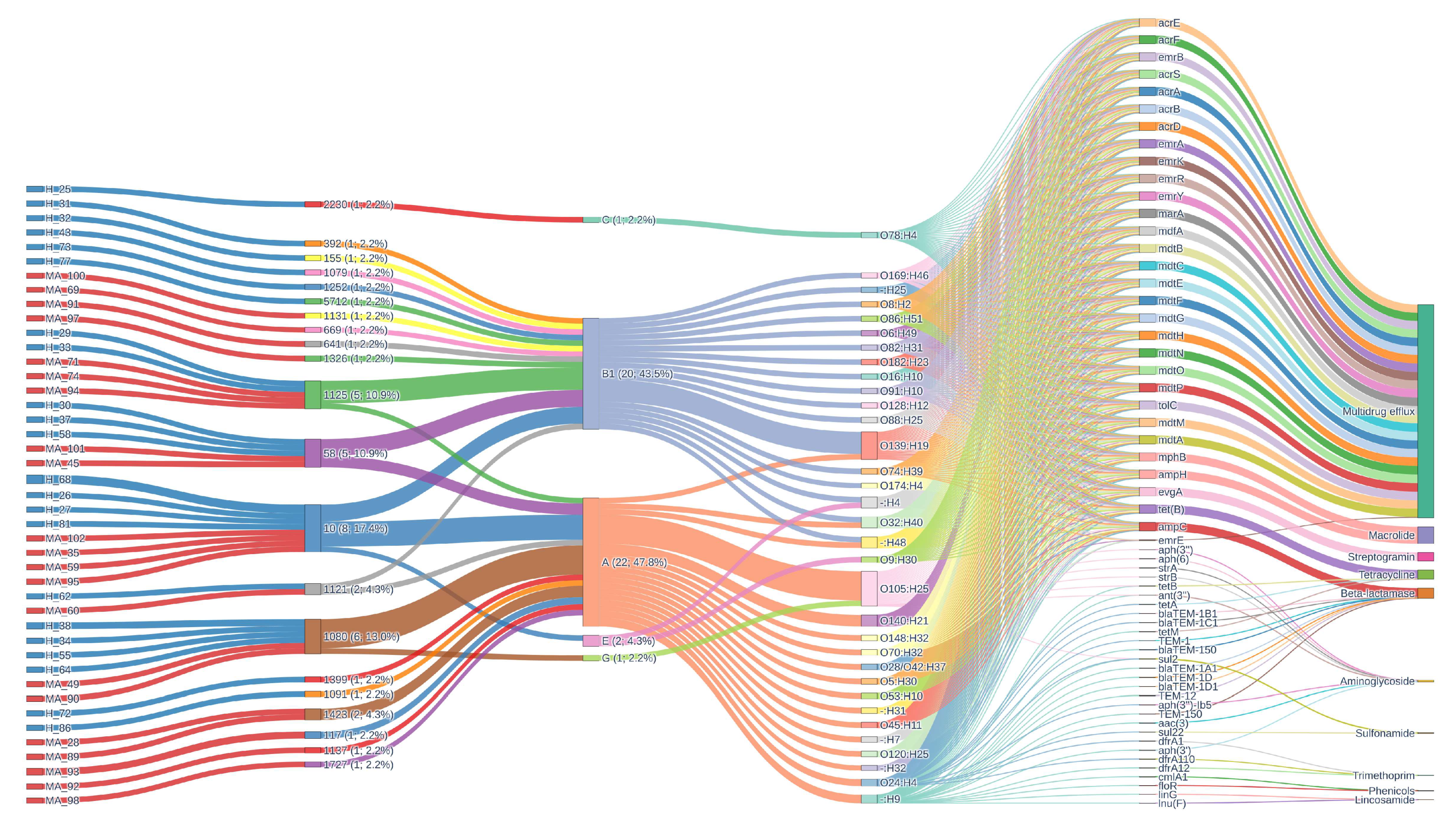
3.3. Antibiotic Resistance
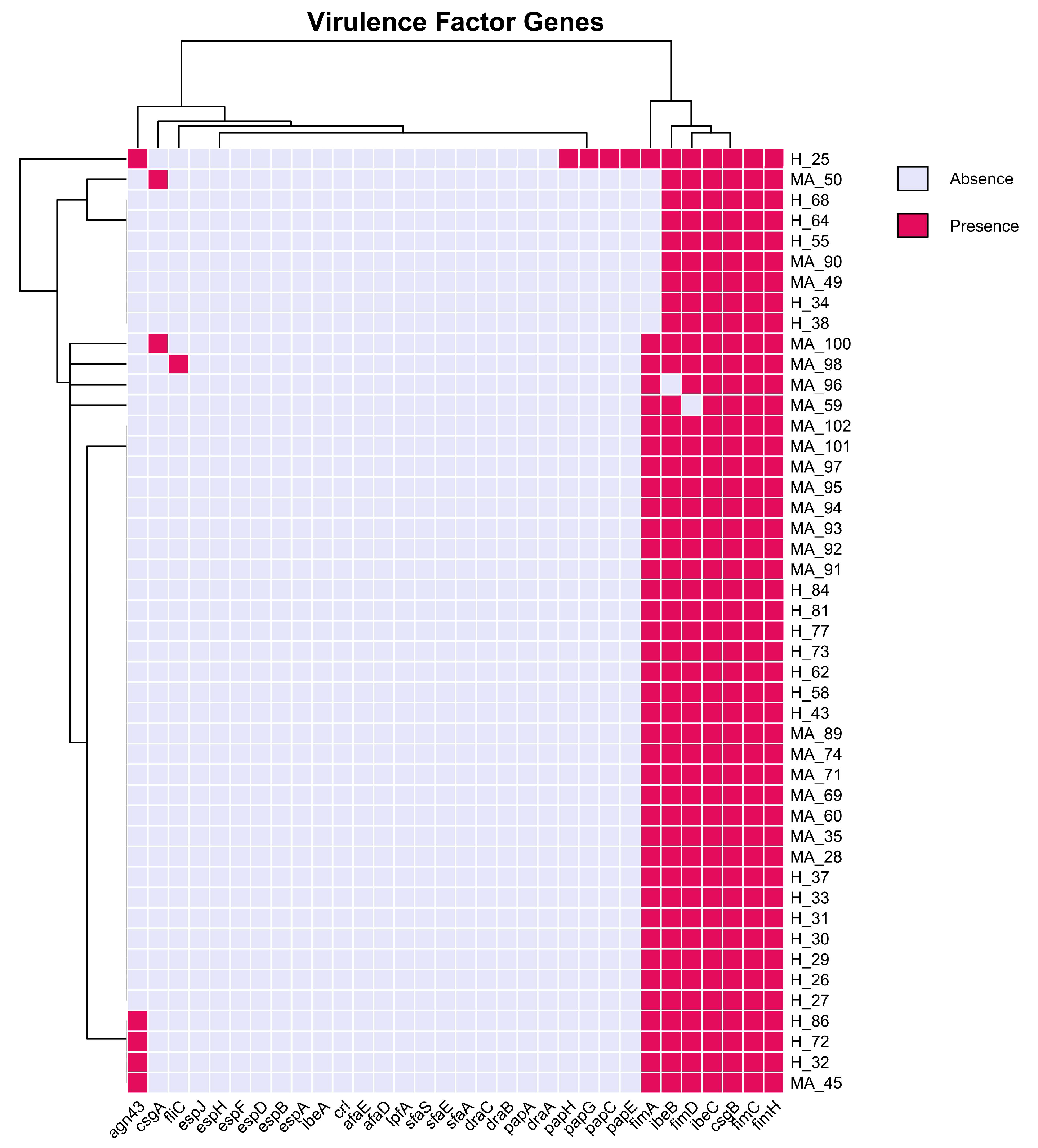
3.4. Virulence Factors
4. Discussion
4.1. Phylogenetic Diversity and Core Genome Structure
4.2. Pan-Genome Composition and Accessory Gene Variability
4.3. Antimicrobial Resistance and Virulence Genes Patterns in Mastitis Pathogenicity
4.4. Ecological and Epidemiological Insights
4.5. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riemann, T.; Bergmann, A. Epidemiology, pathogenesis, treatment and prevention of bovine acute Escherichia coli mastitis, a literature review. DTW Dtsch. Tierarztl. Wochenschr. 2000, 107, 444–454. [Google Scholar]
- Donnenberg, M. (Ed.) Escherichia coli Pathotypes and Principles of Pathogenesis; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-397048-0. [Google Scholar]
- Buitenhuis, B.; Røntved, C.M.; Edwards, S.M.; Ingvartsen, K.L.; Sørensen, P. In Depth Analysis of Genes and Pathways of the Mammary Gland Involved in the Pathogenesis of Bovine Escherichia coli- Mastitis. BMC Genom. 2011, 12, 130. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli Mastitis Is Mainly Determined by Cow Factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, N.Y.; Elazar, S.; Rosenshine, I. Mammary Pathogenic Escherichia coli. Curr. Opin. Microbiol. 2008, 11, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Orsi, H.; Guimarães, F.F.; Leite, D.S.; Guerra, S.T.; Joaquim, S.F.; Pantoja, J.C.F.; Hernandes, R.T.; Lucheis, S.B.; Ribeiro, M.G.; Langoni, H.; et al. Characterization of Mammary Pathogenic Escherichia coli Reveals the Diversity of Escherichia coli Isolates Associated with Bovine Clinical Mastitis in Brazil. J. Dairy Sci. 2023, 106, 1403–1413. [Google Scholar] [CrossRef]
- Campos, F.C.; Castilho, I.G.; Rossi, B.F.; Bonsaglia, É.C.R.; Dantas, S.T.A.; Dias, R.C.B.; Fernandes Júnior, A.; Hernandes, R.T.; Camargo, C.H.; Ribeiro, M.G.; et al. Genetic and Antimicrobial Resistance Profiles of Mammary Pathogenic E. coli (MPEC) Isolates from Bovine Clinical Mastitis. Pathogens 2022, 11, 1435. [Google Scholar] [CrossRef]
- Leimbach, A.; Poehlein, A.; Vollmers, J.; Görlich, D.; Daniel, R.; Dobrindt, U. No Evidence for a Bovine Mastitis Escherichia coli Pathotype. BMC Genom. 2017, 18, 359. [Google Scholar] [CrossRef]
- Dogan, B.; Rishniw, M.; Bruant, G.; Harel, J.; Schukken, Y.H.; Simpson, K.W. Phylogroup and lpfA Influence Epithelial Invasion by Mastitis Associated Escherichia coli. Vet. Microbiol. 2012, 159, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifucks, O.; Leitner, G. Comparison of the Immune Responses Associated with Experimental Bovine Mastitis Caused by Different Strains of Escherichia coli. J. Dairy Res. 2017, 84, 190–197. [Google Scholar] [CrossRef]
- Badis, A.; Heleili, N.; Merradi, M.; Ayachi, A.; Martino, P.A.; Meroni, G.; Soggiu, A. Outbreak of Carbapenem-Resistant High-Risk Clone ST244 of Pseudomonas Aeruginosa in Dogs and Cats in Algeria. Antibiotics 2025, 14, 230. [Google Scholar] [CrossRef]
- Passey, S.; Bradley, A.; Mellor, H. Escherichia coli Isolated from Bovine Mastitis Invade Mammary Cells by a Modified Endocytic Pathway. Vet. Microbiol. 2008, 130, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.; Klaessig, S.; Rishniw, M.; Almeida, R.A.; Oliver, S.P.; Simpson, K.; Schukken, Y.H. Adherent and Invasive Escherichia coli Are Associated with Persistent Bovine Mastitis. Vet. Microbiol. 2006, 116, 270–282. [Google Scholar] [CrossRef]
- Almeida, R.A.; Dogan, B.; Klaessing, S.; Schukken, Y.H.; Oliver, S.P. Intracellular Fate of Strains of Escherichia coli Isolated from Dairy Cows with Acute or Chronic Mastitis. Vet. Res. Commun. 2011, 35, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.A.; Cullimore, C.; Hutchison, W.D.; Grimsrud, A.; Nobrega, D.; De Buck, J.; Barkema, H.W.; Wilson, E.; Pickett, B.E.; Erickson, D.L. Genes Associated with Fitness and Disease Severity in the Pan-Genome of Mastitis-Associated Escherichia coli. Front. Microbiol. 2024, 15, 1452007. [Google Scholar] [CrossRef]
- Kempf, F.; Slugocki, C.; Blum, S.E.; Leitner, G.; Germon, P. Genomic Comparative Study of Bovine Mastitis Escherichia coli. PLoS ONE 2016, 11, e0147954. [Google Scholar] [CrossRef]
- Cruz-Soto, A.S.; Toro-Castillo, V.; Munguía-Magdaleno, C.O.; Torres-Flores, J.E.; Flores-Pantoja, L.E.; Loeza-Lara, P.D.; Jiménez-Mejía, R. Relación Genética, Formación de Biopelículas, Movilidad y Virulencia de Escherichia coli Aislada de Mastitis Bovina. Rev. Mex. Cienc. Pecu. 2020, 11, 167–182. [Google Scholar] [CrossRef]
- Olson, M.A.; Siebach, T.W.; Griffitts, J.S.; Wilson, E.; Erickson, D.L. Genome-Wide Identification of Fitness Factors in Mastitis-Associated Escherichia coli. Appl. Environ. Microbiol. 2018, 84, e02190-17. [Google Scholar] [CrossRef]
- Germon, P.; Foucras, G.; Smith, D.G.E.; Rainard, P. Invited Review: Mastitis Escherichia coli Strains—Mastitis-Associated or Mammo-Pathogenic? J. Dairy Sci. 2025, 108, 4485–4507. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Käppeli, N.; Morach, M.; Eicher, C.; Corti, S.; Stephan, R. Molecular Types, Virulence Profiles and Antimicrobial Resistance of Escherichia coli Causing Bovine Mastitis. Vet. Rec. Open 2019, 6, e000369. [Google Scholar] [CrossRef]
- Goulart, D.B.; Mellata, M. Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, Y.J. Molecular Characteristics of Escherichia coli from Bulk Tank Milk in Korea. J. Vet. Sci. 2022, 23, e9. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.B.C.; Zanardo, L.G.; Galvão, N.N.; Carvalho, I.A.; Nero, L.A.; Moreira, M.A.S. Escherichia coli from Clinical Mastitis: Serotypes and Virulence Factors. J. Vet. Diagn. Investig. 2011, 23, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Lv, Z.; Lian, L.; Wu, Z.; Fu, S.; Zhang, H.; Wu, J.; Pan, Z.; Yu, Y.; Chen, W.; et al. Difference Analysis on Virulence Genes, Biofilms and Antimicrobial Susceptibility of Escherichia coli from Clinical and Subclinical Bovine Mastitis. Vet. Sci. 2025, 12, 132. [Google Scholar] [CrossRef] [PubMed]
| Isolate ID | BioSample | Genome | Yr of Isolation | Assembly Level | Circularity | No. of Fragments | Genome Size (Mbp) | GC (%) | CDS | COV a (x) | Com b (%) | Cont c (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H_EC25 | SAMN47596209 | JBPKIM000000000 | 2023 | Contig | Y | 2 | 4.98 | 51 | 4694 | 222 | 100 | 0.43 |
| H_EC26 | SAMN47596210 | GCA_051912635.1 | 2023 | Contig | Y | 1 | 4.66 | 51 | 4319 | 149 | 100 | 0.31 |
| H_EC27 | SAMN47596211 | JBPKIL000000000 | 2023 | Contig | Y | 3 | 5 | 51 | 4734 | 82 | 100 | 0.5 |
| H_EC29 | SAMN47596212 | JBPKIK000000000 | 2023 | Contig | N | 2 | 4.94 | 51 | 4705 | 199 | 100 | 0.77 |
| H_EC30 | SAMN47596213 | JBPKIJ000000000 | 2023 | Contig | N | 14 | 5.11 | 51 | 4829 | 79 | 100 | 0.18 |
| H_EC31 | SAMN47596214 | JBPKII000000000 | 2023 | Contig | N | 2 | 5.07 | 51 | 4762 | 169 | 100 | 0.24 |
| H_EC32 | SAMN47596215 | JBPKIH000000000 | 2023 | Contig | N | 2 | 4.89 | 51 | 4523 | 134 | 100 | 0.17 |
| H_EC33 | SAMN47596216 | JBPKIG000000000 | 2023 | Contig | N | 6 | 5.02 | 51 | 4751 | 113 | 100 | 0.13 |
| H_EC34 | SAMN47596217 | JBPKIF000000000 | 2023 | Contig | N | 17 | 5.06 | 51 | 4797 | 92 | 100 | 0.17 |
| H_EC37 | SAMN47596218 | JBPKIE000000000 | 2023 | Contig | Y | 2 | 4.97 | 51 | 4663 | 116 | 100 | 0.14 |
| H_EC38 | SAMN47596219 | JBPKID000000000 | 2023 | Contig | Y | 2 | 5.17 | 51 | 4976 | 176 | 100 | 0.18 |
| MA_EC28 | SAMN47596220 | JBPKIC000000000 | 2023 | Contig | N | 5 | 5.09 | 51 | 4889 | 172 | 100 | 0.26 |
| MA_EC35 | SAMN47596221 | JBPKIB000000000 | 2023 | Contig | Y | 2 | 5.13 | 51 | 4837 | 119 | 100 | 0.26 |
| MA_EC45 | SAMN47596222 | JBPKIA000000000 | 2023 | Contig | N | 2 | 4.83 | 51 | 4519 | 167 | 100 | 0.37 |
| MA_EC49 | SAMN47596223 | JBPKHZ000000000 | 2023 | Contig | N | 12 | 5.1 | 51 | 4915 | 119 | 100 | 0.21 |
| MA_EC50 | SAMN47596224 | JBPKHY000000000 | 2023 | Contig | N | 15 | 5.16 | 51 | 4797 | 114 | 100 | 1.24 |
| MA_EC59 | SAMN47596225 | JBPKHX000000000 | 2023 | Contig | Y | 2 | 5.07 | 51 | 4818 | 226 | 100 | 0.12 |
| MA_EC60 | SAMN47596226 | JBPKHW000000000 | 2023 | Contig | N | 4 | 4.73 | 51 | 4467 | 163 | 100 | 0.08 |
| MA_EC69 | SAMN47596227 | JBPKHV000000000 | 2023 | Contig | N | 8 | 4.85 | 51 | 4621 | 275 | 100 | 0.13 |
| MA_EC71 | SAMN47596228 | JBPKHU000000000 | 2024 | Contig | N | 4 | 5.05 | 51 | 4823 | 126 | 100 | 0.34 |
| MA_EC74 | SAMN47596229 | JBPKHT000000000 | 2024 | Contig | N | 3 | 4.83 | 51 | 4535 | 194 | 100 | 0.16 |
| MA_EC89 | SAMN47596230 | JBPKHS000000000 | 2024 | Scaffold | N | 11 | 5.38 | 51 | 5192 | 201 | 100 | 0.5 |
| MA_EC90 | SAMN47596231 | JBPKHR000000000 | 2024 | Contig | N | 3 | 5.09 | 51 | 4837 | 274 | 100 | 0.18 |
| H_EC43 | SAMN47596232 | GCA_051912625.1 | 2023 | Contig | Y | 1 | 4.92 | 51 | 4600 | 318 | 100 | 0.15 |
| H_EC55 | SAMN47596233 | JBPKHQ000000000 | 2023 | Contig | N | 45 | 5.23 | 52 | 4852 | 53 | 100 | 1.29 |
| H_EC58 | SAMN47596234 | JBPKHP000000000 | 2023 | Contig | N | 3 | 4.77 | 51 | 4438 | 114 | 100 | 0.09 |
| H_EC62 | SAMN47596235 | JBPKHO000000000 | 2023 | Contig | N | 9 | 5.1 | 51 | 4923 | 144 | 100 | 0.2 |
| H_EC64 | SAMN47596236 | JBPKHN000000000 | 2023 | Contig | N | 6 | 4.92 | 51 | 4699 | 197 | 100 | 0.16 |
| H_EC68 | SAMN47596237 | JBPKHM000000000 | 2023 | Contig | Y | 3 | 4.84 | 51 | 4531 | 130 | 100 | 0.03 |
| H_EC72 | SAMN47596238 | JBPKHL000000000 | 2024 | Contig | N | 5 | 4.91 | 51 | 4576 | 243 | 100 | 0.21 |
| H_EC73 | SAMN47596239 | JBPKHK000000000 | 2024 | Contig | N | 4 | 5.02 | 51 | 4718 | 145 | 100 | 0.25 |
| H_EC77 | SAMN47596240 | GCA_051912615.1 | 2024 | Scaffold | N | 1 | 4.83 | 51 | 4475 | 233 | 100 | 0.1 |
| H_EC81 | SAMN47596241 | JBPKHJ000000000 | 2024 | Contig | N | 4 | 4.69 | 51 | 4366 | 133 | 100 | 0 |
| H_EC84 | SAMN47596242 | JBPKHI000000000 | 2024 | Contig | N | 2 | 5.02 | 51 | 4821 | 85 | 100 | 0.18 |
| H_EC86 | SAMN47596243 | JBPKHH000000000 | 2024 | Contig | Y | 2 | 4.88 | 51 | 4632 | 394 | 100 | 0.21 |
| MA_EC91 | SAMN47596244 | JBPKHG000000000 | 2024 | Contig | N | 2 | 4.78 | 51 | 4440 | 198 | 100 | 0.04 |
| MA_EC92 | SAMN47596245 | JBPKHF000000000 | 2024 | Contig | N | 2 | 4.83 | 51 | 4562 | 271 | 100 | 0.12 |
| MA_EC93 | SAMN47596246 | JBPKHE000000000 | 2024 | Contig | Y | 6 | 5.41 | 51 | 5121 | 297 | 100 | 0.48 |
| MA_EC94 | SAMN47596247 | JBPKHD000000000 | 2024 | Contig | N | 3 | 5.01 | 51 | 4780 | 153 | 100 | 0.38 |
| MA_EC95 | SAMN47596248 | JBPKHC000000000 | 2024 | Contig | N | 5 | 4.99 | 51 | 4740 | 284 | 100 | 0.12 |
| MA_EC96 | SAMN47596249 | JBPKHB000000000 | 2024 | Contig | N | 5 | 4.6 | 51 | 4257 | 396 | 100 | 0.11 |
| MA_EC97 | SAMN47596250 | JBPKHA000000000 | 2024 | Contig | Y | 3 | 4.85 | 51 | 4500 | 169 | 100 | 0.05 |
| MA_EC98 | SAMN47596251 | JBPKGZ000000000 | 2024 | Contig | N | 5 | 4.83 | 51 | 4480 | 171 | 100 | 0.07 |
| MA_EC100 | SAMN47596252 | JBPKGY000000000 | 2024 | Contig | N | 6 | 5.05 | 51 | 4592 | 167 | 100 | 0.18 |
| MA_EC101 | SAMN47596253 | GCA_051912605.1 | 2024 | Contig | N | 1 | 4.79 | 51 | 4458 | 278 | 100 | 0.08 |
| MA_EC102 | SAMN47596254 | GCA_051912595.1 | 2024 | Contig | N | 1 | 4.79 | 51 | 4504 | 237 | 100 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laterza, G.; Meroni, G.; Soggiu, A.; Martino, P.A.; Sora, V.M.; Zaghen, F.; Bonizzi, L.; Colombo, L.; Zecconi, A. Bovine Clinical E. coli Mastitis in Italian Dairy Herds Is Not Associated with a Specific Pathotype. Pathogens 2025, 14, 1181. https://doi.org/10.3390/pathogens14111181
Laterza G, Meroni G, Soggiu A, Martino PA, Sora VM, Zaghen F, Bonizzi L, Colombo L, Zecconi A. Bovine Clinical E. coli Mastitis in Italian Dairy Herds Is Not Associated with a Specific Pathotype. Pathogens. 2025; 14(11):1181. https://doi.org/10.3390/pathogens14111181
Chicago/Turabian StyleLaterza, Giulia, Gabriele Meroni, Alessio Soggiu, Piera Anna Martino, Valerio Massimo Sora, Francesca Zaghen, Luigi Bonizzi, Luciana Colombo, and Alfonso Zecconi. 2025. "Bovine Clinical E. coli Mastitis in Italian Dairy Herds Is Not Associated with a Specific Pathotype" Pathogens 14, no. 11: 1181. https://doi.org/10.3390/pathogens14111181
APA StyleLaterza, G., Meroni, G., Soggiu, A., Martino, P. A., Sora, V. M., Zaghen, F., Bonizzi, L., Colombo, L., & Zecconi, A. (2025). Bovine Clinical E. coli Mastitis in Italian Dairy Herds Is Not Associated with a Specific Pathotype. Pathogens, 14(11), 1181. https://doi.org/10.3390/pathogens14111181







